Alcohol and cannabis co-use and longitudinal gray matter volumetric changes in early and late adolescence
Abstract
Background
Previous studies have characterized the impact of substance use on cerebral structure and function in adolescents. Yet, the great majority of prior studies employed a small sample, presented cross-sectional findings, and omitted potential sex differences.
Methods
Using data based on 724 adolescents (370 females) curated from the NCANDA study, we investigated how gray matter volumes (GMVs) decline longitudinally as a result of alcohol and cannabis use. The impacts of alcohol and cannabis co-use and how these vary across assigned sex at birth and age were examined. Brain imaging data comprised the GMVs of 34 regions of interest and the results were evaluated with a Bonferroni correction.
Results
Mixed-effects modeling showed faster volumetric declines in the caudal middle frontal cortex, fusiform, inferior frontal, superior temporal (STG), and supramarginal (SMG) gyri, at −0.046 to −0.138 cm3/year in individuals with prior-year alcohol and cannabis co-use, but not those engaged in alcohol or cannabis use only. These findings cannot be explained by more severe alcohol use among co-users. Further, alcohol and cannabis co-use in early versus late adolescence predicted faster volumetric decline in the STG and SMG across assigned sex at birth.
Conclusions
Findings highlight the longitudinal impact of alcohol and cannabis co-use on brain development, especially among youth reporting early adolescent onset of use. The volumetric decline was noted in cortical regions in support of attention, memory, executive control, and social cognition, suggesting the pervasive effect of alcohol and cannabis co-use on brain development.
1 INTRODUCTION
1.1 Impact of alcohol and cannabis use on mental health
Individuals engaged in substance use earlier in life are at greater risk of developing substance use disorders and sustaining cognitive deficits.1, 2 Those who use multiple substances, including alcohol and cannabis co-use, are particularly vulnerable to health consequences.3, 4 Adolescents reporting alcohol and cannabis co-use, but neither use alone, were more likely to engage in non-suicidal self-injury on a given day.5 Co-use of alcohol and cannabis was associated with heavier use of substances, more use-related harms, and symptoms of psychosis in young adulthood.6 Moreover, a report conducted by the National Survey on Drug Use and Health found that a higher frequency of alcohol or cannabis co-use conferred risk for both alcohol use disorder (AUD) and cannabis use disorder (CUD). Thus, alcohol and cannabis co-use may have a more deleterious impact on the brain compared to a single use.
1.2 Cognitive and cerebral markers of alcohol and cannabis use
Many studies have examined how substance use impacts cognitive and neural development among adolescents.8 Adolescent cannabis users relative to non-users demonstrated poorer attention, learning, memory, and aspects of executive functioning.9, 10 For example, cannabis use was associated with bilateral prefrontal cortical thinning in a dose-dependent manner over a 5-year period.11 Further, lifetime use was not correlated with baseline thickness, suggesting that cortical thinning reflected cumulative effects of cannabis consumption. Cannabis use at age 17 was also associated with diminished general cognitive ability at age 23.12 Among youth, cannabis and alcohol co-use relative to alcohol or cannabis use alone were related to diminished attentional capacity13 and compromised integrity of frontolimbic tracts.14 Other studies indicate that AUD, but not CUD, is associated with altered regional activations during cognitive and affective challenges in adolescence.15, 16 Notably, while studies have largely associated cannabis and alcohol co-use with negative health and social outcomes, some findings suggest that the combined use of cannabis and alcohol use may have “protective” effects on neurobiological outcomes, relative to alcohol use alone.17-19
Taken together, these studies support the distinct impact of cannabis use on brain development and the greater impact of alcohol use on brain structure and function.20 Still, the effects of co-use of these substances on the developing brain remain unclear.
1.3 The effects of early versus late use on brain and behavior
Substance use initiated at an early age likely has a more serious impact on mental health.21 Greater impairment in executive functioning has been demonstrated among adolescents compared to frequent adult cannabis users, and abstinence had a weaker impact on alleviating craving in adolescents compared to adults.22 Earlier onset of cannabis use was associated with more severe impairment in verbal intelligence and executive function.23 Both academic and social functions were greater among high school seniors who initiated substance use at a later versus earlier age.24 Individuals 16 to 20 years of age who engaged in early (< 16 years of age) alcohol and cannabis co-use were less likely to successfully transition to adult roles.25 Adolescent cannabis use elevated the risk of psychosis, more severe positive symptoms (e.g., hallucinations), and altered gray matter volume (GMV) in a cerebellar network implicated in the pathogenesis of schizophrenia.26 Thus, the age of initiation is critical in understanding the impact of alcohol and cannabis co-use on the brain.
1.4 Differences across assigned sex at birth relating to effects of alcohol and cannabis use
Assigned sex at birth may also modulate the impact of cannabis and alcohol use on the brain and behavior. One study found that, although young adult females smoked less cannabis while drinking, they experienced the same acute pharmacological and subjective effects as males.27 Men and women with AUD showed different patterns of gray and white matter volumetric reduction28; for instance, only males showed reduced amygdala size compared to controls.29 Moreover, early initiation of cannabis use potentially resulted in more spatial working memory deficits in females than in male adolescents.30 Thus, a substantial body of evidence supports sex differences in the impact of alcohol and/or cannabis use on brain and behavior.
1.5 Longitudinal impacts of alcohol and cannabis use
Adolescent alcohol use accelerated developmental decreases in GMV, particularly in the frontal and cingulate cortices, and decelerated increases in white matter volumes.31 Fewer longitudinal studies exist examining the impact of cannabis use on the brain. Exceptions include prior work showing that the onset and frequency of cannabis use in early adolescence and young adulthood were associated with declining neurocognitive functions.23, 32 Moreover, adolescent cannabis use frequency increased significantly over five biannual assessments along with impairment in motivation.33 Additional work is necessary to understand the longitudinal impact of cannabis use, alcohol use, and co-use of these substances on the brain.
1.6 The present study
This study investigates how GMVs decline longitudinally as a result of alcohol and cannabis use among adolescents enrolled in the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) study. More specifically, the impact of alcohol and cannabis co-use was compared to alcohol and cannabis use alone, as well as to non-users. Further, we investigated how these associations varied across assigned sex at birth and across age (early vs. late adolescents). We hypothesized that alcohol and cannabis co-use would have a greater impact on cerebral GMVs and that this association would vary across males and females and figure more prominently in early versus late adolescents.
2 METHODS AND MATERIALS
2.1 Participants and assessments
Data from the NCANDA study, a five-site, longitudinal neuroimaging study of adolescents at different developmental stages were examined (see Ref.34). The NCANDA study followed an accelerated longitudinal design to assess a sample of youth aged 12–21 (49% male according to assigned sex at birth; 64% White; > 50% at risk for heavy drinking) at baseline and annually for up to 9 years.35 A total of 724 participants who completed the baseline assessment and at least one follow-up visit at years 1, 2, and 3 (679, 625, and 563 participants, respectively) were included in the current study. Participants meeting the criteria for any substance use disorder according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM IV) (1994) at baseline were excluded. Table 1 summarizes the baseline demographics. Participants were classified into two developmental stages—early (< 16 years of age at baseline) and late (≥ 16) adolescents.
| All (N = 724) | Early (N = 381) | Late (N = 343) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 16.02 ± 2.45 | 14.06 ± 1.13 | 18.20 ± 1.49 | <0.001 |
| ICV (cm3) | 1,051.25 ± 118.02 | 1,050.65 ± 117.32 | 1,051.91 ± 118.95 | 0.887 |
| Social-economic status (years) | 16.86 ± 2.42 | 16.94 ± 2.48 | 16.77 ± 2.34 | 0.321 |
| Assigned sex at birth (female, N) | 370 (51.10%) | 193 (50.66%) | 177 (51.60%) | 0.857 |
| Race | 0.158 | |||
| White | 523 (72.24%) | 264 (69.29%) | 259 (75.51%) | |
| Black | 80 (11.05%) | 45 (11.81%) | 35 (10.20%) | |
| Others | 121 (16.71%) | 72 (18.90%) | 49 (14.29%) | |
| Smoking | ||||
| Baseline | 41 (5.82%) | 5 (1.32%) | 36 (11.04%) | <0.001 |
| Ever | 191 (27.09%) | 75 (19.79%) | 116 (35.58%) | <0.001 |
| Alcohol/Cannabis use at baseline | <0.001 | |||
| Non-use controls | 510 (70.44%) | 345 (90.55%) | 165 (48.10%) | |
| Cannabis | 17 (2.35%) | 5 (1.31%) | 12 (3.50%) | |
| Alcohol | 108 (14.92%) | 22 (5.77%) | 86 (25.07%) | |
| Alcohol+Cannabis | 89 (12.29%) | 9 (2.36%) | 80 (23.32%) | |
| Alcohol/Cannabis use ever | <0.001 | |||
| Non-use controls | 175 (24.17%) | 138 (36.22%) | 37 (10.79%) | |
| Cannabis | 22 (3.04%) | 20 (5.25%) | 2 (0.58%) | |
| Alcohol | 164 (22.65%) | 56 (14.70%) | 108 (31.49%) | |
| Alcohol+Cannabis | 363 (50.14%) | 167 (43.83%) | 196 (57.14%) | |
| Volume at baseline (cm3) | ||||
| Superior temporal sulcus | 5.77 ± 0.91 | 5.92 ± 0.91 | 5.60 ± 0.89 | <0.001 |
| Caudal anterior cingulate cortex | 4.83 ± 0.92 | 4.91 ± 0.94 | 4.74 ± 0.89 | 0.014 |
| Caudal middle frontal cortex | 15.13 ± 2.63 | 15.58 ± 2.62 | 14.63 ± 2.56 | <0.001 |
| Cuneus cortex | 6.33 ± 1.04 | 6.48 ± 1.06 | 6.17 ± 0.99 | <0.001 |
| Entorhinal cortex | 3.74 ± 0.70 | 3.75 ± 0.71 | 3.72 ± 0.69 | 0.486 |
| Fusiform gyrus | 23.18 ± 3.19 | 23.66 ± 3.27 | 22.65 ± 3.01 | <0.001 |
| Inferior parietal cortex | 32.41 ± 4.65 | 33.69 ± 4.62 | 30.99 ± 4.27 | <0.001 |
| Inferior temporal gyrus | 24.70 ± 3.95 | 25.38 ± 3.90 | 23.95 ± 3.89 | <0.001 |
| Isthmus-cingulate cortex | 6.09 ± 1.06 | 6.26 ± 1.07 | 5.90 ± 1.03 | <0.001 |
| Lateral occipital cortex | 25.48 ± 3.61 | 26.04 ± 3.51 | 24.85 ± 3.63 | <0.001 |
| Lateral orbital frontal cortex | 17.59 ± 2.39 | 18.18 ± 2.31 | 16.93 ± 2.30 | <0.001 |
| Lingual gyrus | 14.90 ± 2.22 | 15.17 ± 2.28 | 14.60 ± 2.13 | <0.001 |
| Medial orbital frontal cortex | 11.72 ± 1.61 | 12.17 ± 1.61 | 11.22 ± 1.46 | <0.001 |
| Middle temporal gyrus | 25.92 ± 3.43 | 26.65 ± 3.25 | 25.12 ± 3.45 | <0.001 |
| Parahippocampal gyrus | 4.89 ± 0.66 | 4.97 ± 0.65 | 4.80 ± 0.66 | <0.001 |
| Paracentral lobule | 8.59 ± 1.29 | 8.89 ± 1.31 | 8.27 ± 1.19 | <0.001 |
| IFG, pars opercularis | 10.61 ± 1.63 | 10.83 ± 1.59 | 10.37 ± 1.63 | <0.001 |
| IFG, pars orbitalis | 5.83 ± 0.86 | 6.07 ± 0.84 | 5.57 ± 0.81 | <0.001 |
| IFG, pars triangularis | 9.42 ± 1.52 | 9.77 ± 1.47 | 9.03 ± 1.48 | <0.001 |
| Pericalcarine cortex | 4.69 ± 0.88 | 4.71 ± 0.89 | 4.66 ± 0.88 | 0.489 |
| Postcentral gyrus | 21.32 ± 2.89 | 21.80 ± 2.79 | 20.79 ± 2.91 | <0.001 |
| Posterior-cingulate cortex | 7.77 ± 1.11 | 8.01 ± 1.10 | 7.51 ± 1.06 | <0.001 |
| Precentral gyrus | 30.70 ± 3.69 | 31.24 ± 3.63 | 30.10 ± 3.66 | <0.001 |
| Precuneus cortex | 23.11 ± 3.15 | 23.87 ± 3.07 | 22.27 ± 3.03 | <0.001 |
| Rostral anterior cingulate cortex | 5.62 ± 0.93 | 5.70 ± 0.96 | 5.53 ± 0.89 | 0.012 |
| Rostral middle frontal gyrus | 37.85 ± 5.94 | 39.58 ± 5.86 | 35.93 ± 5.42 | <0.001 |
| Superior frontal gyrus | 53.34 ± 6.61 | 55.24 ± 6.31 | 51.23 ± 6.31 | <0.001 |
| Superior parietal cortex | 29.61 ± 4.00 | 30.55 ± 4.04 | 28.57 ± 3.69 | <0.001 |
| Superior temporal gyrus | 26.79 ± 3.20 | 27.37 ± 3.12 | 26.16 ± 3.18 | <0.001 |
| Supramarginal gyrus | 24.93 ± 3.62 | 25.76 ± 3.60 | 24.01 ± 3.41 | <0.001 |
| Frontal pole | 2.32 ± 0.41 | 2.44 ± 0.41 | 2.18 ± 0.36 | <0.001 |
| Temporal pole | 5.36 ± 0.62 | 5.43 ± 0.64 | 5.28 ± 0.59 | 0.001 |
| Transverse temporal cortex | 2.32 ± 0.38 | 2.34 ± 0.38 | 2.30 ± 0.38 | 0.116 |
| Insula | 14.36 ± 1.69 | 14.51 ± 1.69 | 14.18 ± 1.67 | 0.008 |
- Note: p-values were for t- or chi-square tests of the difference between early and late adolescents as appropriate for the variable distributions. ICV: intracranial volume; IFG: inferior frontal gyrus; Early: early adolescents; Late: late adolescents.
2.2 Substance use assessments
The Customary Drinking and Drug Use Record (CDDR; Brown et al., 1998) was administered at each wave. The number of days drinking, using marijuana, and the number of cigarettes smoked in the past year were used to quantify participants' historical substance use. With skewed distributions, these variables were recoded as binary variables (0 = did not use; 1 = used) to form four groups at each wave: non-use controls (N), alcohol-only (A), cannabis-only (C), or alcohol and cannabis (A + C) users. Smoking status during the previous year was included as a covariate in models.
2.3 Imaging data processing
At each visit, MRI scans were acquired on a 3-Tesla GE (Discovery MR750) or Siemens (TIM Trio) scanner. The imaging protocols and data preprocessing procedures were described previously.36, 37 The cerebral cortex was segmented into 34 bilateral regions of interest (ROIs) using the Desikan–Killiany atlas.38 Total volumes of each ROI (bilateral) served as the dependent variable.
2.4 Statistical analysis
2.4.1 Demographic and clinical variables
Descriptive statistics summarize participants' demographics at baseline, and substance use status at baseline and across each follow-up assessment. Chi-square and t-tests were used to examine associations between the variables and developmental stage (early vs. late) and assigned sex at birth. All analyses were conducted using R 3.5.2.
2.4.2 Substance use and longitudinal volumetric changes
To test the associations between substance use and annual rates in volumetric changes of each ROI, F tests were used to examine the overall statistical significance of the interaction of follow-up year and substance use subtype. Namely, we tested the null hypothesis that all coefficients in (1) are zero or that alcohol and cannabis use does not impact the annual volume changes. The F test p-values were adjusted using a Bonferroni correction for 34 ROIs, and those showing corrected p-values < 0.05 were retained for further analysis. We employed t-tests to examine pair-wise differences in annual rates across assigned sex at birth and between substance use subtypes. Statistical significance was assessed by two-sided p-values < 0.05, after Bonferroni corrections, and corrected p-values between 0.05 and 0.06 were considered marginally significant.
2.4.3 Developmental age and assigned sex at birth modulation of the effects of substance use on longitudinal volumetric changes
To assess the robustness of the ROI findings, we also evaluated Models (1) and (2) without smoking status as a covariate.
2.4.4 The effects of alcohol and cannabis use metrics
Alcohol and cannabis co-use, but not alcohol or cannabis use alone, showed significantly faster volumetric declines over time (see “Results”). As the co-use group showed more severe alcohol consumption than those within the alcohol use alone group, one possibility is that these findings may reflect alcohol use severity. To investigate this possibility, we examined the effects of number of drinking ( ) and cannabis ( ) use days in the prior year on the volumetric changes of the ROIs using a Wilcoxon rank sum test to compare these continuous measures among developmental stages, assigned sex at birth, and substance use groups at each visit. In mixed-effects modeling, we replaced categorical substance use types in the main [Equation (1)] and modulation [Equation (2)] model by the continuous alcohol and cannabis use measures and their interaction ( , , ). Similarly, we used t-tests to examine the significance of the coefficients of predictors that involved , , or .
3 RESULTS
3.1 Demographic and clinical variables and volumetrics at baseline
Table 1 shows the summary statistics at baseline for the whole cohort and separately for the two developmental stages. Early and late adolescents did not show significant differences in ICV, socioeconomic status, assigned sex at birth, or race distributions (p-values > 0.158). Significantly more participants smoked at baseline and during follow-ups in late adolescence (p-values < 0.001). The late adolescent group was also comprised of significantly more alcohol and cannabis users across subtypes at baseline and during follow-up (p-values < 0.001). The two groups showed significant volumetric differences at baseline except for the entorhinal, pericalcarine, and transverse temporal cortex (p-values > 0.116). Females and males did not show significant differences in age, developmental stage, baseline smoking, or substance use types at baseline or during follow-up (Table S1). At baseline, males showed larger ICV and ROI volumes (p-values <0.001).
3.2 The effects of substance use on longitudinal volumetric changes
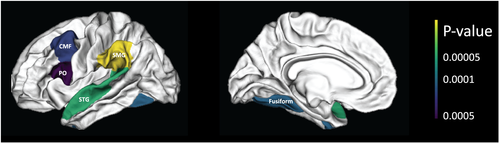
Overall, prior-year alcohol, cannabis use, and co-use were significantly associated with annual volumetric changes in five ROIs: caudal middle frontal cortex (cMFC, Bonferroni-corrected p-value = 0.013), fusiform gyrus (FG, p-value = 0.006), inferior frontal gyrus, pars opercularis (IFGpo, p-value = 0.022), superior temporal gyrus (STG, p-value = 0.002), and supramarginal gyrus (SMG, p-value < 0.001; see Figure 1 and Table 2). Females showed significantly faster volume declines in the STG than males (female–male = −0.060 cm3/year, p-value = 0.020, Bonferroni-corrected; Table 2). Excluding smoking status as a covariate did not change these statistically significant findings (Supplementary Table S2). The results for all ROIs are summarized in Supplementary Table S3.
| Region | Sex | Non-use controls | Cannabis | Alcohol | Alcohol+Cannabis | p-value |
|---|---|---|---|---|---|---|
| Caudal middle frontal cortex | <0.001 | |||||
| Female | −0.361 (−0.394, −0.329) | −0.318 (−0.449, −0.187) | −0.421 (−0.480, −0.362) | −0.455 (−0.510, −0.400) | ||
| Male | −0.376 (−0.409, −0.342) | −0.332 (−0.463, −0.201) | −0.435 (−0.495, −0.375) | −0.469 (−0.524, −0.415) | ||
| Fusiform gyrus | <0.001 | |||||
| Female | −0.379 (−0.413, −0.345) | −0.375 (−0.511, −0.239) | −0.439 (−0.501, −0.378) | −0.485 (−0.542, −0.428) | ||
| Male | −0.367 (−0.402, −0.333) | −0.363 (−0.499, −0.227) | −0.427 (−0.490, −0.365) | −0.473 (−0.530, −0.416) | ||
| IFG, pars opercularis | <0.001 | |||||
| Female | −0.188 (−0.204, −0.172) | −0.157 (−0.222, −0.092) | −0.207 (−0.236, −0.178) | −0.234 (−0.261, −0.206) | ||
| Male | −0.192 (−0.209, −0.175) | −0.161 (−0.226, −0.096) | −0.211 (−0.241, −0.181) | −0.238 (−0.265, −0.210) | ||
| Superior temporal gyrus | <0.001 | |||||
| Female | −0.399 (−0.441, −0.357) | −0.287 (−0.456, −0.119) | −0.440 (−0.516, −0.364) | −0.530 (−0.601, −0.460) | ||
| Male | −0.339 (−0.382, −0.297) | −0.228 (−0.396, −0.060) | −0.381 (−0.458, −0.303) | −0.471 (−0.541, −0.400) | ||
| Supramarginal gyrus | <0.001 | |||||
| Female | −0.615 (−0.658, −0.571) | −0.473 (−0.646, −0.299) | −0.703 (−0.781, −0.624) | −0.752 (−0.825, −0.680) | ||
| Male | −0.638 (−0.683, −0.594) | −0.496 (−0.670, −0.323) | −0.727 (−0.806, −0.647) | −0.776 (−0.849, −0.704) |
- Note: p-values (unadjusted) were for testing the overall associations between annual volume changes and substance use types. Only ROIs with Bonferroni-corrected p-values < 0.05 are shown; the results for all ROIs are shown in Supplementary Table S2. Only superior temporal gyrus showed significant annual change differences between females and males (p-value = 0.004, uncorrected). No other regions showed significant differences across assigned sex at birth.
Associations between annual volumetric changes of these five ROIs and substance use subtypes were examined. Compared with non-users, alcohol and cannabis co-users showed significantly faster volumetric declines in the cMFC (A + C – N = −0.094 cm3/year, Bonferroni-corrected p-value < 0.001), FG (−0.106, p-value < 0.001), IFGpo (−0.046, p-value = 0.003), STG (−0.131, p-value < 0.001), and SMG (−0.138, p-value < 0.001). Alcohol and cannabis co-use was also associated with faster SMG volume declines than cannabis-only use, though only with marginal significance (−0.280, p-value = 0.053). Other group differences were not significant. These findings are summarized in Table 3.
| Region | Substance | Reference | Substance reference | p-value |
|---|---|---|---|---|
| Caudal middle frontal cortex | ||||
| C | N | 0.044 | 0.510 | |
| A | N | −0.060 | 0.019 | |
| A + C | N | −0.094 | <0.001 | |
| A | C | −0.103 | 0.129 | |
| A + C | C | −0.137 | 0.042 | |
| A + C | A | −0.034 | 0.215 | |
| Fusiform gyrus | ||||
| C | N | 0.004 | 0.952 | |
| A | N | −0.060 | 0.023 | |
| A + C | N | −0.106 | <0.001 | |
| A | C | −0.064 | 0.363 | |
| A + C | C | −0.110 | 0.116 | |
| A + C | A | −0.046 | 0.108 | |
| IFG, pars opercularis | ||||
| C | N | 0.031 | 0.348 | |
| A | N | −0.019 | 0.132 | |
| A + C | N | −0.046 | <0.001 | |
| A | C | −0.050 | 0.141 | |
| A + C | C | −0.076 | 0.023 | |
| A + C | A | −0.027 | 0.052 | |
| Superior temporal gyrus | ||||
| C | N | 0.112 | 0.188 | |
| A | N | −0.041 | 0.208 | |
| A + C | N | −0.131 | <0.001 | |
| A | C | −0.153 | 0.080 | |
| A + C | C | −0.243 | 0.005 | |
| A + C | A | −0.090 | 0.011 | |
| Supramarginal gyrus | ||||
| C | N | 0.142 | 0.105 | |
| A | N | −0.088 | 0.009 | |
| A + C | N | −0.138 | <0.001 | |
| A | C | −0.230 | 0.011 | |
| A + C | C | −0.280 | 0.002 | |
| A + C | A | −0.050 | 0.175 | |
- Note: N: non-use controls; C: cannabis; A: alcohol; A + C: alcohol and cannabis co-use. p-values (unadjusted) were for testing the differences in annual rates. Bonferroni-corrected p-values < 0.05 are bolded. All regions in Table 2 were tested but only those with significant p-values are reported here.
3.3 Modulation by assigned sex at birth and developmental age
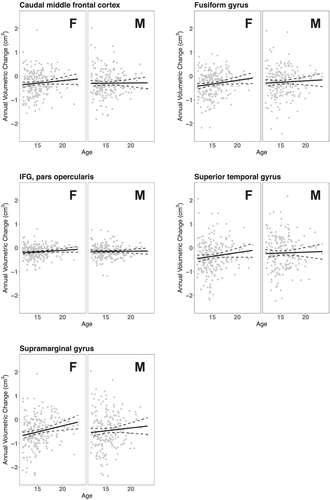
Annual volume changes versus age among female and male controls are shown in Figure 2 for the five ROIs identified from the main model. Only the slope for SMG among females was significant (p-value = 0.044, Bonferroni-corrected; > 0.313, all other slopes).
After Bonferroni corrections, assigned sex at birth did not modulate the effects of substance use subtype on volumetric changes (p-values > 0.125).
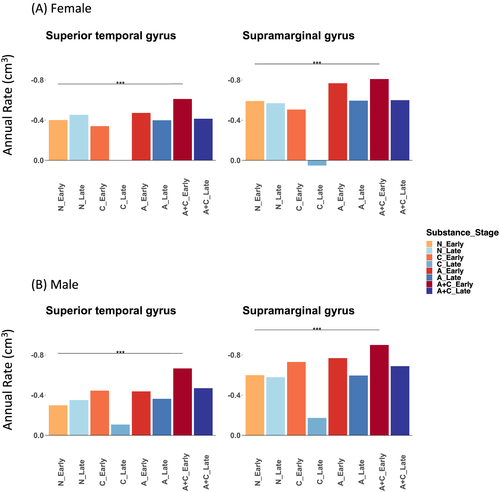
F tests showed that the developmental stage significantly modulated the associations between substance use and annual volume change in the STG (p-value = 0.004, Bonferroni-corrected) and SMG (p-value = 0.014) but not any other regions (Table 4). Alcohol and cannabis co-use versus non-use among early adolescents predicted significantly faster volume declines only in the STG (females: −0.210 cm3/year, Bonferroni-corrected p-value = 0.022; males: −0.368 cm3/year, p-value < 10−7) and SMG (females: −0.218 cm3/year, p-value = 0.020; males: −0.300 cm3/year, p-value < 10−4) (Figure 3, Supplementary Table S4). In contrast, both females and males in late adolescence did not show significant associations between alcohol and cannabis co-use and volume decline rates in any regions (corrected p-values > 0.832). Excluding smoking status as a covariate did not change these statistically significant findings (Supplementary Table S5). Among alcohol and cannabis co-users, the early versus late adolescent group also showed faster declines for both regions and across assigned sex at birth. However, the differences were only marginally significant (Bonferroni-corrected p-value < 0.06) (Supplementary Table S4).
| Region | Sex | Stage | Non-use controls | Cannabis | Alcohol | Alcohol+Cannabis | Stage modulation (p-value) | Sex modulation (p-value) |
|---|---|---|---|---|---|---|---|---|
| Caudal middle frontal cortex | 0.406 | 0.597 | ||||||
| Female | Early | −0.356 (−0.394, −0.318) | −0.343 (−0.566, −0.120) | −0.464 (−0.556, −0.371) | −0.453 (−0.543, −0.363) | |||
| Female | Late | −0.365 (−0.447, −0.283) | −0.168 (−0.469, 0.132) | −0.410 (−0.512, −0.309) | −0.416 (−0.511, −0.321) | |||
| Male | Early | −0.365 (−0.404, −0.326) | −0.409 (−0.614, −0.203) | −0.447 (−0.544, −0.350) | −0.506 (−0.594, −0.418) | |||
| Male | Late | −0.374 (−0.457, −0.292) | −0.234 (−0.508, 0.039) | −0.393 (−0.498, −0.288) | −0.468 (−0.559, −0.377) | |||
| Fusiform gyrus | 0.317 | 0.383 | ||||||
| Female | Early | −0.380 (−0.419, −0.340) | −0.381 (−0.612, −0.150) | −0.420 (−0.516, −0.325) | −0.517 (−0.611, −0.423) | |||
| Female | Late | −0.377 (−0.462, −0.291) | −0.153 (−0.464, 0.159) | −0.388 (−0.493, −0.282) | −0.442 (−0.541, −0.344) | |||
| Male | Early | −0.349 (−0.390, −0.308) | −0.474 (−0.687, −0.261) | −0.464 (−0.564, −0.363) | −0.521 (−0.612, −0.429) | |||
| Male | Late | −0.346 (−0.431, −0.260) | −0.245 (−0.529, 0.038) | −0.431 (−0.540, −0.322) | −0.446 (−0.541, −0.352) | |||
| IFG, pars opercularis | 0.115 | 0.353 | ||||||
| Female | Early | −0.191 (−0.210, −0.172) | −0.169 (−0.279, −0.058) | −0.233 (−0.279, −0.187) | −0.239 (−0.284, −0.195) | |||
| Female | Late | −0.206 (−0.247, −0.166) | −0.057 (−0.205, 0.092) | −0.201 (−0.251, −0.150) | −0.225 (−0.272, −0.177) | |||
| Male | Early | −0.186 (−0.205, −0.166) | −0.236 (−0.338, −0.134) | −0.243 (−0.291, −0.194) | −0.266 (−0.310, −0.223) | |||
| Male | Late | −0.201 (−0.242, −0.160) | −0.124 (−0.260, 0.011) | −0.210 (−0.263, −0.158) | −0.251 (−0.297, −0.206) | |||
| Superior temporal gyrus | <0.001 | 0.025 | ||||||
| Female | Early | −0.401 (−0.450, −0.353) | −0.340 (−0.624, −0.056) | −0.473 (−0.591, −0.355) | −0.611 (−0.726, −0.496) | |||
| Female | Late | −0.454 (−0.558, −0.349) | −0.002 (−0.386, 0.382) | −0.399 (−0.530, −0.269) | −0.414 (−0.536, −0.293) | |||
| Male | Early | −0.298 (−0.348, −0.248) | −0.445 (−0.707, −0.183) | −0.437 (−0.560, −0.313) | −0.666 (−0.779, −0.554) | |||
| Male | Late | −0.351 (−0.456, −0.245) | −0.107 (−0.456, 0.242) | −0.363 (−0.497, −0.229) | −0.470 (−0.586, −0.354) | |||
| Supramarginal gyrus | <0.001 | 0.308 | ||||||
| Female | Early | −0.591 (−0.641, −0.540) | −0.507 (−0.800, −0.213) | −0.768 (−0.889, −0.646) | −0.809 (−0.928, −0.690) | |||
| Female | Late | −0.569 (−0.678, −0.461) | 0.052 (−0.345, 0.448) | −0.595 (−0.729, −0.460) | −0.599 (−0.725, −0.473) | |||
| Male | Early | −0.600 (−0.652, −0.548) | −0.731 (−1.002, −0.460) | −0.769 (−0.897, −0.641) | −0.899 (−1.016, −0.783) | |||
| Male | Late | −0.579 (−0.687, −0.470) | −0.173 (−0.533, 0.188) | −0.596 (−0.734, −0.457) | −0.689 (−0.809, −0.569) |
- Note: p-values were for F test whether either developmental stage or assigned sex at birth moderated the associations between annual volumetric changes and substance use. Bonferroni-corrected p-values < 0.05 are bolded.
3.4 Analysis of continuous alcohol and cannabis use measures
The number of drinking and cannabis use days were significantly higher in the late versus the early adolescent group for all visits (Supplementary Table S6), but none were significantly different between females and males after Bonferroni correction (Supplementary Table S7). The number of drinking days was also significantly higher in the A + C versus A and versus C group across all visits, but the number of cannabis use days was not significantly different between A + C and C for any visit, after Bonferroni correction (Supplementary Table S8).
The longitudinal models showed that none of the quantitative use measures or their interaction terms were significant in modulating longitudinal volume change rates for the cMFC, FG, IFGpo, STG, or SMG (all p-values > 0.157, uncorrected) across the developmental period.
4 DISCUSSION
The current study revealed two main findings. First, alcohol and cannabis co-use, but not alcohol or cannabis use alone, is associated with a significant longitudinal regional volumetric decline. Importantly, this cannot be accounted for by more severe alcohol use in the co-use group. Second, alcohol and cannabis co-use in early adolescence predicted significantly faster volumetric decline in the superior temporal gyrus and supramarginal gyrus, in both females and males. In contrast, no longitudinal volumetric changes were noted for late adolescents for any substance use groups. Taken together, these findings suggest that alcohol and cannabis co-use is a distinct phenotype and that the effects of alcohol and cannabis use figure more prominently on the brain when onset occurs during early adolescence.
4.1 The effects of alcohol and cannabis co-use on the volumetric decline in adolescents
Individuals engaged in alcohol and cannabis co-use relative to non-drug users showed significantly faster volumetric declines in the caudal middle frontal cortex, fusiform gyrus, inferior frontal gyrus, pars opercularis, superior temporal gyrus, and supramarginal gyrus. These brain regions are implicated in attention, cognitive control, and emotion processing,39 suggesting a broad impact of alcohol and cannabis co-use on cognitive and affective functions. Cannabis use alone versus non-use did not appear to incur volumetric decline, consistent with relatively minor effects of cannabis exposure on cognitive functioning. Surprisingly, alcohol use alone versus non-use did not demonstrate significant differences in volumetric decline. This finding appears at odds with a vast literature demonstrating volumetric deficits from heavy drinking both in adults28, 29, 40-42 and adolescents [see8 for a review]. For instance, prior work indicates smaller GMVs in brain regions, including the hippocampus,43 and left frontal, temporal, and parietal cortices44 in adolescents engaged in problem alcohol use. However, it is important to note that our approach aimed to identify a longitudinal pattern of changes rather than cross-sectional effects. Thus, alcohol misuse may manifest in diminished GMVs in a given year, but does not show a significant annual decrement over time.
Indeed, brain regions may increase or decrease in GMVs during development.45 For instance, while more children demonstrate an increase in GMVs in the rostral anterior cingulate and superior temporal gyrus with development, the opposite is true of the cuneus and rostral middle frontal gyrus.45 To account for these developmental changes, including U- or inverted U-shaped patterns, of GMVs, we accounted for the effects of age and age squared in mixed-effects modeling. Further, by modeling the GMV changes from prior to current year, we were able to query the yearly difference irrespective of overall patterns. Thus, the findings of volumetric decline here suggest that alcohol and cannabis co-use impedes or even reverses the typical trend of age-related volumetric increases for some brain regions and accelerates the trend of age-related decreases for others. The findings support the importance of modeling brain volumes longitudinally, which may yield insight on brain development that eludes cross-sectional findings.
4.2 Developmental period but not assigned sex at birth modulates the effects of alcohol and cannabis co-use on the volumetric decline in adolescents
Consistent with prior work,46 alcohol use and alcohol and cannabis co-use, but not cannabis use alone, was significantly more prevalent in the late than the early adolescent group. The early but not late adolescent group demonstrated volumetric decline due to alcohol and cannabis co-use. This suggests that early adolescence is a developmental period characterized by increased vulnerability to alcohol and cannabis co-use.
Alcohol and cannabis co-use during early adolescence predicted significantly faster volumetric decline in the superior temporal gyrus and supramarginal gyrus, in both females and males. Across all substance use subtypes, females showed significantly faster volume declines in the STG. However, assigned sex at birth did not modulate the effects of substance use subtype on volumetric changes. Thus, assigned sex at birth demonstrated a limited modulating effect on the impact of alcohol and cannabis use on brain development. This finding does not negate the importance of studying assigned sex at birth differences in the influences of substance use, which may transpire earlier or only unfold later in the lifespan.
4.3 General implications of the findings
Adolescence represents a developmental period marked by significant behavioral vulnerability and complex structural and functional changes in the brain.47 The current study highlights the importance of characterizing the longitudinal patterns of brain changes during adolescence. The current work not only adds to the literature of cross-sectional findings but also suggests the possibility of cross-sectional studies misrepresenting the effects of substance use, which are likely nonlinear and area-dependent. It would be of particular interest to investigate how longitudinal brain changes support cognitive and behavioral development across this critical lifespan. The longitudinal dataset of the NCANDA and Adolescent Brain Cognition and Development projects provides ample opportunities to this end.
4.4 Limitations, other considerations, and conclusions
A number of limitations need to be considered for the study. First, substance use data were self-reported and thus were subject to recall bias. Second, there are many other clinical variables, including childhood adversity, that can influence brain volume changes. A much larger longitudinal dataset would be required to thoroughly address the impact of these other factors on brain development. Third, the current findings were based on alcohol and cannabis use as categorical variables. Yet, findings modeling these variables continuously did not yield significant results. This likely reflects the nonlinear compounding effects of alcohol and cannabis co-use. For example, the STG volume showed an annual decline of −0.131 cm3/year (Table 3) with prior-year alcohol and cannabis co-use, but statistically nonsignificant changes of 0.112 cm3/year and −0.041 cm3/year for cannabis and alcohol use only, respectively. Thus, the volumetric decline in co-use cannot be explained simply by the sum of the changes in cannabis and alcohol use alone.
Although we demonstrated longitudinal brain changes, this is a not a randomized trial and the findings should not be taken to imply causality of the effects of alcohol and cannabis use. That is, substance use precedes structural brain changes but temporal order alone is not sufficient to determine causation. Thus, alternative explanations should be explored further.
In summary, this work demonstrates the longitudinal impact of alcohol and cannabis co-use on volumetric brain development and a more significant impact with the onset of use in early than in late adolescence and potentially in females than in males. The volumetric decline was noted in cortical regions in support of attention, memory, executive control, and social cognition, suggesting potentially pervasive influences of alcohol and cannabis co-use on brain development. Alcohol and cannabis co-use may represent a distinct phenotype of importance to addiction neuroscience and medicine. More research is needed to address how these volumetric markers predict alcohol and cannabis use and co-use during a critical period in the developmental lifespan.35
ACKNOWLEDGEMENTS
The study is supported by the National Institute of Biomedical Imaging and Bioengineering grant R01EB022911 (Luo), National Institute on Drug Abuse grants R01DA051922 (Li) and R01DA049154 (Buu), and National Institute on Minority Health and Health Disparities grant U54MD012393 (Trucco). NCANDA data collection was supported by NIH grants AA021697, AA021697-04S1, AA021695, AA021692, AA021696, AA021681, AA021690, and AA021691. The Institutional Review Board (IRB) of the local collection sites approved the data collection in accordance with Department of Health and Human Services regulations at 45 CFR Part 46.
AUTHOR CONTRIBUTIONS
XL, AB, and CL were responsible for the study concept and design. XL, JJY, and AB contributed to the acquisition and management of data. XL and JJY performed the statistical analysis. JJY, AB, and CL assisted with data analysis and interpretation of findings. XL and CL drafted the manuscript. EMT provided critical feedback and contributed to the final version of the manuscript. All authors critically reviewed the content and approved the final version for publication.
DISCLOSURES
The authors report no financial interests or potential conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) and National Institute on Alcohol Abuse and Alcoholism (NIAAA). Restrictions apply to the availability of these data, which were used under a data distribution agreement for this study.




