Self-control impacts symptoms defining Internet gaming disorder through dorsal anterior cingulate–ventral striatal pathway
Funding information: This study was supported by the National Natural Science Foundation of China (Grant numbers: 81971245, 62077042, and 81871426) and Qianjiang Talent Plan of Zhejiang Province (Grant number: D-2019).
Abstract
Self-control is important for long-term success and could be a protective factor against maladaptive behaviours such as excessive gaming activity or Internet gaming disorder (IGD). However, the neurobiological basis of self-control and its relationship to IGD remain elusive. Using resting-state fMRI data from 89 participants aged from 18 to 26, we found that self-control and the number of IGD symptoms (IGD-S) were positively and negatively correlated with functional connectivity between right ventral striatum (rVS) and dorsal anterior cingulate cortex (dACC), respectively. A mediation analysis indicated that self-control influenced IGD-S partially through the rVS-dACC connectivity. In addition, step-wise regression analyses revealed that the rVS connectivity in a reward-anticipation limbic pathway contributed to IGD-S but not self-control, independent of the dACC pathway. These results suggest that the cingulate–ventral striatal functional connectivity may serve as an important neurobiological underpinning of self-control to regulate maladaptive behaviours such as these manifesting IGD through striatal circuitry balance.
1 INTRODUCTION
To address the increasing public concern regarding negative consequences of excessive digital gaming, the World Health Organization has suggested the diagnosis of Gaming Disorder, and the American Psychiatric Association has also listed Internet gaming disorder (IGD) as a condition for future consideration.1 Further, after the outbreak of COVID-19, students of all ages suddenly access to the Internet for much longer time to take online courses or make social connections.2 As self-control and underlying neural substrates are still under development in children and adolescents,3 sudden and prolonged access to the Internet may greatly increase their risk of developing maladaptive Internet use behaviours including IGD. The outbreak of COVID-19, therefore, made this issue even more concerned. While the term self-control is ordinarily used to describe decisions between alternatives arriving at different times,4 contemporary psychologists refer it to as the ability to adjust one's impulses/responses to support the pursuit of long-term goals.5 As an important cognitive ability that supports our daily activities, high self-control can predict good adjustment, better grades, and interpersonal success.6 In contrast, low level of self-control is associated with undesired behaviours such as alcohol abuse, gambling,7 and IGD.8, 9 Using a behavioural paradigm, Bailey et al.10 found that video game experience had a negative influence on proactive cognitive control, a critical cognitive construct related to self-control. In addition, individuals with IGD showed defective emotional regulation or adjustment,11, 12 poor executive function,13 and suboptimal decision under risk and weakened ability to delay gratification.14, 15 Further, the level of self-reported self-control ability is inversely related to IGD.16, 17
Regarding the neural basis of IGD, abnormalities in brain structure and function in default mode network (DMN), frontoparietal network (FPN), limbic system, anterior cingulate cortex (ACC), and many other areas have been documented.18-20 In contrast, neuroimaging studies demonstrate that cognitive control is associated with prefrontal cortex (PFC), ACC, and caudate nucleus.21 Particularly, IGD subjects showed diminished brain activation in ACC in a response inhibition task,22 which is similar to findings from a study on drug addiction.23 Relatedly, alterations of functional connectivity in executive control networks (ECNs) and fronto-striatal circuits have also been implicated in self-control and addiction.24, 25
Apart from impaired self-control, reward-related sensitization is also an important component contributing to IGD from a dual-process perspective.21, 26 Resting-state functional connectivity between nucleus accumbens (NAc) and reward networks (including amygdala, striatum, and orbitofrontal regions) have been found significantly higher in addictive populations than that in control groups.27 While the sensitization to drug and drug cues is thought to be underpinned by a ‘hot’ system including the reward network, its counterpart process, self-control, is related to a ‘cold’ system mainly composed by dorsal ACC (dACC), dorsolateral PFC (DLPFC), presupplementary area, dorsal premotor area, anterior insula, and the intraparietal cortex.21, 28
Anatomically, the striatum receives both glutamatergic and dopaminergic projections from multiple cortical and midbrain regions, respectively,29, 30 indicating that the striatum may be an integrative hub for both self-control and reward-related sensitization. Indeed, the activation of ventral striatum (VS) was increased during a decision-making task and decreased during a response inhibition task in pathological gambling subjects compared to that in healthy controls.31 Such task-related hypoactivation and hyperactivation may result from weakened and potentiated inputs from the ‘cold’ and ‘hot’ systems, respectively. Relatedly, the functional connectivity between VS and dACC has been found negatively correlated with nicotine dependence severity32 and compulsive cocaine use,33 which may reflect a weakened ‘cold’ system in addictive populations. However, whether self-control and addictive gaming behaviour are related to the same VS-dACC circuit remain a lack. Whether the striatal connectivity in the reward ‘hot’ system also contributes to self-control and IGD in dependent of that in the ‘cold’ system is not clear, either.
The goal of this work was to address these questions by examining the relationships among self-control, IGD, and resting-state striatal functional connectivity. Based on previous studies mentioned above, we hypothesized that the VS connectivity in areas within cognitive control network would associate with both self-control and IGD, and the VS connectivity with reward-related area would contribute to IGD independent of the cognitive pathway.
2 METHODS
2.1 Participants
One hundred and twenty-eight young adults were recruited through online advertisement. Information regarding symptoms of gaming disorder, self-control, anxiety, depression, and demography were surveyed. Particularly, we asked the subjects to report if they played Internet games or not. We also designed two catch trials to exclude individuals who filled the survey carelessly. The participants who reported “do not play internet games” (n = 35), scored in anxiety, depression, or self-control away from the group mean more than 3 standard divisions, or made incorrect response in catch trails were considered outliers and had also been excluded (n = 4). With these exclusion criteria, 89 gamers aged from 18 to 26 were left for fMRI analysis (Table 1).
| All participants | Gamers | ||
|---|---|---|---|
| N | 124 | 89 | |
| Age (years) | M (SD) | 20.21 (2.03) | 20.07 (1.94) |
| Range | [18, 26] | [18, 26] | |
| Sex | male | 56 | 53 |
| female | 68 | 36 | |
| IGD-S | M (SD) | 1.69 (2.06) | 2.36 (2.09) |
| ≥5 | 18 | 18 | |
| ≥5 male | 12 | 12 | |
| ≥5 female | 6 | 6 | |
| SCS | M (SD) | 16.01 (2.62) | 15.85 (2.63) |
| HM | M (SD) | 0.1445 (0.0430) | 0.1479 (0.0453) |
| Anxiety | M (SD) | 10.94 (3.32) | 10.80 (3.14) |
| Depression | M (SD) | 9.48 (3.45) | 9.75 (3.66) |
- Note: N, number of participants; M, mean value; SD, standard deviation; HM, head motion parameters; SCS, self-control scale; IGD-S, number of Internet Gaming Disorder symptoms.
This study was approved by the Ethic Committee of Zhejiang University. Written informed consent was obtained from all individuals prior to study enrollment, and participants received financial compensation. The fMRI experiment was conducted between August 2020 and October 2020 at the Center for Brain Imaging Science and Technology (CBIST), Zhejiang University.
2.2 Questionnaire assessments
2.2.1 Self-control
The Chinese version of Self Control Scale (SCS)6, 34 was used to measure self-control. The SCS contains five dimensions, namely, impulse control, healthy habit, resistance to temptation, work focus, and appropriate entertainment. The number of items in each dimension is 6, 3, 4, 3, and 3, a total of 19 items. For each item, a graded response was selected from 1 (not at all) to 5 (very much). Score of each dimension is the mean score of items in this dimension and total score is the sum of all dimension's scores.
2.2.2 Symptoms of IGD
The nine criteria recommended in DSM-51, 35 were used to assess the severity of gaming disorder. A previous study has tested the psychometric properties of the Chinese version Internet Gaming Disorder scale and demonstrated good internal consistency and test-retest reliability.36 Participants were instructed to report ‘yes’ or ‘no’ about whether they showed a symptom in the last 12 months. Online gambling was excluded from this criterion. The total number of symptoms a participant met was recorded for the participant. Instead of a dichotomic diagnosis, it has been recommended to characterize IGD with multiple severity levels in DSM-5.1 Similar to our previous studies,33, 37 the present study used the number of IGD symptoms (IGD-S), as a proxy for IGD risk.
Measures used as confounding variables
Anxiety and depression dimensions in the Brief Symptom Inventory (BSI)38 were used to characterize symptoms of anxiety and depression. Each dimension includes six items. For each item, a graded response was selected from 1 (not at all) to 5 (extremely). The bigger sum of six items means the more severe symptom.
2.3 FMRI data acquisition
All participants underwent a resting-state fMRI scan in a 3T scanner (MAGNETOM Prisma, Siemens Healthcare Erlangen, Germany) using gradient echo planar image (EPI) sequence with multiband (MB) acceleration. The sequence parameters were as follows: Repetition time (TR) = 1000 ms, echo time (TE) = 34 ms, flip angle = 50°, slice thickness = 2.50 mm, size of per voxel = 2.5 × 2.5 × 2.5 mm3, field of view = 230 × 230 mm2, 52 slices per volume, matrix = 92 × 92, and MB = 4. Each run included 480 imaging volumes for each participant. All participants were instructed to lie quietly in the scanner, keep awake, stare at a white crossing on the screen and try not to think of anything specific. An anatomic T1-weighted image was collected for each participant for spatial normalization with the following parameters: TR = 2300 ms, TE = 2.32 ms, flip angle = 8°, size of per voxel = 0.938 × 0.937 × 0.9 mm3, field of view = 240 × 240 mm2, 208 slices in the sagittal panel, matrix = 256 × 256.
2.4 FMRI data preprocessing
Resting-state imaging data were analysed using AFNI39 and ANTs.40 The preprocessing steps included slice timing correction, realignment, segmentation, normalization, smoothing (FWHM = 5 mm), nuisance regression (with 6 head motion parameters and their temporal depravities as well as the first 5 principal components from the white matter and CSF regions), and band pass filtering (0.01–0.1 Hz).
2.5 Seed selection and first-level functional connectivity computation
The resultant z maps were defined as resting-state functional connectivity (rsFC) maps.
2.6 Statistical Analyses
To examine the relationships between striatal functional connectivity and self-control as well as IGD-S, voxel-wise regression analyses were conducted on the rVS-seeded connectivity maps against SCS and IGD-S in the participants who reported playing games, respectively. Age, sex, anxiety, depression, and head motion (the average frame displacement) were included as covariates. The same voxel-wise regression analyses were conducted on the lVS-seeded connectivity maps. A cognitive control brain network was generated using Neurosynth (https://neurosynth.org/) by searching the term ‘cognitive control’ (Figure S1a). The voxel-wise regression analysis was constrained by this cognitive control brain mask, correcting for multiple comparisons based on AFNI's autocorrelation function (ACF) model.41 Specifically, we simulated noise volume assuming the ACF given by a mixed-model of the form ‘a * exp(−r * r/(2 * b * b)) + (1 − a) * exp(−r/c)’, where a, b, and c are three parameters to be estimated by AFNI command 3dFWHMx, and the minimum cluster size, which was 17 voxels in the present study, was determined using 3dClustSim with the condition of voxel-level p < 0.001.
To test the hypothesis that there is a common neural basis that underlies self-control and IGD-S, a conjunction analysis was carried out to identify brain regions whose connectivity with rVS was associated with both self-control and IGD-S. Then, the self-control was inputted as the primary variable (X) in a mediation model to predict the outcome IGD-S (variable Y) with the connectivity extracted from the overlapped region (variable M) as a possible mediator. The mediation analysis was conducted using SPSS Process based on Hayes and Preacher's work.42 Sex, age, anxiety, depression, and frame-displacement in head motion parameters (HM for short) were included as covariates and the model was bootstrapped 5000 times.
To test the hypothesis that both self-control and IGD-S would be also influenced by the limbic ‘hot’ system, step-wise multiple linear regressions were performed to regress the SCS and IGD-S with functional connectivity strength between rVS and the cognitive control region as well as the reward-anticipation ‘hot’ region as predictors. The brain network related to reward anticipation was defined in Neurosynth (https://neurosynth.org/) by searching the term ‘reward anticipation’. The resultant network mainly consisted of middle brain ventral tegmental area (VTA), VS, and right amygdala-parahippocampus complex (AmyPara for short) (Supplementary Figure S1b). As our seed VS was part of the striatum, we selected the right AmyPara and VTA as ROIs to test the hypothesis that regions of the ‘hot’ system connecting with VS would contribute to IGD-S and self-control independent of regions of the ‘cold’ system. Age, sex, anxiety, depression, and HM were included as covariates in the multiple regression models.
3 RESULTS
3.1 Test of the correlation between self-control and IGD-S
The demographic information was shown in Table 1. Controlling for the impacts of age, sex, anxiety, depression, and head motion (HM) inside MRI scanner, the SCS score was found to negatively correlate with IGD-S (partial r = −0.364, p < 0.001).
3.2 The relationship between VS connectivity and SCS
The result of multiple regression on rVS rsFC against SCS was shown in Figure 1 with corrections for multiple comparisons (i.e., per-voxel p value <0.001 and cluster size >17 voxels). The connectivity strength between rVS and dACC was significantly correlated with self-reported self-control (i.e., SCS score) after controlling for HM, anxiety, depression, age, and sex (Figure 1A). A partial residual plot with above confounds being removed was shown in Figure 1B. As for the lVS seed, the same multiple regression against self-control yielded no region of significance.
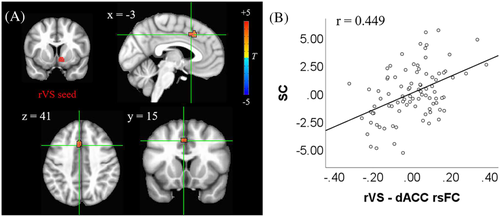
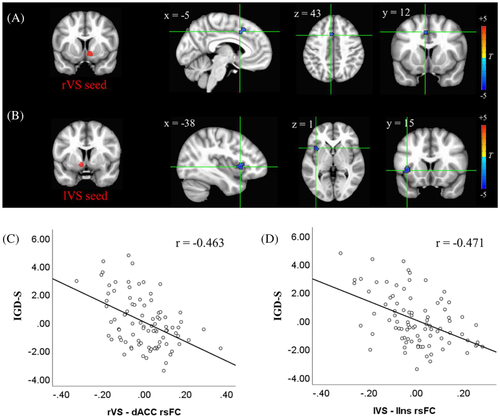
3.3 The relationship between VS connectivity and IGD-S
The result of multiple regression on rVS rsFC against IGD-S was shown in Figure 2A. The connectivity strength between rVS and an area of dACC was significantly correlated with IGD-S after controlling for HM, anxiety, depression, age, and sex. As for the lVS seed, its connectivity with an area in left insula was significantly correlated with IGD-S after controlling for HM, anxiety, depression, age, and sex (Figure 2B). The partial residual plots for the relations between IGD-S and rsFC were shown in Figure 2C,D.
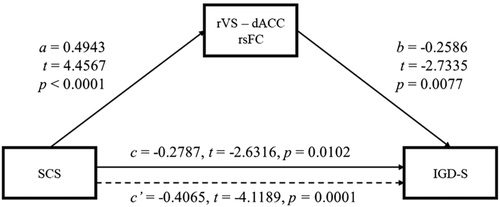
3.4 The mediation role of rVS-dACC rsFC in the relationship between self-control and IGD-S
The mediation analysis showed that the rVS connectivity with the dACC region relating to both SCS and IGD-S (i.e., the common region in Figures 1A and 2A) partially mediated the relationship between self-control and IGD-S (Figure 3 and Table 2). The SCS score could significantly predict the IGD-S both directly (t = −2.6316, p = 0.0102) and indirectly through the rVS-dACC connectivity (Effect = −0.1016, BootSE = 0.0421, 95% BootLLCI = −0.1923, 95% BootULCI = −0.0267).
| Model | Coeff | SE | Std coeff | t | p | LLCI | ULCI |
|---|---|---|---|---|---|---|---|
| rVS-dACC rsFC | |||||||
| SCS | 0.0262 | 0.0059 | 0.4943 | 4.4567 | 0.0000 | 0.0145 | 0.0379 |
| HM | 0.4192 | 0.3215 | 0.1363 | 1.3038 | 0.1960 | −0.2204 | 1.0587 |
| Sex | 0.0094 | 0.0305 | 0.0333 | 0.3085 | 0.7585 | −0.0513 | 0.0701 |
| Age | 0.0054 | 0.0072 | 0.0752 | 0.7489 | 0.4561 | −0.0089 | 0.0197 |
| Anxiety | −0.0032 | 0.0056 | −0.0720 | −0.5670 | 0.5723 | −0.0144 | 0.0080 |
| Depression | 0.0076 | 0.0049 | 0.1989 | 1.5316 | 0.1295 | −0.0023 | 0.0174 |
| IGD-S | |||||||
| SCS | −0.2216 | 0.0842 | −0.2787 | −2.6316 | 0.0102 | −0.3891 | −0.0540 |
| rVS-dACC rsFC | −3.8769 | 1.4183 | −0.2586 | −2.7335 | 0.0077 | −6.6989 | −1.0549 |
| HM | 14.2697 | 4.1717 | 0.3094 | 3.4206 | 0.0010 | 5.9693 | 22.5701 |
| Sex | −0.5838 | 0.3921 | −0.1378 | −1.4889 | 0.1404 | −1.3639 | 0.1964 |
| Age | −0.0801 | 0.0929 | −0.0744 | −0.8622 | 0.3911 | −0.2650 | 0.1048 |
| Anxiety | 0.0489 | 0.0726 | 0.0734 | 0.6736 | 0.5025 | −0.0955 | 0.1933 |
| Depression | 0.0592 | 0.0644 | 0.1037 | 0.9188 | 0.3609 | −0.0690 | 0.1874 |
- Note: Model rVS-dACC rsFC dependent variable: rVS-dACC rsFC; predictor: (constant), head motion (HM), anxiety, depression age, sex, SCS score; Model IGD-S dependent variable: IGD-S; predictor: (constant), SCS score, rVS-dACC rsFC, head motion (HM), sex, age, anxiety, and depression.
3.5 Additional contributions of the reward network to IGD-S and self-control
A stepwise regression model was used to test whether the rVS rsFC with the ‘hot’ system (AmyPara and VTA, Figure S1b) can explain additional variance in SCS and IGD-S after controlling for the contribution of the rsFC of the ‘cold’ circuit rVS-dACC. The results showed that the rVS-AmyPara rsFC could significantly predict the IGD-S (Tables 3 and 4, t = 2.209, p = 0.046) independent of the rVS-dACC rsFC. But the rVS-AmyPara rsFC could not predict the SCS score (t = 1.164, p = 0.248) independent of the rVS-dACC rsFC. In addition, the rVS-VTA rsFC was not correlated with neither self-control nor IGD-S.
| Model | R2 | Adjusted R2 | ΔR2 | ΔF | Sig. ΔF | p |
|---|---|---|---|---|---|---|
| 1 | 0.372 | 0.350 | 0.029 | 4.118 | 0.046 | <0.001 |
| 2 | 0.402 | 0.373 | <0.001 |
- Note: Dependent variable: IGD-S. Model 1 predictors: (constant), HM, rVS-dACC rsFC, depression; Model 2 predictors: (constant), HM, rVS-dACC rsFC, depression, rVS-AmyPara rsFC.
| Model | Unstandardized | Standardized | t | p | |
|---|---|---|---|---|---|
| Beta | SE | Beta | |||
| 1 | |||||
| HM | 17.799 | 3.990 | 0.386 | 4.461 | <0.001 |
| rVS-dACC rsFC | −5.636 | 1.289 | −0.376 | −4.373 | <0.001 |
| Depression | 0.149 | 0.049 | 0.261 | 3.015 | 0.003 |
| 2 | |||||
| HM | 17.988 | 3.920 | 0.390 | 4.589 | <0.001 |
| rVS-dACC rsFC | −5.413 | 1.271 | −0.361 | −4.260 | <0.001 |
| Depression | 0.146 | 0.049 | 0.256 | 3.013 | 0.003 |
| rVS-AmyPara rsFC | 2.942 | 1.450 | 0.172 | 2.029 | 0.046 |
- Note: Dependent variable: IGD-S. Model 1 predictors: (constant), HM, rVS-dACC rsFC, depression; Model 2 predictors: (constant), HM, rVS-dACC rsFC, depression, rVS-AmyPara rsFC. Nonsignificant predictors including age, sex, and anxiety were excluded from the table.
4 DISCUSSION
Using resting-state functional magnetic resonance imaging, we observed that self-control and IGD-S were positively and negatively correlated with functional connectivity between rVS and dACC, respectively. The mediation analysis demonstrated that self-control influenced IGD-S partially through the rVS-dACC connectivity. In addition, the step-wise regression analyses revealed that the AmyPara limbic pathway contributed to IGD-S but not self-control, independent of the cognitive dACC pathway.
Self-control has been found related to ventral medial PFC, lateral PFC, VS, dACC, and caudate.21, 33, 43-47 According to the force-field model and the reward-based model,21 self-control is composed by two competing processes. One is the driving force induced by immediate reward, emotional arousal, and other automatic reactive processes that are underpinned by a ‘hot’ system. In contrast, the other one is the restraining force associated with the mental effort to maximize the expected value of control that is underpinned by a ‘cold’ system. Previous studies suggested that dACC, one node of the ‘cold’ system, is a core region for monitoring and solving conflicts,48, 49 whereas the VS, a typical region of the ‘hot’ system, is related to the expectation/representation of reward50, 51 and motivation.52 Anatomically, VS receives projections from both ‘hot’ and ‘cold’ systems,30 making it a possible integrative interface for top-down controls over bottom-up impulsions. Although a detailed picture about how different projections interact with each other at the synaptic level remains unclear, rsFC seems to be able to capture the influence of the neural activity of a top-down control region (e.g., dACC) on that of the VS, with a consistent behavioural relevance for self-control. For example, our previous studies have found that the rsFC between dACC and VS is negatively correlated with out-of-control behaviours in drug addiction.33, 53 In the present work, we demonstrated that the dACC-VS rsFC was negatively and positively correlated with the number of IGD symptoms and self-control, respectively. Particularly, the correlation between dACC-VS rsFC and its mediating effect in the relationship between self-control and IGD-S provided direct evidence on the regulatory role of the pathway in addiction. In addition, we found that the rsFC between rVS and amygdala-parahippocampus contributed to IGD-S independent of the dACC pathway, which further supported the idea that the VS may serve as an interface in which top-down and bottom-up signals can be integrated to generate a behaviour output. It is well known that VS is a major target of dopaminergic projection that plays an crucial role in the motivation21, 54 and goal-directed behaviours.55-57 But in the present study, the rsFC between VS and ventral tegmental area (VTA) was not correlated with IGD-S nor SCS. However, we are cautious about the interpretation on this null relationship because this result could be confounded by the spatial limitation of fMRI technique when considering small structure such as VTA. In addition, we found the functional connectivity between lVS and left anterior insular, rather than dACC, was correlated with IGD-S. The functional laterality of ventral striatal connectivity warrants further investigation.
The present study has several implications for IGD prevention and intervention. First, the dACC-VS rsFC may be used as a biomarker for risky behaviours such as excessive gaming. Second, models that jointly consider reward sensitivity to digital games and self-control may have better performance in prediction of IGD risks. Third, self-control and the rVS-dACC pathway may serve as important target for IGD interventions using cognitive-behavioural-treatment (CBT) and neural modulation strategies, respectively. Indeed, behavioural self-control training has been suggested effective in controlling drinking in a long-term follow-up study,58 and mindfulness-based training has been shown to increase the neural activity of dACC and reduce smoking.59 In addition, neural modulation of dACC has been showed to improve cognitive control in the Stroop paradigm,60 and reduce the relapse in alcohol dependence.61, 62
The present study also has several limitations. First, we included gender as a covariate when examining the brain–behaviour relationships. But gender effects on both IGD risk and self-control ability have been reported.63, 64 In addition, gender differences in the mesocorticolimbic system when playing computer game have also been documented,65 and males demonstrated greater activation of the right striatum before gaming.66 The results suggest the differences in both behavioural characteristics and brain functions between males and females in IGD population. However, no significant difference in the rsFC between male and female gamers was found in the present study. One possible reason is that the gender effect on striatal activation is task dependent, and the present study could not detect such gender effect because it is diminished without task modulation. Second, the present study regards IGD as a single dimensional diagnosis and takes the total number of IGD symptoms as a continuous variable to characterize IGD risk. Future study contrasting rsFC of the same striatal circuits between clinically diagnosed IGD patients and controls is warranted.
In conclusion, the present study showed that the functional connectivity between ventral striatal and dACC mediates the relationship between self-control and maladaptive gaming behaviours. While the rVS-dACC is a regulatory pathway for IGD risk, the rVS-limbic pathway may play an opposite role to potentiate it. In short, these results provide converging evidence that the cingulate–ventral striatal functional connectivity may serve as an important neurobiological underpinning of self-control to regulate maladaptive behaviours not only in substance abuse but also in behavioural addiction.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Conceptualization: L.G. and Y.H.; Formal analysis: L.G., H.Z., and Y.H.; Investigation: L.G., C.S., W.X., B.T.; Initial draft writing: G.L.; Review and critical editing: F.G., K.Y., M.Z., and Y.H.; Finding acquisition: F.G., K.Y. and Y.H.
Open Research
DATA AVAILABILITY STATEMENT
The data are available from the corresponding author upon reasonable request.




