The glucagon-like peptide-1 system is modulated by acute and chronic alcohol exposure: Findings from human laboratory experiments and a post-mortem brain study
Funding information: This work was supported by NIH intramural funding (ZIA-DA000635 and ZIA-AA000218, Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, LL) jointly supported by the NIDA Intramural Research Program and the NIAAA Division of Intramural Clinical and Biological Research, the National Center for Advancing Translational Sciences (NCATS) (UH2/UH3 grant: TR000963; LL and FA), the Brain and Behavior Research Foundation (BBRF; formerly NARSAD) grant #17325 (LL), a fellowship from the Center on Compulsive Behaviors funded by the NIH Deputy Director for Intramural Research Innovation Award (MF) and a Pathways Research Award® funded by the Alkermes Pathways Research Awards Program® (MF). The development of the Computerized Alcohol Infusion System (CAIS) software was supported by Dr. Vijay Ramchandani's Section on Human Psychopharmacology in the NIAAA DICBR and by the NIAAA-funded Indiana Alcohol Research Center (AA007611). Brain tissues were received from the New South Wales Brain Tissue Resource Centre (NSWBTRC) at the University of Sydney, which is supported by NIAAA under award number R28AA012725 and Neuroscience Research Australia. The funding organizations did not have any role in the study design, execution or interpretation of the results. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abstract
Growing evidence suggests that the glucagon-like peptide-1 (GLP-1) system modulates alcohol seeking and consumption, and GLP-1 analogues may represent novel pharmacotherapies for alcohol use disorder (AUD). Accordingly, it is important to understand the potential effects of alcohol on the endogenous GLP-1 system. In a series of secondary analyses of previous human laboratory experiments, we first examined the effects of alcohol administration, with different doses and routes of administration, on peripheral active GLP-1 concentrations in heavy-drinking individuals with AUD enrolled in placebo-controlled pharmacological studies (only placebo conditions were analysed here). Alcohol administration resulted in a significant reduction of GLP-1 levels across the four experiments (oral alcohol, variable dose: F3,28 = 6.52, p = 0.002; oral alcohol, fixed dose: F7,75.94 = 5.08, p < 0.001; intravenous alcohol, variable dose: F4,37.03 = 20.72, p < 0.001; intravenous alcohol, fixed dose: F4,13.92 = 10.44, p < 0.001). Next, central expression of the GLP-1 receptor (GLP-1R) in post-mortem brain tissues (amygdala, ventral tegmental area, nucleus accumbens, hippocampus and prefrontal cortex) was compared between individuals with AUD and controls. Fold change of GLP-1R mRNA in the hippocampus was significantly higher in individuals with AUD, compared to controls (F1,21 = 6.80, p = 0.01). A trend-level effect with the same direction was also found in the prefrontal cortex (F1,20 = 3.07, p = 0.09). Exploratory analyses showed that GLP-1R gene expression levels were correlated with behavioural measures of alcohol drinking (hippocampus) and cigarette smoking (hippocampus and prefrontal cortex). Collectively, these data provide novel information on the crosstalk between alcohol and GLP-1 in a clinically relevant sample. Further studies are needed to understand the underlying mechanisms of this link.
1 INTRODUCTION
Glucagon-like peptide-1 (GLP-1) is a multifaceted hormone and neuropeptide primarily produced by enteroendocrine cells in the intestines and preproglucagon (PPG) neurons of the nucleus tractus solitarius (NTS) in the brainstem.1, 2 The GLP-1 system plays a key role in glucose homeostasis and in food intake regulation via a combination of peripheral and central mechanisms. For example, GLP-1 reduces blood glucose levels by stimulating insulin secretion and inhibiting glucagon release from the pancreas, and it decreases food intake by slowing gastric emptying and suppressing appetitive signals in the hypothalamus.3-6 For these reasons, GLP-1 analogues have been approved and are being used for the treatment of type 2 diabetes and obesity.7-9
Growing data from preclinical and clinical research suggest that the GLP-1 system is also involved in neurobiological pathways that are linked to alcohol use and addictive behaviours.10 GLP-1 is synthesized by neurons of the solitary tract that project to mesolimbic regions, such as the nucleus accumbens (NAc) and ventral tegmental area (VTA), that express GLP-1 receptors (GLP-1Rs).11, 12 These regions have a well-documented role not only in food reward but also in the rewarding properties of alcohol and other addictive substances.13 GLP-1Rs are also widely expressed in other extrahypothalamic brain regions, such as the amygdala and hippocampus,14-16 and modulate biobehavioural processes, such as stress response and cognition.17, 18 As such, several preclinical studies have examined pharmacotherapeutic potential of the GLP-1 system as a novel target to reduce alcohol consumption.19 Overall, administration of GLP-1 or GLP-1 analogues attenuates alcohol-induced locomotor activity, accumbal dopamine release and conditioned place preference (CPP) and suppresses alcohol self-administration (ASA) in rodents and non-human primates.20-31
Despite growing data from animal research, clinical evidence on the role of the GLP-1 system in alcohol use and other related outcomes is scarce. In one of the few studies, our group found that genetic variation leading to reduced function of the GLP-1R was associated with a higher risk of having an alcohol use disorder (AUD) diagnosis, increased amount of intravenous ASA and higher brain functional activity (fMRI BOLD response) in the right globus pallidus during a monetary incentive delay (MID) task.21 This study also included a preclinical experiment, where intraperitoneal injection of a GLP-1 analogue (AC3174), compared to vehicle, reduced voluntary alcohol consumption in male C57BL/6 mice that were made dependent on alcohol.21 To our knowledge, there are no published studies testing whether pharmacological manipulations of the GLP-1 system, such as administering GLP-1 analogues and/or dipeptidyl peptidase 4 (DPP4) inhibitors already approved for other indications, may reduce alcohol intake in humans, but clinical trials are under way (see Antonsen et al.32).
To obtain a deeper insight into the crosstalk between GLP-1 and alcohol, further research is needed to understand the potential impact of alcohol itself on the endogenous GLP-1 system. This question is particularly important in light of the growing interest in targeting the GLP-1 system as a pharmacotherapy for AUD.33 A previous study found significantly higher GLP-1R gene expression levels in the NAc of high versus low-alcohol-consuming rats that were given intermittent access to alcohol for 12 weeks.34 In the present study, our aim was to examine the effects of alcohol on different components of the GLP-1 system in a clinically relevant sample of heavy-drinking individuals with AUD. Specifically, we first tested, in a series of secondary analyses, the effect of acute exposure to alcohol (administered orally or intravenously with a variable or fixed dose) on peripheral active GLP-1 concentrations among heavy-drinking individuals who took part in placebo-controlled human laboratory experiments with different pharmacological interventions (only placebo conditions were analysed here). Next, we studied the impact of chronic exposure to alcohol (operationalized as a diagnosis of AUD) on the central expression of the GLP-1R gene in selected human brain regions involved in the initiation and maintenance of AUD. It is important to note that there is a high comorbidity between AUD and cigarette smoking, and some evidence points to a role of the GLP-1 system in nicotine use as well35, 36 —an aspect relevant to our post-mortem brain study (see below).
2 METHODS AND MATERIALS
2.1 Study 1
2.1.1 Design, setting and participants
Data were collected from four human laboratory experiments conducted at the National Institute on Alcohol Abuse and Alcoholism (NIAAA) in Bethesda, Maryland. The parent studies were approved by the National Institutes of Health (NIH) Addictions Institutional Review Board (IRB) and registered at Clinicaltrials.gov (NCT01751386, NCT02039349 and NCT01779024). For a detailed description of the experiments, see the references cited here.37-39 Although the experiments were independent and tested different medications/pharmacological challenges in a placebo-controlled fashion, they all included alcohol administration to nontreatment-seeking, heavy-drinking adults, and only the placebo conditions of these experiments were included in the present secondary analyses. Also, data on plasma GLP-1 concentrations were not available at the time of earlier publications,37-39 and measurements were performed later for the present study. Potential participants were recruited through advertisements and word of mouth. Interested candidates were first pre-screened through a phone interview and then brought to the NIH Clinical Center for an in-person screening visit. Individuals were then evaluated for inclusion in the studies based on respective eligibility criteria (see Appendixes S1–S3). Eligible individuals were enrolled after providing written informed consent and received compensation in proportion to their time and participation in each respective study and consistent with NIH and IRB guidelines and policies. A brief description of each experimental session is outlined below. Following the completion of alcohol administration, participants were admitted to an inpatient unit, closely monitored until their breath alcohol concentration (BrAC) reached 0 mg% and discharged the day after.
Oral alcohol, variable dose
This experiment was conducted as part of a randomized, between-subjects, double-blind, placebo-controlled human laboratory study testing the effects of baclofen on alcohol-related outcomes.37 Only the placebo group was used for this analysis (n = 13). Participants first underwent an alcohol cue-reactivity procedure conducted in a bar-like setting, during which they were exposed to visual, tactile and olfactory stimuli associated with their alcohol-containing drink of choice (and water as a neutral cue) and self-reported their craving level. Participants were then provided with a priming drink (preferred alcohol and mixer) and were instructed to consume the drink in 5 min or less. The quantity of alcohol in the priming drink was calculated based on each participant's total body water to raise the blood alcohol concentration (BAC) to 30 mg%. Forty minutes later, the ASA experiment began, during which participants were given the choice to consume up to eight mini drinks or receive an alternative reinforcer of $3.00 per mini drink not consumed, leading to a different amount of alcohol intake across participants. Each mini drink was composed of half the alcohol content of the priming drink and was designed to raise the BAC by 15 mg%. The ASA experiment lasted 120 min, and a safety limit was implemented so that BrAC would not exceed 120 mg%.
Oral alcohol, fixed dose
This experiment was conducted as part of a Phase 1b, within-subjects, dose-escalating, single-blind, placebo-controlled human laboratory study testing the safety and pharmacokinetic/pharmacodynamic characteristics of a ghrelin receptor inverse agonist (PF-5190457) co-administered with alcohol.39 Only the placebo condition was used for this analysis (n = 12). The alcohol challenge, performed on the third day after inpatient admission, included administration of a drink containing the same type of alcohol (Smirnoff vodka, 40% alcohol by volume) and each participant's preferred mixer selected from a list of seven common mixers. The quantity of alcohol for each participant was calculated based on total body water to achieve a BAC of 60 mg%.
IV alcohol, variable dose
This experiment was conducted as part of a randomized, within-subjects, double-blind, placebo-controlled human laboratory study testing the effects of IV ghrelin administration on alcohol-related outcomes.38 Only the placebo condition was used for this analysis (n = 11). During the IV-ASA experiment, participants were given the opportunity to press a button to receive IV alcohol infusions using the computerized alcohol infusion system (CAIS). Incremental infusion rates were calculated individually based on total body water to raise the BAC by 7.5 mg% over 2.5 min, followed by a decrease of 0.5 mg%/min. A progressive-ratio schedule was employed, requiring the participants to press the button for an increasing number of times to receive subsequent alcohol infusions, leading to a different amount of alcohol intake across participants. The IV-ASA experiment lasted for 120 min, and a safety limit was implemented so that BrAC would not exceed 120 mg%.
IV alcohol, fixed dose
This experiment was conducted as part of the same IV ghrelin study,38 and only the placebo condition was used for this analysis (n = 6). Here, participants received a fixed dose of IV alcohol, calculated based on total body water, which was designed to linearly increase the BAC to 80 mg% within 20 min and maintain (clamp) the BAC at this level for an additional 15 min (35 min in total). This procedure typically leads to stable BAC levels during the clamp period.
2.1.2 Blood sampling and analysis
Repeated blood samples were collected during each session. Figure 1 outlines the timing of blood sampling in relation to the meals and alcohol, where time 0 indicates the start of alcohol administration in each experiment. Of note, only the first sample of the oral alcohol variable-dose experiment was collected under fasting conditions. The timing and content of the meals were standardized within and across participants, but not across different studies (see Appendixes S4–S6 and Figure 1). At each time point, blood was collected into a K2EDTA tube (BD Vacutainer®) pre-treated with the following inhibitors: 4-(2-aminoethyl)benzene sulfonyl fluoride hydrochloride (Pefabloc®, Roche Diagnostics GmbH, Germany), dipeptidyl peptidase IV inhibitor (EMD Millipore Corp., Billerica, MA) and protease inhibitor cocktail (Sigma-Aldrich Inc., Saint Louis, MO). Samples were centrifuged within 30 min post-collection (relative centrifugal force: 1700 × g, temperature: 4°C, centrifugation time: 15 min), and the extracted plasma was stored at −80°C until analysis. The Millipore Human Metabolic Hormone Magnetic Bead Panel 96-Well Plate MILLIPLEX® MAP kit (EMD Millipore Corp., Billerica, MA, Cat. #HMHEMAG-34K) was used to measure active GLP-13, 40 concentrations, along with other analytes (acyl-ghrelin, active amylin, gastric inhibitory peptide, insulin, leptin, pancreatic polypeptide and peptide YY). Intra- and inter-assay %CVs for all MILLIPLEX analytes were <10% and <15%, respectively, as determined by the manufacturer. Values below the lower limit of quantitation (LLOQ) were set to 1/2 of the LLOQ.41

2.2 Study 2
2.2.1 Design, setting and participants
Human post-mortem brain tissues were obtained from the New South Wales Tissue Resource Centre (NSWBTRC) at the University of Sydney, Australia. This study was approved by the NIAAA Scientific Advisory Board and the NIH Office of Human Subjects Research Protections, which also determined it to be exempt from review by the NIH IRB. Tissues from five brain regions were available: amygdala, VTA, NAc, hippocampus and prefrontal cortex (PFC). Participants were male individuals with a DSM-5 diagnosis of severe AUD who also smoked (n = 11) and controls who did not have an AUD diagnosis (n = 16). Details on methods used at the NSWBTRC to collect demographic, alcohol-related and other behavioural data have been previously reported.42 Briefly, a clinician evaluated alcohol intake by retrospectively reviewing all available medical files and contacting the next of kin who completed a questionnaire. Alcohol-related questions were based on DSM diagnostic criteria and the Alcohol Use Disorders Identification Test (AUDIT).43 Number of drinks per week and per day were calculated based on an Australian standard drinking unit, which contains 10 g of pure alcohol. Information on the frequency and quantity of smoking was also calculated, based on which cigarette pack years were calculated (number of cigarette packs smoked per day multiplied by the number of years of smoking).
2.2.2 RNA extraction and analysis
Total RNA was extracted from the brain tissue, using an RNeasy Lipid Tissue Mini kit (Qiagen, Hilden, Germany), and nucleic acid extraction was performed according to the manufacturer's protocols (http://www.qiagen.com). Tissue samples were manually disrupted using a rotary homogenizer, and homogenate was passed through an RNeasy mini column containing an RNeasy silica gel membrane, which selectively binds RNA. Total RNA was subsequently resuspended in RNase-free water. RNA concentration and quality were determined using an Agilent 2100 Bioanalyzer and an RNA 6000 Nano kit. RNA samples were stored at −80°C until analysis. To quantify GLP-1R gene expression levels, 1 μg of total RNA was reverse-transcribed using a SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR kit (Invitrogen, Waltham, MA). Reverse-transcription reactions were incubated at 25°C for 10 min, 50°C for 30 min and 85°C for 5 min. Real-time quantitative polymerase chain reaction (PCR) was run in the ViiA™ 7 Real-Time PCR System using the GLP-1R (Hs00157705_m1) TaqMan Gene Expression Assay. The GADPH gene was also used as an endogenous control. Data were analysed using the ViiA™ 7 software (Applied Biosystems, Foster City, CA). Automatic cycle threshold (Ct) detection was normalized with the endogenous GAPDH gene by subtracting the Ct for GAPDH from the Ct for GLP-1R mRNA for each brain region in each participant, yielding a ΔCt. Next, ΔΔCt was calculated by subtracting the control group's average ΔCt from each individual ΔCt. Finally, the fold change in GLP-1R gene expression was calculated using the formula 2−ΔΔCt for each brain region per participant.
2.3 Statistical methods
Baseline characteristics of each study sample were summarized with descriptive statistics (mean and standard deviation for continuous variables; number and percent for categorical variables). All data were checked for normal distribution, and statistical outliers (defined as outside of the ±1.5 interquartile range) were removed prior to analysis. For Study 1, changes in plasma GLP-1 concentrations during the four experimental sessions with alcohol administrations were examined using linear mixed-effects models, with repeated time points as a fixed effect, individual participants as a random effect and GLP-1 concentrations as the outcome. A list of covariates that could potentially influence alcohol-related outcomes and/or peripheral GLP-1 concentrations (including age, gender, race and BMI) was also tested in the initial run of each model and, if significant, was included in the final model. For Study 2, group differences in fold change of GLP-1R mRNA were examined using linear mixed-effects models, with group (case vs. control) as a fixed effect, individual participants as a random effect and GLP-1R gene expression levels as the outcome. A list of covariates that could potentially influence alcohol-related outcomes, GLP-1R gene expression levels and/or the quality of post-mortem brain tissue (including age, BMI, cigarette pack years, brain pH, brain weight and post-mortem interval) was also tested in the initial run of each model and, if significant, was included in the final model. As an exploratory outcome, bivariate Pearson's correlations were also performed to examine the association between behavioural measures (including age of onset of alcohol drinking, daily alcohol intake and cigarette pack years) and GLP-1R gene expression levels (only the hippocampus and PFC, given the results of the case–control analysis). The Statistical Package for the Social Sciences (SPSS) software (IBM Corp., version 25, Armonk, NY) was used for data analysis, and the significance level was set at p < 0.05 (two-tailed).
3 RESULTS
3.1 Study 1
Table 1 summarizes baseline characteristics of the samples across the four experiments. Overall, participants were predominantly male and Black/African American, and the mean age was approximately 40 years old. Of note, although a diagnosis of AUD was an inclusion criterion only for the oral alcohol, variable-dose experiment (Appendix S1), all participants, except for one (from the oral alcohol, fixed-dose experiment), fulfilled AUD diagnostic criteria. They were all heavy drinkers as indicated by their AUDIT score and 90-day alcohol timeline follow-back (TLFB) data presented in Table 1.
| Oral alcohol, variable dose (n = 13) | Oral alcohol, fixed dose (n = 12) | IV alcohol, variable dose (n = 11) | IV alcohol, fixed dose (n = 6) | |
|---|---|---|---|---|
| Gender, male, n (%) | 11 (84.61) | 11 (91.66) | 8 (72.72) | 5 (83.33) |
| Race, Black/African American, n (%) | 10 (76.92) | 11 (91.66) | 9 (81.81) | 5 (83.33) |
| Age, years, M (SD) | 42.08 (11.28) | 40.45 (13.05) | 39.86 (11.76) | 39.45 (7.51) |
| BMI, kg/m2, M (SD) | 28.19 (4.51) | 26.94 (4.67) | 25.56 (3.20) | 25.41 (2.64) |
| Years of education, M (SD) | 13.08 (2.59) | 12.09 (3.56) | 13.09 (1.37) | 13.17 (2.04) |
| Current cigarette smoker, n (%) | 5 (38.46) | 9 (75.00) | 8 (72.72) | 5 (83.33) |
| Total AUDIT score, M (SD) | 22.42 (5.96) | 20.92 (7.09) | 20.40 (7.44) | 27.00 (7.03) |
| 90-day alcohol TLFB, M (SD) | ||||
| Drinksa per drinking days | 8.28 (4.38) | 11.34 (4.67) | 8.26 (7.34) | 10.05 (6.77) |
| Heavy-drinking daysb | 49.62 (22.05) | 66.00 (17.12) | 47.27 (25.60) | 52.67 (28.22) |
- Abbreviations: AUDIT, Alcohol Use Disorder Identification Test; BMI, body mass index; IV, intravenous; TLFB, timeline follow-back.
- a Standard drinking unit (14 g of alcohol in the United States).
- b >3 or 4 standard drinking unit per day for females or males, respectively.
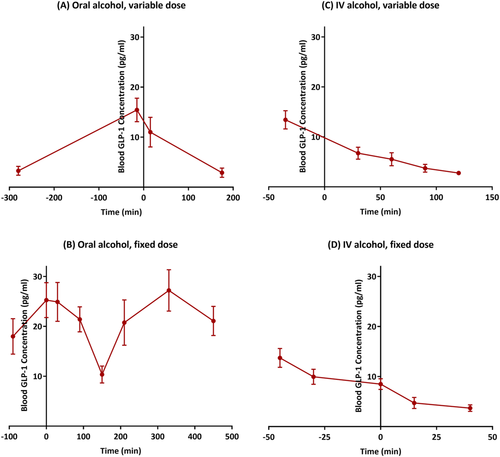
Figure 2 demonstrates plasma GLP-1 concentrations measured at repeated time points during each experimental session. GLP-1 concentrations reduced following alcohol administration (marked at time 0) in all four experiments, as depicted in Figure 2 and indicated by significant time point effects (oral alcohol, variable dose: F3,28 = 6.52, p = 0.002; oral alcohol, fixed dose: F7,75.94 = 5.08, p < 0.001; IV alcohol, variable dose: F4,37.03 = 20.72, p < 0.001; IV alcohol, fixed dose: F4,13.92 = 10.44, p < 0.001). Detailed results of the linear mixed-effects models are presented in Appendix S7. Mean, standard deviation and standard error of plasma GLP-1 concentrations per time point are also presented in Appendix S8.
3.2 Study 2
Table 2 summarizes baseline characteristics of the two groups. Participants were all male, predominantly of European ethnic origin, and the mean age was approximately 50 years old (Table 2). As expected, the AUD group showed higher daily and weekly alcohol intake and lower age of onset of alcohol drinking, compared to the control group (Table 2).
| AUD group (n = 11) | Control group (n = 16) | |
|---|---|---|
| Age, years, M (SD) | 50.55 (6.07) | 49.94 (11.32) |
| BMI, kg/m2, M (SD) | 24.64 (5.40) | 33.00 (10.45) |
| Ethnic origin, European, n (%) | 11 (100) | 14 (87.50) |
| Daily alcohol intake, g, M (SD) | 233.27 (118.09) | 18.51 (19.87) |
| Standard drinksa per week, M (SD) | 125.36 (89.99) | 9.81 (9.60) |
| Age of onset of alcohol drinking, M (SD) | 18.55 (4.13) | 24.00 (4.86) |
| Cigarette pack years, M (SD) | 45.09 (19.22) | 4.07 (13.87) |
| Brain pH, M (SD) | 6.56 (0.37) | 6.66 (0.19) |
| Post-mortem interval,b h, M (SD) | 38.91 (12.69) | 31.06 (13.94) |
| Cause of death, cardiac, n (%) | 4 (36.36) | 13 (81.25) |
- Abbreviations: AUD, alcohol use disorder; BMI, body mass index.
- a Standard drinking unit contains 10 g of alcohol in Australia (where the post-mortem brain samples were obtained from).
- b Post-mortem interval (PMI) refers to the time between death and tissue sampling. With increasing PMI, RNA progressively degrades. Therefore, a short PMI optimizes molecular research. Of note, cases and controls were matched on PMI and brain tissue pH in order to minimize potential confounding effects.
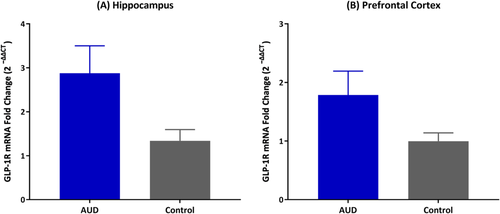
Fold change in GLP-1R mRNA in the hippocampus was significantly higher in individuals with AUD, compared to controls (F1,21 = 6.80, p = 0.01; Figure 3A). A trend-level increase in GLP-1R mRNA fold change in PFC was also found in the AUD group (F1,20 = 3.07, p = 0.09; Figure 3B). No significant differences were shown in the other brain regions (amygdala: F1,20 = 0.42, p = 0.52; VTA: F1,19 = 0.03, p = 0.84; NAc: F1,20 = 0.76, p = 0.39). Detailed results of the linear mixed-effects models are presented in Appendix S9.
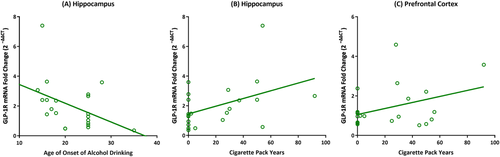
Exploratory analyses showed that earlier age of onset of alcohol drinking was associated with higher GLP-1R gene expression in the hippocampus (r = −0.43, p = 0.05; Figure 4A). Higher cigarette pack years were also associated with higher GLP-1R gene expression in the hippocampus (r = 0.43, p = 0.04; Figure 4B) and PFC (r = 0.37, p = 0.08; Figure 4C). For the full correlation matrix, see Table S1.
4 DISCUSSION
In the present study, plasma GLP-1 concentrations were reduced after alcohol administration (Figure 2), and central expression of the GLP-1R gene was higher among individuals with AUD than among controls (Figure 3). Albeit preliminary, these results provide novel information about the impact of acute and chronic alcohol exposure on the GLP-1 system, both peripherally and centrally, and are important considering the role of GLP-1 in modulating alcohol-related behaviours and its potential pharmacotherapeutic implications.10
In Study 1, participants were all nontreatment-seeking, heavy-drinking individuals, the majority of whom (except one participant) had a current diagnosis of AUD. Repeated blood samples were collected, and active GLP-1 concentrations were measured with a multiplex kit. Active GLP-1 includes GLP-1(7–36) and GLP-1(7–37), which are the GLP-1 forms that bind to the GLP-1R, leading to the activation of cellular signalling and biological activity. Other forms of GLP-1, such as GLP-1(9–36) and GLP-1(9–37), can also bind to the GLP-1R but normally do not lead to the same biological activity; they may antagonize the effects of active GLP-1 by competition for the receptor.44 Total GLP-1 would include all these types and possibly some fragments. By measuring active GLP-1 in this study, we focused on the biologically active and relevant form of the peptide.3, 40
For these secondary analyses, only the placebo conditions of the parent pharmacological studies were included in order to avoid possible confounding effects of the treatments under investigation. We did not have a matched placebo for alcohol, a major limitation due to the secondary nature of these analyses, which does not allow us to fully disentangle the effects of alcohol versus time. Nonetheless, the repeated measurements of GLP-1 concentrations and consistent findings across the four experiments increase our confidence in the observed patterns. Notably, the first time point in Figure 2A was the only one done under fasting conditions; a significant increase in GLP-1 levels was observed from the first to the second time point (prior to alcohol administration), most likely due to feeding in that period (see Figure 1A). Another key event that took place between these two time points was the alcohol cue-reactivity. Therefore, the increase in GLP-1 levels could be due to feeding, cue exposure, a combination of both or none—a set of questions that cannot be answered in our study but may generate hypotheses for future research. Although similar evidence in the alcohol field is very limited, a previous study found trend-level reduced GLP-1 concentrations following exposure to visual food cues.45 This study45 and our present study were designed to address different questions and employed different designs; hence, they are not comparable side by side, but the opposite direction of change in GLP-1 levels further highlights the need for future well-controlled studies. Blood samples were collected for a longer duration following the oral fixed-dose experiment compared with the other experiments. As demonstrated in Figure 2B, there was an increase in GLP-1 concentrations after approximately 200 min following alcohol administration, which could be a rebound effect in response to the initial reduction in GLP-1 concentrations. This increase in GLP-1 concentrations could also be due to lunch, which was served between the +150 and +210 time points (Figure 1B), similar to the increase observed between the first two time points of the oral alcohol, variable-dose experiment (Figure 2A). In general, due to the inherent limitations of the present study (e.g., small sample sizes, secondary analyses and varying methodologies across the four experiments), we encourage the readers to focus more on the pattern and direction of changes in GLP-1 levels (depicted in Figure 2), rather than pure statistical results (presented in Appendix S7), consistent with the overall goal here to evaluate the pattern of change in blood GLP-1 concentrations following alcohol administration.
Unlike oral administration, IV alcohol bypasses the first-pass metabolism and results in less variability in blood alcohol levels across participants. Oral alcohol, on the other hand, may have a local direct impact on the gastrointestinal tract that relates to GLP-1 physiology. Despite different routes of administration and dosages, alcohol reduced blood GLP-1 concentrations across the four experimental sessions, an observation that (A) can be considered an internal replication of the results and (B) suggests that any potential local effect of alcohol in the gut on GLP-1, if any, is negligible in this context. A previous study in a different sample (12 healthy, non-heavy-drinking, Caucasian males) found no significant differences between GLP-1 responses to intragastric ethanol infusion and an isoethanolaemic IV ethanol infusion.46 However, unlike in our present study, they did not find significant changes in blood GLP-1 concentrations following either oral or IV alcohol administration,46 which could be explained, at least partially, by multiple differences across the two studies, such as design, study sample, setting and/or timeline.
GLP-1 is not only an incretin with peripheral functions but is also a neuropeptide, and both the peptide and its receptor are expressed in the brain.12, 47-49 The central GLP-1 system modulates brain regions involved in reward processing, stress regulation and cognition, all of which are linked to addictive behaviours.18, 50-53 In our post-mortem brain study, the most robust finding was in the hippocampus, where individuals with AUD had significantly higher GLP-1R gene expression levels, compared to controls. The hippocampus plays a key role in memory, emotion processing and social cognition; alcohol use and AUD may lead to deficits in these cognitive domains, which may increase the risk of relapse.54 Furthermore, studies have shown that the amnesia effects of alcohol on memory are linked to the hippocampus and that alcohol can suppress neurogenesis in the hippocampus, highlighting the importance of this brain structure in the pathophysiology of AUD.55, 56 In addition to the hippocampus, a trend-level increase in GLP-1R gene expression was also found in the PFC among individuals with AUD, compared to controls. The PFC also plays a prominent role in addiction and is influenced by addictive substances, including alcohol. Dysfunction of the PFC can cause problems with self-control related to harmful actions and can contribute to the development of craving and preference for alcohol and other drugs.57, 58 The present post-mortem brain findings must be considered preliminary, as the study design did not enable us to disentangle causation versus correlation—an important question that must be probed in future research, possibly by back-translating this work in preclinical models of chronic alcohol exposure.
Exploratory analyses also found that GLP-1R gene expression levels were positively associated with higher severity of alcohol use (as indicated by lower age of onset of alcohol drinking) and cigarette smoking (as indicated by higher cigarette pack years). The significant bivariate correlation with age of onset of alcohol drinking is consistent with our case–control analysis, further suggesting that chronic exposure to alcohol results in overexpression of the GLP-1R gene in the hippocampus. The positive association with cigarette pack years is also interesting, as research has found the GLP-1 system to be involved in nicotine use as well.35, 36 It is important to note that our post-mortem brain study was not able to tease out the effects of alcohol versus nicotine due to an inherent limitation: all participants in the AUD group (100%), but only two participants in the control group (12.5%), were cigarette smokers. We did control for cigarette pack years as a covariate in our case–control analysis, but disentangling the effects of alcohol versus nicotine would require a fully factorial design with participants who use one drug, but not the other.
To our knowledge, this is the first human study reporting how alcohol influences the GLP-1 system, both the peptide and the receptor, in heavy-drinking individuals with AUD. A previous preclinical study investigated GLP-1R gene expression levels in rats with high versus low alcohol consumption. GLP-1R gene expression was quantified via PCR in six brain regions (NAc, VTA, amygdala, hippocampus, PFC and striatum), using the RNeasy Lipid Tissue Mini Kit. Results showed that the high-alcohol-consuming rats had significantly higher GLP-1R gene expression in the NAc than the low-alcohol-consuming rats, but little difference was observed in the other brain regions.34 In our study, although not statistically significant, participants with AUD had higher GLP-1R gene expression levels in the NAc, compared to healthy controls (fold change of 1.377 vs. 1.073)—a trend that is consistent with the aforementioned study.34 Nonetheless, from a statistical standpoint, the results did not fully replicate across the two studies, which could be related to the different designs, kits and/or species. For example, in our study, participants either had a lifetime diagnosis of AUD or were controls, whereas the other study compared rats who had consumed various amounts of alcohol over a 12-week period.
The studies presented in this report had several limitations, some of which have been outlined above. Both studies had relatively small sample sizes, and the results must be considered preliminary. Because of the secondary nature of Study 1 and the lack of a control condition for alcohol, it is difficult to confidently relate the observed change in GLP-1 concentrations to alcohol alone. An ideal design would have a control arm to tease out the effect of alcohol from other possible factors (e.g., time). However, the experimental conditions, such as timing and food intake, were standardized before and during each session, which reduces the risk of confounding effects. Study 1 also used predominantly male subjects around the age of 40. Because of the small sample size and limited demographics, the results may not necessarily generalize to other populations, such as women or younger/older individuals. Similarly, Study 2 only included male individuals and had a small sample, potentially limiting the generalizability of the findings to larger and more diverse populations. As previously mentioned, the composition of our case and control groups did not allow us to distinguish the effects of alcohol versus nicotine use in this sample. Any post-mortem study would also pose some limitations, as it is difficult to control for all potential variables at play, such as nutrition and lifestyle, and reporting biases (e.g., the inevitable use of information from a next to kin) may limit an accurate history of alcohol use.
In summary, our results suggest that alcohol intake reduces peripheral GLP-1 concentrations in heavy-drinking individuals with AUD, and certain brain regions show higher GLP-1R gene expression in individuals with AUD than in controls. To address some of the study limitations, future studies with a priori designs and larger and more diverse samples are needed.
ACKNOWLEDGEMENTS
We thank the clinical and research staff involved in patient care, data collection/analysis and technical support in the joint NIDA/NIAAA Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section (in particular, Ms. Sarah Min, Dr. Petra Suchankova and Dr. Mary Lee), in the NIAAA clinical programme of the Division of Intramural Clinical and Biological Research (DICBR) (in particular, the NIAAA Office of the Clinical Director and the NIAAA Clinical Core Laboratory), and in the NIH Clinical Center (Departments of Nursing, Nutrition, and Pharmacy). We would also like to thank Dr. Vijay Ramchandani (Section on Human Psychopharmacology, NIAAA DICBR) and Dr. Reza Momenan (Clinical NeuroImaging Research Core, NIAAA DICBR) for their support in the execution of the parent studies from which some of these analyses stemmed; Ms. Donna Sheedy, Dr. Jillian Kril and Dr. Greg Sutherland (New South Wales Tissue Resource Centre (NSWBTRC), University of Sydney, Australia) for providing the human post-mortem brain tissue for this project; and Dr. Gail Seabold (NIDA, NIH) for proofreading the article. The authors would also like to express their gratitude to the participants who took part in these studies.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
N/A.


