Alcohol's effects on the mouse brain are modulated by age and sex
Funding information: This work was supported by Grants R01 AA005965 and U01 AA013521 by the U.S. Department of Health and Human Services [NIH] National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Abstract
Binge alcohol consumption is common among adolescents and may impair normal brain development. Emerging, longitudinal studies in adolescents suggest that the effects of binge alcohol exposure on brain structure differ between sexes. To test the hypothesis that the effects of binge alcohol exposure on developmental brain growth trajectories are influenced by age of exposure and sex, adolescent and adult, male and female C57Bl/6 mice (n = 32), were exposed to a binge-like ethanol (EtOH) exposure paradigm (i.e., 5 cycles of 2 on/2 off days of 5 g/kg EtOH intraperitoneal) or served as saline controls. Longitudinal structural magnetic resonance imaging was acquired at baseline, following binge EtOH exposure, and after 2 weeks of recovery. Alcohol treatment showed interactions with age and sex in altering whole brain volume: adolescents of both sexes demonstrated inhibited whole brain growth relative to their control counterparts, although significance was only attained in female mice which showed a larger magnitude response to EtOH compared to male mice. In region of interest analyses, the somatosensory cortex and cerebellum showed inhibited growth in male and female adolescent mice exposed to EtOH, but the difference relative to controls did not reach multiple comparison-corrected statistical significance. These data suggest that in mice exposed to binge EtOH treatment, adolescent age of exposure and female sex may confer a higher risk to the detrimental effects of EtOH on brain structure and reinforce the need for direct testing of both sexes.
1 INTRODUCTION
Adolescence is characterised by remarkable changes in the structure and function of the brain (i.e., developmental plasticity) that underlie maturation of cognitive, affective, and other behavioural functions.1, 2 Trajectories of neural circuit development are precisely orchestrated with somatic maturation to maintain function during adolescence whilst the brain and body mature.2 As adolescence progresses and an adult phenotype emerges, neural plasticity is thought to decline.2 Behaviours often observed in emerging adults include increased risk taking and ingestion of substances of abuse, including alcohol.3 Estimates suggest that a majority of 13–18 year old adolescents have tried alcohol and as many as 24% drink alcohol in binge-like patterns.4 Disruption of developmental neural trajectories by alcohol may permanently disorder circuits and increase lifetime risks for cognitive and affective dysfunctions, psychopathology, and alcohol use disorders (AUD). Given that adolescent alcohol consumption is widespread and can disrupt normal brain development, it is a pressing public health need to advance a mechanistic understanding of how drinking affects brain development, if normal development is resumed upon abstention, and what biological variables contribute to individual differences in vulnerability or resilience.5
The pubertal rise in gonadal hormones is a useful marker for the onset of adolescence in animal models and is associated with the expression of many sex differences and inflection points in neurodevelopmental trajectories.1, 2, 6, 7 The severity of effects or regional vulnerability of the brain's response to alcohol during adolescence may be linked to the timing of these maturational events. In adults, some studies find that men with AUD show greater regional volume loss than women,8, 9 whereas others report that women and men show similar volume deficits despite lower lifetime exposure to alcohol in women.10, 11 In adolescence, emerging evidence suggests that mid to late adolescent binge drinking is associated with reduced frontal cortical thickness in boys but not girls.12, 13 Age at initial alcohol exposure may interact with sex to further determine brain responses. Animal studies suggest that adolescents exposed to similar quantities and patterns of alcohol may have worse brain outcomes than adults,14-16 but less is known about sex differences in this domain. Female adult rodents of several strains and species drink more than their male counterparts17 (an effect that may interact with age18), but male rats may suffer from more severe withdrawal symptoms.19 Further, a previous study from this lab suggests that young adult female rats may be less vulnerable to alcohol than young male rats in whole brain grey matter volumes.16 As observed in rats, mouse regional brain volumes are affected by adolescent alcohol exposure20, 21; however, little is known about how sex influences age-related brain vulnerability directly in either species, particularly in adolescents. Because adolescence is a period of ongoing sexual differentiation, alcohol exposure during this period may induce fundamentally different effects on the brain compared to the same exposure in adulthood, when active neurodevelopment has slowed.
Animal models permit experimental control over age, sex, timing and length of ethanol (EtOH) exposure, which is not possible in humans. The present study used an animal model of binge alcohol exposure to experimentally control the amount and timing of alcohol exposure across sex and age groups to determine if these biological variables affect EtOH effects on the brain. To increase translation to humans with respect to the extensive human neuroimaging literature in alcohol-related neuroadaptations, we used longitudinal in vivo magnetic resonance imaging (MRI) to test the hypothesis that younger age of exposure and female sex would be associated with heightened vulnerability of the central nervous system to binge EtOH exposure.
2 METHODS
2.1 Ethics statement
All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Institutional Animal Care and Use Committees at SRI International and Stanford University approved all procedures.
2.2 Animals
A total of 32 C57Bl/6j mice (Jackson Laboratories, Sacramento, CA) were acquired when 21 days (adolescent group, n = 16, 8 female) or 12 weeks (adult group, n = 16, 8 female) old. Mice were housed four per cage, maintained in a pathogen-free facility on a 12-h light/dark cycle, and had ad libitum access to regular chow and water.
2.3 Experimental design
Following 1 week of acclimation to the vivarium, each mouse received a baseline scan at postnatal day (P) 29–30 for the adolescent group (approximate start of puberty2) and P104–129 for the adult group. To model binge ethanol (EtOH) exposure and maximise blood ethanol content (BEC), eight adolescent (four female) and eight adult (four female) mice were exposed to five consecutive cycles of 2 days on, 2 days off EtOH (5 g/kg, 25% in 0.9% saline, intraperitoneal). This EtOH treatment paradigm was adapted from previous animal literature15, 20, 22 and is intended to mimic repeated binge drinking episodes commonly observed in adolescent humans. Control animals were injected with saline (0.9%) at the same volume as that received by EtOH animals in the same age group. All mice were scanned again 24 h (“binge” timepoint) and 2 weeks (“recovery” timepoint) after the final injection.
For the determination of BEC, each mouse was briefly anesthetised with isoflurane gas (3%) 30 min after the second injection (day 2 of cycle 1) and ~50 mL of blood was collected via tail nick. Blood was frozen at −20°c for <1 month before analysis so that blood samples from all mice could be run in parallel to avoid inter-assay variability. Short duration freezing does not affect accuracy of BEC measurements.23 BECs were determined by direct reaction with ethanol oxidase using a GM7 analyser (Analox Instruments Ltd., London, UK). Samples were run in duplicate and averaged.
Two adolescent male mice (one control, one EtOH-exposed) and one adolescent female mouse (EtOH-exposed) died during the experiment; their data were removed from all analyses. The final cohorts thus included seven adolescent control (four female), six adolescent EtOH-exposed (three female), eight adult control (four female), and eight adult EtOH-exposed (four female) mice.
2.4 Magnetic resonance scanning procedures and data analysis
Before each scan, mice were anesthetised with isoflurane (3% for induction; ~0.5–2% for maintenance) and body weight was acquired. Animals were then placed on an animal cradle base with built-in water circulation for body temperature control. Respiration was monitored throughout each ∼1-h scan. All animals received 0.5 cc subcutaneous saline (0.9%) for hydration at the end of the scan.
MR data were collected on a Bruker 70/16 US AVANCE III 7.0 T system (Karlsruhe, Germany) with 380-mT/m gradient strength on each (X, Y, and Z) axis, slew rate of 3420 T/m/s, 16-cm bore size using a Bruker mouse head volume coil (23 mm) and ParaVision 6.1 software. A gradient-recalled echo localiser scan was used to position the animals in the scanner and for graphical prescription of the subsequent scans. Structural analysis was based on acquisition of T2-weighted, high-resolution, TurboRare sequences: repetition time (TR) = 6774.8 ms; echo time (TE) = 33 ms; field of view (FOV) = 18 × 18; matrix = 144 × 144; pixel size = 0.125 × 0.125 × 0.5 mm; four averages; echo spacing = 11 ms; rare factor (i.e., echo train length) = 8; slice thickness = 0.5 mm; 40 slices.
Preprocessing of each image included removal of noise24 and inhomogeneity correction via ANTS 2.1.0.25 Each image was skull stripped by aligning a template to the scan via symmetric diffeomorphic registration,26 and the resulting deformation map was applied to the brain mask of the template. Image inhomogeneity correction was repeated on skull-stripped images. Structural images were segmented into cerebrospinal fluid (CSF), grey matter and white matter with finite mixture modelling segmentation (ANTS atropos) producing a probability for each of the three tissue types for each voxel in the brain. The final unit of measure for each tissue type was the integrated probability over the entire brain, yielding whole brain grey matter, white matter and CSF volumes. In parallel, each brain was parcellated into 42 regions-of-interest (ROIs) by registering the Allen Reference Atlas (ARA)27 to our template and then transforming the parcellation to the space of each image using the prior deformation map. Finally, tissue segmentation and ARA parcellation were resampled in the template space by rigidly aligning the bias-corrected skull-stripped image to the template. Volume of the three tissue types within each ROI was calculated by Computational Morphometry Toolkit.28 A histogram of image intensities was fit with three Gaussians yielding the probability of grey matter, white matter and CSF on a voxel-by-voxel basis; a CSF mask was constructed comprising all voxels in which the probability of CSF was the largest of the three values. This mask was then projected onto the ARA parcellation map.
Of the 42 ARA atlas-defined ROIs, one ROI – for cerebellar nuclei – was removed from analysis as the measure was deemed unreliable. The remaining 41 ROIs were reduced to 20 ROIs by adding individual volumes as follows: fronto-orbital cortex = frontal pole cerebral cortex + orbital area; motor cortex = primary motor area + secondary motor area; somatosensory cortex = primary somatosensory area trunk, lower limb, nose, upper limb, barrel field, mouth + supplemental somatosensory area; insular cortex = gustatory areas + visceral area + agranular insular area; temporal cortex = auditory areas + temporal association areas; visual cortex = visual areas + retrosplenial area + posterior parietal association areas; cingulate cortex = anterior cingulate area + infralimbic area + prelimbic area; hippocampal formation = ectorhinal area + perirhinal area + hippocampal region + retrohippocampal region; striatum = striatum dorsal region + striatum ventral region + lateral septal complex + striatum-like amygdalar nuclei; thalamus = thalamus sensory-motor cortex related + thalamus polymodal association cortex related. The remaining volumes (i.e., olfactory bulb, olfactory cortex, cortical subplate, pallidum, hypothalamus, midbrain, pons, medulla, cerebellar cortex and CSF) were treated individually (i.e., not combined). Finally, whole brain volume was the sum of the 20 ROIs multiplied by the total by a factor of 1.15557 to account for white matter.
2.5 Statistics
Statistical analyses were conducted using R (version 4.1.0) [htpp://www.r-project.org/]. Bodyweight and brain measures were analysed using age (adolescent/adult) × sex (m/f) × treatment (EtOH/saline) × timepoint (baseline, post treatment and recovery) mixed model analysis of variance (ANOVA) using the rstatix package. Because this study is the first to evaluate alcohol's effects on brain volumes whilst considering age and sex in mice, effect sizes [generalised eta squared (ges)] for the primary analyses of interest were provided, in addition to p values, to guide future studies. BEC statistics were based on a single measure per animal; consequently, an age × sex × treatment ANOVA was used. Significant three- or four-way interactions were followed by age × sex ANOVAs or t tests where relevant. For analyses of ROIs, a Bonferroni corrected p value of 0.0026 was required (i.e., 0.05/20).
3 RESULTS
3.1 Body weight
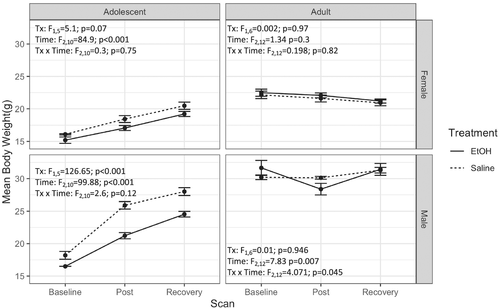
The full age × sex × treatment × time ANOVA was not significant (F2,44 = 0.08, p = 0.93). Treatment × sex × time (F2,44 = 4.2, p = 0.02) and age × sex × time (F2,44 = 18.1, p < 0.001) ANOVAs, however, were significant (Figure 1). Both female (F2,10 = 84.9, p < 0.001) and male (F2,10 = 99.9, p < 0.001) adolescent animals showed a significant main effect of time demonstrating body growth during the experiment; adolescent males also showed an effect of treatment (F1,5 = 126.7, p < 0.001), but no time × treatment interaction. Whilst follow-up ANOVAs for adult females were not significant, adult males showed a time × treatment interaction (F2,12 = 4.1, p = 0.045), suggesting that male weight in both age groups was more affected by EtOH treatment than was female weight.
3.2 Blood ethanol concentrations (BECs)
EtOH-treated adolescent male mice had a mean BEC of 299.2 ± 7.6 mg/dL; adolescent females reached 318.7 ± 15.5 mg/dL. BEC of the control males was 9.3 ± 1.2, whereas BEC of the control females was 5.5 ± 2.1 mg/dL. EtOH-treated adult male mice had a mean BEC of 277.7 ± 18.9, and females reached 299.6 ± 23.8 mg/dL (male and female controls had 9.2 ± 1.4 and 4.7 ± 1.0 mg/dL, respectively). An age × sex × treatment ANOVA showed a main effect of treatment only (F1,30 = 727.6, p < 0.001).
3.3 Whole brain volume
The full model ANOVA (age × sex × treatment × time; Table 1) for whole brain volume was significant (F2,42 = 3.3, p < 0.05, ges = 0.009) (Figure 2). Simpler models follow for each age × sex group. Within group means for whole brain volume, and each ROI is reported in the supporting information Table S1.
| Region | Subregion | Age | Sex | Treatment | Time | Age × sex | Age x treatment | Age × time | Sex × treatment | Sex × time | Treatment × time | Age × sex × treatment | Age × sex × time | Age × treatment × time | Sex × treatment × time | Age × sex × treatment × time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isocortex (Cerebral cortex/Cortical plate) | ||||||||||||||||
| Fronto-orbital1 |
F(1,21) = 5.845; p = 0.025 |
F(1,21) = 1.503; p = 0.234 |
F(1,21) = 2.358; p = 0.14 |
F(2,42) = 0.947; p = 0.396 |
F(1,21) = 0.476; p = 0.498 |
F(1,21) = 0.524; p = 0.477 |
F(2,42) = 0.849; p = 0.435 |
F(1,21) = 2.787; p = 0.11 |
F(2,42) = 0.246; p = 0.783 |
F(2,42) = 3.019; p = 0.06 |
F(1,21) = 0.255; p = 0.619 |
F(2,42) = 0.086; p = 0.918 |
F(2,42) = 0.326; p = 0.724 |
F(2,42) = 0.123; p = 0.885 |
F(2,42) = 0.378; p = 0.687 |
|
| Motor2 |
F(1,21) = 48.947; p < 0.0001 |
F(1,21) = 5.092; p = 0.035 |
F(1,21) = 0.293; p = 0.594 |
F(2,42) = 8.815; p = 6e-04 |
F(1,21) = 0.02; p = 0.888 |
F(1,21) = 0.501; p = 0.487 |
F(2,42) = 4.961; p = 0.012 |
F(1,21) = 0.7; p = 0.412 |
F(2,42) = 0.272; p = 0.763 |
F(2,42) = 1.306; p = 0.282 |
F(1,21) = 0.02; p = 0.89 |
F(2,42) = 0.326; p = 0.724 |
F(2,42) = 4.52; p = 0.017 |
F(2,42) = 1.331; p = 0.275 |
F(2,42) = 0.054; p = 0.948 |
|
| Somatosensory3 |
F(1,21) = 11.322; p = 0.003 |
F(1,21) = 1.1; p = 0.306 |
F(1,21) = 1.207; p = 0.284 |
F(2,42) = 14.009; p < 0.0001 |
F(1,21) = 2.456; p = 0.132 |
F(1,21) = 1.12; p = 0.302 |
F(2,42) = 5.968; p = 0.005 |
F(1,21) = 0.132; p = 0.72 |
F(2,42) = 0.751; p = 0.478 |
F(2,42) = 4.18; p = 0.022 |
F(1,21) = 1.429; p = 0.245 |
F(2,42) = 0.675; p = 0.515 |
F(2,42) = 6.302; p = 0.004 |
F(2,42) = 3.718; p = 0.033 |
F(2,42) = 6.368; p = 0.004 |
|
| Insula4 |
F(1,21) = 0.463; p = 0.504 |
F(1,21) = 3.247; p = 0.086 |
F(1,21) = 4.384; p = 0.049 |
F(2,42) = 12.661; p < 0.0001 |
F(1,21) = 1.213; p = 0.283 |
F(1,21) = 4.386; p = 0.049 |
F(2,42) = 8.968; p = 6e-04 |
F(1,21) = 0.268; p = 0.61 |
F(2,42) = 1.319; p = 0.278 |
F(2,42) = 1.252; p = 0.296 |
F(1,21) = 0.093; p = 0.763 |
F(2,42) = 1.965; p = 0.153 |
F(2,42) = 1.658; p = 0.203 |
F(2,42) = 2.401; p = 0.103 |
F(2,42) = 3.568; p = 0.037 |
|
| Temporal5 |
F(1,21) = 26.445; p < 0.0001 |
F(1,21) = 2.681; p = 0.116 |
F(1,21) = 0.958; p = 0.339 |
F(2,42) = 0.081; p = 0.922 |
F(1,21) = 4.42; p = 0.048 |
F(1,21) = 1.999; p = 0.172 |
F(2,42) = 1.629; p = 0.208 |
F(1,21) = 0.17; p = 0.684 |
F(2,42) = 0.062; p = 0.94 |
F(2,42) = 5.662; p = 0.007 |
F(1,21) = 1.005; p = 0.327 |
F(2,42) = 1.261; p = 0.294 |
F(2,42) = 2.754; p = 0.075 |
F(2,42) = 1.411; p = 0.255 |
F(2,42) = 4.674; p = 0.015 |
|
| Visual6 |
F(1,21) = 12.071; p = 0.002 |
F(1,21) = 0.176; p = 0.679 |
F(1,21) = 0.655; p = 0.428 |
F(2,42) = 41.376; p < 0.0001 |
F(1,21) = 0.339; p = 0.566 |
F(1,21) = 0.582; p = 0.454 |
F(2,42) = 0.784; p = 0.463 |
F(1,21) = 0.17; p = 0.684 |
F(2,42) = 0.351; p = 0.706 |
F(2,42) = 1.062; p = 0.355 |
F(1,21) = 0.065; p = 0.801 |
F(2,42) = 0.266; p = 0.768 |
F(2,42) = 3.039; p = 0.059 |
F(2,42) = 3.497; p = 0.039 |
F(2,42) = 1.399; p = 0.258 |
|
| Cingulate7 |
F(1,21) = 7.778; p = 0.011 |
F(1,21) = 0.002; p = 0.963 |
F(1,21) = 0.049; p = 0.827 |
F(2,42) = 15.27; p < 0.0001 |
F(1,21) = 0.127; p = 0.725 |
F(1,21) = 0.205; p = 0.656 |
F(2,42) = 2.405; p = 0.103 |
F(1,21) = 0.723; p = 0.405 |
F(2,42) = 1.612; p = 0.212 |
F(2,42) = 3.194; p = 0.051 |
F(1,21) = 4e-04; p = 0.985 |
F(2,42) = 7.897; p = 0.001 |
F(2,42) = 5.968; p = 0.005 |
F(2,42) = 0.097; p = 0.908 |
F(2,42) = 4.435; p = 0.018 |
|
| Olfactory areas | ||||||||||||||||
| Olfactory bulb |
F(1,21) = 133.48; p < 0.0001 |
F(1,21) = 18.098; p = 4e-04 |
F(1,21) = 2.052; p = 0.167 |
F(2,42) = 90.11; p < 0.0001 |
F(1,21) = 0.013; p = 0.909 |
F(1,21) = 0.314; p = 0.581 |
F(2,42) = 62.846; p < 0.0001 |
F(1,21) = 0.217; p = 0.646 |
F(2,42) = 0.652; p = 0.526 |
F(2,42) = 1.754; p = 0.186 |
F(1,21) = 0.648; p = 0.43 |
F(2,42) = 1.341; p = 0.273 |
F(2,42) = 1.086; p = 0.347 |
F(2,42) = 0.178; p = 0.838 |
F(2,42) = 0.013; p = 0.987 |
|
| Olfactory cortex |
F(1,21) = 22.468; p = 1e-04 |
F(1,21) = 15.839; p = 7e-04 |
F(1,21) = 2.267; p = 0.147 |
F(2,42) = 41.475; p < 0.0001 |
F(1,21) = 0.003; p = 0.96 |
F(1,21) = 8.002; p = 0.01 |
F(2,42) = 59.62; p < 0.0001 |
F(1,21) = 0.058; p = 0.811 |
F(2,42) = 1.513; p = 0.232 |
F(2,42) = 1.622; p = 0.21 |
F(1,21) = 0.24; p = 0.629 |
F(2,42) = 0.274; p = 0.762 |
F(2,42) = 1.979; p = 0.151 |
F(2,42) = 1.816; p = 0.175 |
F(2,42) = 3.854; p = 0.029 |
|
| Hippocampal formation8 |
F(1,21) = 19.969; p = 2e-04 |
F(1,21) = 6.98; p = 0.015 |
F(1,21) = 0.08; p = 0.78 |
F(2,42) = 12.525; p = 1e-04 |
F(1,21) = 0.844; p = 0.369 |
F(1,21) = 3.614; p = 0.071 |
F(2,42) = 7.819; p = 0.001 |
F(1,21) = 0.004; p = 0.953 |
F(2,42) = 0.45; p = 0.64 |
F(2,42) = 4.951; p = 0.012 |
F(1,21) = 0.237; p = 0.632 |
F(2,42) = 0.089; p = 0.915 |
F(2,42) = 0.028; p = 0.973 |
F(2,42) = 3.151; p = 0.053 |
F(2,42) = 0.878; p = 0.423 |
|
| Cortical subplate |
F(1,21) = 97.36; p < 0.0001 |
F(1,21) = 10.942; p = 0.003 |
F(1,21) = 0.001; p = 0.976 |
F(2,42) = 18.846; p < 0.0001 |
F(1,21) = 0.533; p = 0.473 |
F(1,21) = 4.511; p = 0.046 |
F(2,42) = 19.987; p < 0.0001 |
F(1,21) = 0.017; p = 0.898 |
F(2,42) = 4.762; p = 0.014 |
F(2,42) = 0.396; p = 0.676 |
F(1,21) = 0.061; p = 0.807 |
F(2,42) = 0.036; p = 0.965 |
F(2,42) = 1.123; p = 0.335 |
F(2,42) = 0.39; p = 0.679 |
F(2,42) = 1.798; p = 0.178 |
|
| Cerebral nuclei | ||||||||||||||||
| Striatum9 |
F(1,21) = 61.71; p < 0.0001 |
F(1,21) = 3.315; p = 0.083 |
F(1,21) = 0.181; p = 0.675 |
F(2,42) = 31.85; p < 0.0001 |
F(1,21) = 0.31; p = 0.584 |
F(1,21) = 3.459; p = 0.077 |
F(2,42) = 16.583; p < 0.0001 |
F(1,21) = 0.115; p = 0.738 |
F(2,42) = 3.069; p = 0.057 |
F(2,42) = 5.778; p = 0.006 |
F(1,21) = 0.504; p = 0.485 |
F(2,42) = 0.389; p = 0.68 |
F(2,42) = 0.753; p = 0.477 |
F(2,42) = 0.275; p = 0.761 |
F(2,42) = 0.066; p = 0.936 |
|
| Pallidum |
F(1,21) = 179.384; p < 0.0001 |
F(1,21) = 1.328; p = 0.262 |
F(1,21) = 0.318; p = 0.579 |
F(2,42) = 20.543; p < 0.0001 |
F(1,21) = 0.017; p = 0.898 |
F(1,21) = 2.522; p = 0.127 |
F(2,42) = 0.304; p = 0.74 |
F(1,21) = 2.846; p = 0.106 |
F(2,42) = 0.819; p = 0.448 |
F(2,42) = 1.577; p = 0.219 |
F(1,21) = 1.383; p = 0.253 |
F(2,42) = 0.359; p = 0.701 |
F(2,42) = 2.299; p = 0.113 |
F(2,42) = 2.548; p = 0.09 |
F(2,42) = 4.372; p = 0.019 |
|
| Diencephalon | ||||||||||||||||
| Thalamus10 |
F(1,21) = 35.778; p < 0.0001 |
F(1,21) = 1.37; p = 0.255 |
F(1,21) = 2.421; p = 0.135 |
F(2,42) = 6.298; p = 0.004 |
F(1,21) = 0.077; p = 0.784 |
F(1,21) = 4.69; p = 0.042 |
F(2,42) = 22.408; p < 0.0001 |
F(1,21) = 0.558; p = 0.463 |
F(2,42) = 0.34; p = 0.714 |
F(2,42) = 6.152; p = 0.005 |
F(1,21) = 0; p = 0.997 |
F(2,42) = 2.329; p = 0.11 |
F(2,42) = 1.749; p = 0.186 |
F(2,42) = 1.583; p = 0.217 |
F(2,42) = 0.032; p = 0.969 |
|
| Hypothalamus |
F(1,21) = 36.667; p < 0.0001 |
F(1,21) = 9.486; p = 0.006 |
F(1,21) = 0.069; p = 0.796 |
F(2,42) = 30.711; p < 0.0001 |
F(1,21) = 0.167; p = 0.687 |
F(1,21) = 3.388; p = 0.08 |
F(2,42) = 11.45; p = 1e-04 |
F(1,21) = 0.463; p = 0.503 |
F(2,42) = 0.919; p = 0.407 |
F(2,42) = 4.323; p = 0.02 |
F(1,21) = 0.153; p = 0.699 |
F(2,42) = 0.642; p = 0.531 |
F(2,42) = 3.34; p = 0.045 |
F(2,42) = 0.937; p = 0.4 |
F(2,42) = 2.132; p = 0.131 |
|
| Midbrain |
F(1,21) = 7.21; p = 0.014 |
F(1,21) = 7.01; p = 0.015 |
F(1,21) = 1.425; p = 0.246 |
F(2,42) = 14.976; p < 0.0001 |
F(1,21) = 0.549; p = 0.467 |
F(1,21) = 2.229; p = 0.15 |
F(2,42) = 8.955; p = 6e-04 |
F(1,21) = 0.04; p = 0.843 |
F(2,42) = 0.062; p = 0.94 |
F(2,42) = 7.001; p = 0.002 |
F(1,21) = 0.188; p = 0.669 |
F(2,42) = 2.79; p = 0.073 |
F(2,42) = 0.494; p = 0.613 |
F(2,42) = 0.612; p = 0.547 |
F(2,42) = 0.365; p = 0.696 |
|
| Brainstem | ||||||||||||||||
| Pons |
F(1,21) = 44.357; p < 0.0001 |
F(1,21) = 5.299; p = 0.032 |
F(1,21) = 0.616; p = 0.441 |
F(2,42) = 27.154; p < 0.0001 |
F(1,21) = 0.074; p = 0.788 |
F(1,21) = 2.545; p = 0.126 |
F(2,42) = 24.696; p < 0.0001 |
F(1,21) = 0.052; p = 0.821 |
F(2,42) = 4.408; p = 0.018 |
F(2,42) = 1.295; p = 0.285 |
F(1,21) = 0.002; p = 0.964 |
F(2,42) = 0.827; p = 0.444 |
F(2,42) = 3.964; p = 0.026 |
F(2,42) = 1.549; p = 0.224 |
F(2,42) = 1.21; p = 0.308 |
|
| Medulla |
F(1,21) = 52.94; p < 0.0001 |
F(1,21) = 5.09; p = 0.035 |
F(1,21) = 0.681; p = 0.418 |
F(2,42) = 33.469; p < 0.0001 |
F(1,21) = 0.023; p = 0.881 |
F(1,21) = 1.44; p = 0.244 |
F(2,42) = 16.402; p < 0.0001 |
F(1,21) = 0.894; p = 0.355 |
F(2,42) = 2.952; p = 0.063 |
F(2,42) = 2.711; p = 0.078 |
F(1,21) = 0.098; p = 0.758 |
F(2,42) = 0.098; p = 0.907 |
F(2,42) = 0.605; p = 0.551 |
F(2,42) = 0.277; p = 0.759 |
F(2,42) = 1.68; p = 0.199 |
|
| Cerebellar cortex |
F(1,21) = 13.355; p = 0.001 |
F(1,21) = 2.102; p = 0.162 |
F(1,21) = 0.526; p = 0.476 |
F(2,42) = 0.058; p = 0.944 |
F(1,21) = 2.717; p = 0.114 |
F(1,21) = 3.451; p = 0.077 |
F(2,42) = 19.922; p < 0.0001 |
F(1,21) = 0.611; p = 0.443 |
F(2,42) = 1.892; p = 0.163 |
F(2,42) = 3.684; p = 0.034 |
F(1,21) = 0.012; p = 0.915 |
F(2,42) = 1.78; p = 0.181 |
F(2,42) = 3.787; p = 0.031 |
F(2,42) = 3.677; p = 0.034 |
F(2,42) = 6.617; p = 0.003 |
|
| CSF |
F(1,21) = 39.64; p < 0.0001 |
F(1,21) = 2.839; p = 0.107 |
F(1,21) = 0.017; p = 0.898 |
F(2,42) = 26.003; p < 0.0001 |
F(1,21) = 0.034; p = 0.855 |
F(1,21) = 12.71; p = 0.002 |
F(2,42) = 6.013; p = 0.005 |
F(1,21) = 3.123; p = 0.092 |
F(2,42) = 0.91; p = 0.41 |
F(2,42) = 2.289; p = 0.114 |
F(1,21) = 2.293; p = 0.145 |
F(2,42) = 0.382; p = 0.685 |
F(2,42) = 0.338; p = 0.715 |
F(2,42) = 0.746; p = 0.481 |
F(2,42) = 0.271; p = 0.764 |
|
| Whole brain volume ⨍ |
F(1,21) = 42.308; p < 0.0001 |
F(1,21) = 4.151; p = 0.054 |
F(1,21) = 0.784; p = 0.386 |
F(2,42) = 24.632; p < 0.0001 |
F(1,21) = 0.474; p = 0.499 |
F(1,21) = 3.688; p = 0.068 |
F(2,42) = 42.772; p < 0.0001 |
F(1,21) = 0.069; p = 0.796 |
F(2,42) = 1.546; p = 0.225 |
F(2,42) = 10.309; p = 2e-04 |
F(1,21) = 0.026; p = 0.872 |
F(2,42) = 0.731; p = 0.487 |
F(2,42) = 3.313; p = 0.046 |
F(2,42) = 2.109; p = 0.134 |
F(2,42) = 3.315; p = 0.046 |
|
- ⨍ calculated as the sum of 19 regions*1.15557 to account for exclusion of white matter by ATLAS; + results of repeated measures, 4-group, 3-timepoint mixed model ANOVAs; 1 frontal pole cerebral cortex + orbital area; 2 primary motor area + secondary motor area; 3 primary somatosensory area: trunk, lower limb, nose, upper limb, barrel field, mouth + supplemental somatosensory area; 4 gustatory areas + visceral area + agranular insular area; 5 auditory areas + temporal association areas; 6 visual areas + retrosplenial area + posterior parietal association areas; 7 anterior cingulate area + infralimbic area + prelimbic area; 8 ectorhinal area + perirhinal area + hippocampal region + retrohippocampal region; 9 striatum dorsal region + striatum ventral region + lateral septal complex + striatum-like amygdalar nuclei; 10 thalamus sensory-motor cortex related + thalamus polymodal association cortex related.
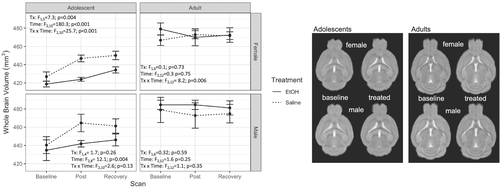
3.3.1 Adolescent males
There was a main effect of time (F2,8 = 12.0, p = 0.004, ges = 0.285) but not treatment (F1,4 = 1.73, p = 0.26, ges = 0.273) or interaction (F2,8 = 2.64, p = 0.131, ges = 0.080). Control male adolescent mice gained 5.5% brain volume between baseline and post-treatment timepoints, and lost 0.7% volume from post-treatment to recovery timepoints for a total 4.8% increase in whole brain volume from baseline to recovery (6 weeks apart). EtOH treated adolescent males gained 1.6% in brain volume from baseline to post-treatment and showed a 1.0% increase from post-treatment to recovery timepoints for a net increase of 2.6%.
3.3.2 Adult males
There were no time (F2,12 = 1.57; p = 0.25, ges = 0.009), treatment (F1,6 = 0.32; p = 0.59, ges = 0.05), or interaction (F2,12 = 1.13; p = 0.35, ges = 0.006) effects.
3.3.3 Adolescent females
There was an effect of treatment (F1,5 = 7.3, p = 0.04, ges = 0.583), time (F2,10 = 180.3, p < 0.001, ges = 0.596) and an interaction (F2,10 = 25.7, p < 0.001, ges = 0.174). This interaction was driven by an increase of 4.7% in brain volume from baseline to post-treatment timepoints in control animals compared to a 1.2% increase in EtOH treated mice. Controls increased an additional 0.7% from post-treatment to recovery timepoints whilst brain volume in EtOH treated mice increased 2.4% during the same period. In total, from baseline to recovery, control females gained 5.4% brain volume compared to a 3.7% increase in volume in EtOH treated females. Mean brain volumes did not differ at baseline (t5 = 1.22, p = 0.278) but did differ at post-treatment (t5 = 4.85, p = 0.005) and recovery timepoints (t5 = 2.57, p < 0.05) (smaller in EtOH treated mice).
3.3.4 Adult females
There was a treatment × time interaction (F2,12 = 8.2; p = 0.006, ges = 0.074) but no main effects of treatment (F1,6 = 0.13; p = 0.73, ges = 0.074) or time (F2,12 = 0.3; p = 0.75, ges = 0.003). Control female adult mice showed a 1.3% brain volume increase between baseline and binge scans, whilst EtOH-treated female adult mice lost 1.9% of total brain volume during the same interval. However, mean brain volumes in EtOH-treated female adult mice were not different from those of controls at baseline (t6 = −1.39, p = 0.21), post-treatment (t6 = 0;29, p = 0.79) or recovery (t6 = −0.07, p = 0.95), suggesting that interaction effect may have been driven by baseline differences.
3.4 Brain ROI volumes
Mean ROI volumes and full ANOVA results are presented in Table 1. Among the 20 regions evaluated, only the somatosensory cortex (F2,42 = 6.4, p = 0.004, ges = 0.055; Figure 3A) and cerebellar cortex (F2,42 = 6.6, p = 0.003, ges = 0.039; Figure 3B) showed a trend toward significance in the overall model after Bonferroni correction. For cerebellar cortex, all groups but the adolescent male mice showed a treatment × time interaction. In adolescent female mice (F2,10 = 7.2, p = 0.01, ges = 0.159), the volume of the cerebellar cortex was not different at baseline (t5 = 1.1, p = 0.3) but was smaller in the EtOH-exposed group than in the saline controls at both the binge (t5 = 4.3, p = 0.008) and recovery (t5 = 3.0, p = 0.03) timepoints. Although the treatment × time interaction was significant (F2,12 = 5.4, p = 0.02, ges = 0.057), adult male mice in control and EtOH-exposed groups did not differ in cerebellar cortex volume at baseline (t6 = −0.02 p = 0.99), binge (t6 = −1.3, p = 0.25) or recovery (t6 = −0.9, p = 0.40). Similar results were observed in adult female mice [i.e., significant treatment × time interaction (F2,12 = 4.9, p = 0.03, ges = 0.127) but no group differences at baseline (t6 = −1.4, p = 0.20), binge (t6 = 0.8, p = 0.43) or recovery (t6 = −0.003, p = 0.99)].
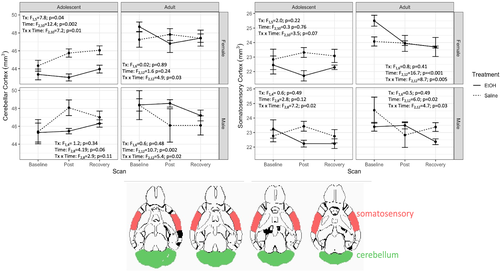
For somatosensory cortex, adolescent female mice showed a trend towards significance for the treatment × time interaction (F2,10 = 3.5, p = 0.07, ges = 0.092); adolescent male mice showed an interaction (F2,8 = 7.2, p = 0.02, ges = 0.252), driven by significant group differences at the binge (t4 = 2.915, p = 0.04) but not at baseline (t4 = −0.63, p = 0.56) or recovery (t4 = 0.90, p = 0.42). Adult male mice also demonstrated a treatment × time interaction (F2,12 = 4.7, p = 0.03, ges = 0.157), but this was driven by group differences at the recovery timepoint (t6 = 2.81, p = 0.03) [not at baseline (t6 = 1.2, p = 0.27) or binge (t6 = −0.8, p = 0.48) timepoints]. The significant treatment × time interaction in adult female mice (F2,12 = 8.726, p < 0.001, ges = 0.186) was due to baseline differences in somatosensory cortex volume (t6 = −2.9, p = 0.03) [not at binge (t6 = 0.034, p = 0.97) or recovery (t6 = −0.008, p = 0.99)].
4 DISCUSSION
The present study was designed to test the hypothesis that sex and age are critical factors in influencing the brain's response to binge EtOH exposure. Further, we hypothesised that stronger effects would be observed in the developing brain, with female mice at greater risk than male mice. Indeed, age and sex interacted with EtOH to influence whole-brain volumes across time, suggesting that both factors influence the brain's response to binge EtOH. Analysing the effects within groups revealed treatment × time interactions in adolescent females with EtOH resulting in decreased brain growth at both the post-treatment and recovery timepoints. Whilst adolescent males showed a similar pattern, the treatment × time interaction failed to reach significance in this group, and adolescent females showed an effect size almost double than that of adolescent males (0.174 vs 0.08).
In adults, males showed no interaction effect whilst adult females did; however, there were no significant differences of EtOH treatment within timepoints, suggesting that this interaction will need to be explored further before strong conclusions are drawn. Similar difficulties exist in interpreting the data for the somatosensory and cerebellar cortices in adult males and females.
Heavy drinking is associated with regional brain volume reductions in adult29-32 and adolescent13, 20, 30, 33-35 humans and rats across a variety of regions. Several studies suggest that adolescents exposed to similar quantities and patterns of alcohol consumption may have worse brain outcomes than adults, including declines in prefrontal cortex volume,36 hippocampal volume36 and physiology,14 changes to cholinergic neuron number15 and whole brain grey matter.16 Further, adult women show similar brain volume deficits as men in whole brain grey matter and corpus callosum area but with less total lifetime alcohol exposure.10, 11 In adolescents, some studies have demonstrated that EtOH results in smaller prefrontal cortical volume and thickness13, 33, 37 in females. Nonetheless, sex differences in EtOH's effects on the adolescent brain are not consistently reported,5, 38 and thus, it is unclear how readily the adolescent studies comport with those performed in adults. The rigorously controlled pattern and dosage of EtOH exposure in the present study uncovered clear whole brain volume effects of EtOH in adolescent females but borderline effects in the males. Whilst the current study does not demonstrate strong sex differences in adolescent mice, the data do suggest a larger effect size in females than males. Future higher powered studies aimed at uncovering subtle whole brain and ROI specific effects will be necessary to make definitive claims in support of sex differences. A previous study from this lab in fisher 344 rats suggests that females may be less vulnerable to brain health outcomes after binge EtOH in both cerebral ventricular and grey matter volumes.16 That report also found that brain structure of younger rats were more vulnerable than older rats, indicating that further research is required to study how species interacts with sex, age and alcohol treatment.
During the recovery phase, adolescent female mice treated with EtOH showed partial recovery from ethanol exposure. It is unclear, however, if the 2 weeks of recovery time afforded each mouse was sufficient to determine whether a full recovery is possible. In men and women with alcohol use disorder (AUD), MRI visible EtOH-related brain shrinkage is reversible within days to weeks of abstinence39 but that further recovery is possible with longer sobriety.40 However, studies in animals demonstrate that EtOH exposure in adolescence can cause long-term neurobiological and behavioural changes; cross-sectional studies of rats treated with intermittent EtOH during adolescence and measured in adulthood have found EtOH-related differences in cortical thickness41 and alterations to the microstructure of white matter tracts.42 Whilst previous studies reported that male rats and mice did not show changes to whole brain volumes in adulthood,20, 42 regional brain volume differences were observed across several different regions.20 These studies demonstrate that adolescent EtOH exposure can induce persistent neurobiological changes to the structure or volume of the brain; however, those studies were conducted in male rodents of a single age, which precludes any conclusions about interactions between sex and age on outcome measures.
After correction for multiple comparisons, the cerebellar cortex and somatosensory cortex each approached having a significant age × sex × treatment × time interaction. Interestingly, adult males showed alcohol effects on both of these regions despite showing no overall effects on whole brain volume. Further, there were no clear distinctions between age or sex on the alcohol effects on volumes of these two regions. Future higher-powered studies will be required to delineate these effects and to uncover more subtle effects on individual ROIs.
Previous literature in humans has shown that cerebellar structure is vulnerable to alcohol.31, 43 Although previous animal studies have not reported changes to cerebellum grey matter volume, there are reports that alcohol exposure alters diffusion tensor imaging metrics in rat cerebellum.42, 43 Recent studies have also discovered that chronic intermittent alcohol exposure decreases somatosensory cortex volume in adult male mice44 and increases mean diffusivity in adult male rats,45 both of which were accompanied by markers of neuroinflammation. One of the most consistent effects observed in both humans and rats is an alcohol-induced increase in cerebral ventricle size in men and women with AUD and EtOH-treated rats,46, 47 which resolves after abstention in both species.39, 46 We did not observe a significant effect on ventricular volumes suggesting that mice may be resilient to these changes after EtOH treatment. Because of differences between species, ages and treatment paradigms used across studies, further research will be required before broad conclusions about how EtOH affects these regions can be drawn.
There are limitations to the present study that also inform interpretation of the results. The binge treatment paradigm used here resulted in ~300 mg/dL BECs, which closely mimic those in previous animal studies,18 but represents a relatively high dosage of EtOH in humans. Peak BECs were similar in all groups, suggesting the injections were properly adjusted for bodyweight; however, BECs were measured only at one timepoint, and so it is not known if groups differed in rate of EtOH clearance that would result in differential cumulative EtOH exposure. There are reports that EtOH metabolism is slower in adult women, resulting in higher cumulative EtOH exposure than men,48 whilst studies in adult rodents suggest that females have faster clearance,49 discounting this as an explanation for increased vulnerability in female mice. These studies suggest that greater EtOH exposure of females is unlikely to explain the stronger effects observed on female brains. However, it is unknown if sex differences in EtOH clearance are absent in adolescent mice; thus, it is unclear if age effects could be explained through this mechanism. Further, this treatment accomplished very high BECs, well in excess of the formal definition of binge drinking. However, humans do routinely present with BECs in the range accomplished in this study.50 As such, this model may mimic heavy binge drinking in adolescents rather than more casual use. Finally, future studies should aim to boost sample sizes to increase power in discovery effects across numerous ROIs and to follow recovery time periods for longer durations. The results from the present experiment justify further large-scale studies in rodents.
Despite its limitations, the present experiment establishes that sex and age are critical biological variables that influence the brain's vulnerability to EtOH. Given that disruptions to normal brain development during adolescence may induce permanent untoward neuroadaptations, it is critical to understand the nuances of these effects with longer-term investigations of the aging brain with a history of EtOH bingeing.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualisation: DJP, NMZ and AP; methodology: DJP, NMZ, QZ and AP; formal analysis: DJP and NMZ; investigation: DJP; resources: NMZ, EVS and AP; data curation: QZ and AP; writing—original draft preparation: DJP; writing—review and editing: NMZ, EVS and AP; project administration: DJP, NMS and AP; funding acquisition: NMZ, EVS and AP. All authors have read and agreed to the published version of the manuscript.
INSTITUTIONAL REVIEW BOARD STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Animal Care and Use Committees at SRI International and Stanford University. SRI International protocol 01019. Stanford University protocol 8800.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study will be openly available in https://data.mendeley.com/.




