Compulsive-like eating of high-fat high-sugar food is associated with ‘addiction-like’ glutamatergic dysfunction in obesity prone rats
Abstract
Chronic overeating is a core feature of diet-induced obesity. There is increasing evidence that in vulnerable individuals, such overeating could become compulsive, resembling an addictive disorder. The transition to compulsive substance use has been linked with changes at glutamatergic synapses in the nucleus accumbens. In this study, we investigated a potential link between such glutamatergic dysregulation and compulsive-like eating using a rat model of diet-induced obesity. A conditioned suppression task demonstrated that diet-induced obese rats display eating despite negative consequences, as their consumption was insensitive to an aversive cue. Moreover, nucleus accumbens expression of GluA1 and xCT proteins was upregulated in diet-induced obese animals. Lastly, both a computed ‘addiction score’ (based on performance across three criteria) and weight gain were positively correlated with changes in GluA1 and xCT expression in the nucleus accumbens. These data demonstrate that the propensity for diet-induced obesity is associated with compulsive-like eating of highly palatable food and is accompanied by ‘addiction-like’ glutamatergic dysregulation in the nucleus accumbens, thus providing neurobiological evidence of addiction-like pathology in this model of obesity.
1 INTRODUCTION
Obesity is a chronic and relapsing condition, typically driven by overconsumption of foods high in fat and sugar (HFHS). People suffering from diet-induced obesity often continue to overeat despite knowledge of its negative health consequences, resembling a hallmark diagnostic feature of addiction—the persistence of behaviour despite adverse consequences. Indeed, increasing evidence suggests that the brain's reward circuitry is dysregulated in diet-induced obesity.1-3 To study this phenomenon, a rodent model of diet-induced obesity can be used.4 Previous work using this model has demonstrated that diet-induced obese (DIO) rats exhibit addiction-like behaviour towards HFHS food compared to their diet-resistant (DR) counterparts. This is evidenced by (1) heightened motivation for palatable food, (2) binge-like consumption (3) and a lack of control over HFHS food-seeking during periods of signalled food-unavailability.5, 6 We aimed to investigate whether DIO rats show another important diagnostic feature of addictive behaviour: difficulty to restrain from consumption in the face of negative consequences and a hallmark of both substance use disorder and compulsive overeating.7-10 The presence of this specific hallmark has not yet been investigated in an animal model of DIO. To assess this, we used a conditioned suppression paradigm where a mild foot shock was utilized as a negative consequence.11
Previously, Brown et al.5 demonstrated that addiction-like behaviour towards HFHS food in DIO rats is associated with glutamatergic plasticity deficits in the nucleus accumbens (NAc). This joined a body of evidence implicating dysregulated glutamate transmission in the nucleus accumbens in the development of addictive behaviours associated with chronic consumption of drugs of abuse and, more recently, of palatable foods.7-9 Thus, we decided to assess changes in the expression of some glutamate proteins that may underlie the observed deficits in accumbal glutamatergic plasticity in this DIO model. Our first target was the GluN2B subunit of the N-methyl-D-aspartate (NMDA) receptor as upregulation of this subunit in the NAc is observed in animal models of drug relapse.8, 10, 11 Further, NMDAR antagonism in the NAc shell has been shown to suppress compulsive-like food intake.12 NMDARs participate in synapse-membrane homeostatic crosstalk with α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors.13 Trafficking of GluA1-containing AMPA receptors in the NAc plays an important role in drug-induced neuroplasticity,14, 15 and increased levels of GluA2-lacking AMPA receptors have been implicated in increased sensitivity of NAc neurons to drug-associated cues, as well as relapse to cocaine-seeking.16
Another possible mechanism underlying the behaviour observed in DIO animals after HFHS diet is altered glutamate homeostasis. The maintenance of glutamate homeostasis is achieved via transporters and exchangers, such as the cystine-glutamate exchanger and the EAAT2 transporter (rodent GLT1). The cystine-glutamate exchanger exports glutamate to maintain extra-synaptic levels of glutamate,17 while the GLT1 transporter performs re-uptake.18, 19 Changes in expression of the cystine-glutamate exchanger catalytic subunit, xCT, as well as GLT1 have been implicated vulnerability to drug relapse and the transition to addiction.17, 18, 20, 21 Importantly, changes in expression of these proteins are linked to synaptic plasticity adaptations in NAc. We also investigated changes in the dorsal striatum, part of the brain that plays an important role in the development of habitual and compulsive behaviours.22
Our first aim was to extend previous findings of addiction-like behaviour towards palatable food in in the DIO model and assess the presence of eating despite negative consequences. We hypothesized that even without food restriction, some DIO rats would display difficulty restraining from HFHS food consumption in the conditioned suppression paradigm where a mild foot shock was utilized as a negative consequence.23 The second aim was to investigate brain changes potentially associated with the transition to compulsive-eating behaviour in DIO rats. We hypothesized that DIO rats would show altered striatal expression of one or more of the glutamatergic proteins GluN2B, GluA1, GLT1 and xCT and these changes would be associated with compulsive-like behaviour towards HFHS food.
2 MATERIALS AND METHODS
2.1 Experimental subjects
Outbred male Sprague–Dawley rats (340 ± 20 g, 9–10 weeks old, n = 60) (ARC, Canning Wale) were housed individually with nesting/enrichment material at a room temperature of 20 ± 2°C on a reverse 12 h light/dark cycle (lights off at 7:00 AM). Rats were provided ad libitum access to water and either standard rodent diet (Chow, 18% kcal fat; total density = 3.17 kcal g−1, Barastoc, Australia) or a high-fat high-sugar (HFHS) diet, as described below. Rats were allowed to acclimate for 7 days before experimentation began. All procedures performed were in accordance with the Prevention of Cruelty to Animals Act (2004), under the guidelines of the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of animals for Experimental Purposes (2013) and approved by The Florey Institute of Neuroscience and Mental Health Animal Ethics Committee.
2.2 Model of diet-induced obesity
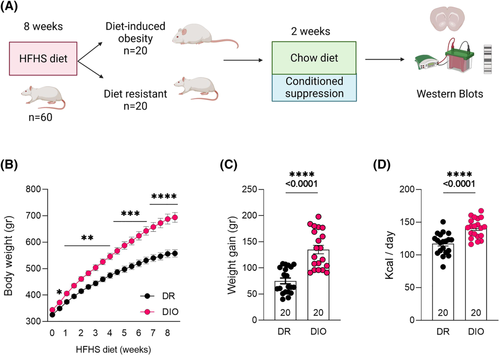
Sixty rats were placed on an 8-week HFHS diet (SF04-001, 45% kcal from fat; total density = 4.73 kcal g−1, Specialty Feeds, Glen Forrest, Australia). Food intake and body weight were measured twice per week. Rats were then separated into DIO (top third, n = 20) and DR (bottom third, n = 20) groups based on weight gain from weeks 3 to 8 to avoid weight gain due to normal development during the first 2 weeks.4, 5 For the subsequent duration of the experiment, rats were placed back on standard chow diet.
2.3 Conditioned suppression test
Rats underwent a conditioned suppression task 2 days after being placed back on the standard chow diet (Figure 1A). In fear conditioning chambers (MED Associates; St. Albans, VT), rats were given access to HFHS food (20 g) for 30 min, made available in a plastic tray affixed to the grid floor. Food was weighed before and after the session to establish baseline HFHS intake; this was repeated daily until the daily food intake no longer varied beyond 10% from total average.23 Average training time was 13 days. Once the baseline was established, we ran a single shock session. Rats were placed in the same fear chambers, this time without any food. During the first and last 10 min of the 30 min session, a flashing cue light (conditioned stimulus, CS) was accompanied by a 1 s electric shock (0.5 mA, unconditioned stimulus, US) every minute. During minutes 11 to 20, there was no US or CS presentations. The following day, rats were placed back into the chambers with HFHS pellet, and the effect of CS presentation (cue light) on food consumption was measured. We recorded baseline and test day latencies to commence eating, time spent interacting with food (scored manually via NIR video recording) and total amount of food consumed during these sessions (kcal and grammes).
2.4 Western blots
GluN2B (NMDAR2B, NR2B), GluA1 (GluR1, GRIA1), GLT1 and xCT expression in the dorsal striatum and nucleus accumbens was determined by western blot analysis, according to previously described methods24; the membrane subfraction protocol was adapted from Reissner et al.20 At conclusion of experiment, half of the rats were transferred to another experiment, and half were culled via i.p. injection of pentobarbital (1.00 ml) followed by rapid decapitation (DIO n = 12, DR n = 13). Snap-frozen brains from DIO, DR and control Chow were cut into 1 mm thick slices at Bregma +2.2 mm,25 and 2 mm diameter patches were collected. A control group, never exposed to the HFHS diet, was included to normalize western blot analysis (Chow, n = 14), all protein expression data were normalized to protein expression levels in this Chow control group. The Chow group was matched by age and weight. Brain punches were homogenized on ice in sucrose buffer containing protease (cOmplete™, Roche, IN) and phosphatase inhibitors (PhosSTOP EASYpack, Roche, IN). Some brain punches were lost due to freezer failure. For nucleus accumbens, we analysed punches from 10 DR, 6 DIO, 9 Chow, for dorsal striatum 4 DR, 4 DIO, 5 Chow. The membrane protein fraction was separated in two steps. First, the 1000g, 4°C for 10 min to separate supernatant from tissue debris and the final membrane fraction was obtained by centrifugation at 12 000g, 4°C for 20 in and resuspending protein in 35 μl of sucrose buffer. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein (20 μg) was separated on Tris/Glycine 4–20% gels and transferred onto a PVDF membrane. Membranes were cut in three parts. Part A was incubated with mouse anti-GluN2B (1:2000; Merck 05-920), mouse anti-GluA1 antibodies (1:1000; Merck SAB6201086), part B with rabbit anti-GLT1 antibody (1:5000; gift from David Pow, RMIT University, Australia) and part C with rabbit anti-xCT (1:1000; Novus NB300-318, Lot M-1 and N) diluted in 1% skimmed milk in Signal Enhancer solution 1 (SignalBoost Immunoreaction Enhancer kit, Merck Millipore, Cat# 407207) and mouse anti-β-actin antibodies (1–10 000; Sigma-Aldrich, USA). Part A was incubated with goat anti-mouse (1:10 000, IRDye®800cw [Green], LI-COR, USA) for NR2B and GluA1. Part B: goat anti-rabbit (1:10 000, IRDye®680cw [Red], LI-COR, USA) was used for GLT1 detection. Part C: First, xCT was detected with goat anti-rabbit (1:10 000, IRDye®800cw [Green], LI-COR, USA) diluted in 5% skimmed milk in Signal Enhancer Solution 2 (SignalBoost Immunoreaction Enhancer kit, Merck Millipore, Cat# 407207) and then goat anti-mouse (1:10 000, IRDye®680cw [Red], LI-COR, USA) for β-actin. Expressions of GluN2B, GLT1, GluA1, xCT and β-actin bands were quantified with ImageJ ver. 1.52p (NIH).
2.5 Addiction score
The ‘addiction score’ was calculated using an approach previously described5, 26, 27 for three behavioural criteria: (1) overeating palatable food measuring kcal intake during the HFHS diet period, (2) lack of control to refrain from eating despite negative consequences, measuring latency to start eating HFHS on the test day in the presence of an aversive signal (CS previously associated with electrical shock), and (3) binge-like eating by measuring kcal intake of palatable pellets in a 30 min session. Standardization of a score for each behaviour was done based on data obtained from the whole group: subtracting the average from each data point and dividing by the standard deviation. The addiction score for each rat equals the sum of the standardized scores in the 3 behaviours. According to DSM-5,28 substance use disorder is categorized ‘mild’, ‘moderate’ or ‘severe’ according to the number of criteria fulfilled. In line with this, animals in our study that were positive for the maximum number of criteria (3) we will consider severe, two criteria moderate, one criterion mild and zero criteria not addicted.
2.6 Statistical analysis
Statistical analyses were performed using GraphPad Prism 9.1 (GraphPad Software Inc., San Diego, California). Two-way repeated measures analysis of variance (two-way RM ANOVAs) were used to examine group (DIO vs. DR) differences in weight, latency to food consumption and total kcal intake. For western blotting, target protein expression was first normalized to β-actin expression and next divided by mean expression in the Chow group. The weight gain after 8 weeks of HFHS diet, daily kcal intake on the final week of HFHS diet, time spent eating in the first 5 min of the test day session, the addiction score and western blot results were analysed by unpaired two-tailed Student t tests. Post hoc Bonferroni's analyses were performed where indicated. Alpha values of <0.05 were considered significant. For correlations, linear regressions were performed, and F test determined whether the linear regression slope was different from 0 (P values and goodness of fit [R2] values, not F values, are displayed on graphs).
3 RESULTS
3.1 Establishing DIO and diet resistant subpopulations
A two-way ANOVA conducted on body weight over the 8 weeks of access to palatable food (Figure 1B) found main effects of Group (F (1, 38) = 24.68, p < 0.0001), and a significant Time X Group interaction (F (17, 646) = 34.10, p < 0.0001). Bonferroni post hoc test found that prior to palatable food diet (Week 0), there were no group differences in weight, but by Week 8, DIO rats were heavier than DR rats (p < 0.0001). Before the diet, regular chow intake did not differ between groups (week 0) (data not shown; t (38) = 1.348, p = 0.186). Total amount of weight gain was greater in the DIO rats (Figure 1C; t (38) = 6.096, p < 0.0001). DIO rats also consumed more HFHS food (t (38) = 4.819, p < 0.0001; Figure 1D) (overeating in the home cage was used as the first criterion for the addiction score).
3.2 DIO rats display pervasive consumption of HFHS food despite aversive stimuli
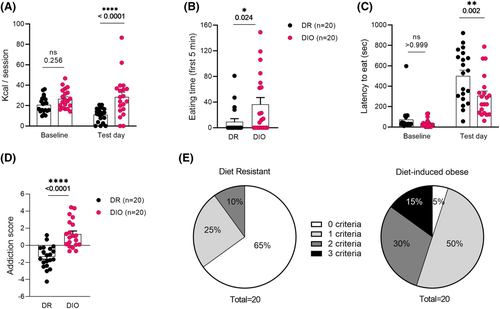
After 8 weeks of ad libitum access to HFHS diet, animals were placed on standard chow, and access was restricted to 30 min daily during conditioned suppression baseline sessions (see Figure 1A). On the test day, the CS was presented, without the US, and HFHS food was available. A two-way RM-ANOVA computed on food intake (Figure 2A) found a main effect of Group (F (1, 38) = 12.67, p = 0.001), no main effect of Shock and a significant Group X Shock interaction (F (1, 38) = 7.568, p = 0.009). Bonferroni's multiple comparisons test showed that while the Baseline consumption was similar between DIO and DR groups (p = 0.256), the Test Day consumption was higher in DIO group (p < 0.0001), accordingly comparison of consumption during Baseline versus Test Day was different only in DR (p = 0.005) and unchanged in DIO (p > 0.999) group. DIO rats spent more time eating in the presence of the aversive cue (Figure 2B; unpaired t test, t (38) = 2.355, p = 0.024). Both groups increased their latency to approach food during test (second criterion for the addiction score), but DIO accessed HFHS pellets earlier than DR (Figure 2C; two-way RM-ANOVA: main effect of Group, F (1, 38) = 7.821, p = 0.008, main effect of Shock, F (1, 38) = 86.45, p < 0.0001, Group X Shock interactions, F (1, 38) = 4.452, p = 0.042). Bonferroni's multiple comparisons found no group differences at baseline (p > 0.999), but a difference on test day (p = 0.002).
3.3 DIO rats display a higher addiction score compared to DR rats
The final criterion, binge-like eating of HFHS in 30 min, was higher in DIO rats as revealed by unpaired t test of the baseline data (t (38) = 2.182, p = 0.035, data not shown). When computing performance on these three criteria (binge-like eating during baseline consumption, overeating of HFHS in homecage and eating despite negative consequences), it was found that DIO animals had a higher addiction score compared to DR rats (Figure 2D; unpaired two tailed t test: t (38) = 5.687, p < 0.0001).
3.4 DIO rats display increased expression of GluA1 and xCT protein in nucleus accumbens
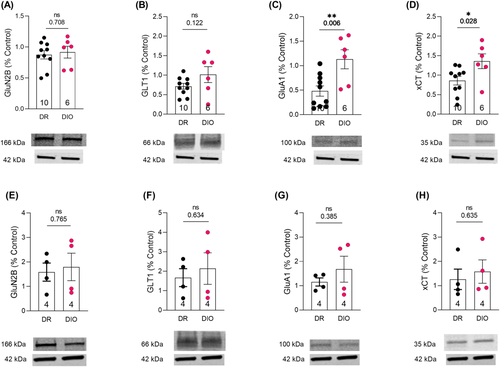
Western blots of membrane fraction from NAc revealed that DIO and DR animals did not show any significant differences in GluN2B or GLT1 expression (Figure 3A,B). However, DIO showed increased expression of both GluA1 (t (14) = 4.230, p = 0.0008; Figure 3C) and xCT (t (14) = 2.382, p = 0.032; Figure 3D). There was no significant difference in the expression of any of the proteins in dorsal striatum (Figure 3E–K).
3.5 Addiction score is positively correlated with weight gain, GluA1 and xCT expression
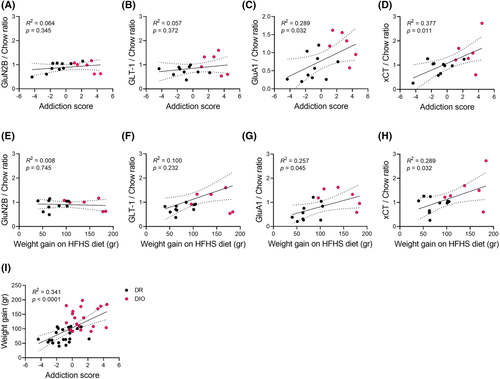
Both addiction score and weight gain were positively correlated with GluA1 (Figure 4C; R2 = 0.289, p = 0.0316, Figure 4G; R2 = 0.257, p = 0.045) and xCT expression in the nucleus accumbens (Figure 4D; R2 = 0.377, p = 0.011, Figure 4H; R2 = 0.289, p = 0.0317). There were no correlations with GluN2B and GLT1 (Figure 4A,E: GluN2B, p = 0.345, p = 0.745; Figure 4B,F: GLT1, p = 0.372, p = 0.232, respectively). Addiction score and propensity for weight gain were positively correlated (Figure 4L; R2 = 0.341, p < 0.0001).
4 DISCUSSION
The goal of this study was to examine whether the propensity for diet-induced obesity was associated with compulsive-like behaviour towards HFHS food and glutamatergic dysregulation in the striatum. We show that DIO rats exhibit an impaired ability to refrain from eating in the presence of cues that signal an aversive consequence (foot shock): one of the key hallmarks of addiction (consumption of the substance despite known negative consequences).28 Further, addiction-like behaviour towards HFHS food was associated with glutamatergic dysfunction in the NAc of DIO rats, and the extent of this dysfunction predicted the extent of addiction-like behaviour.
Although to date, the theory of food addiction remains somewhat controversial, there is growing evidence that diet-induced obesity is often associated with addiction-like behaviour towards HFHS food in both humans and animal models.23, 29 We have previously shown that DIO but not DR animals display binge-like eating, loss-of-control over food-seeking behaviour and increased willingness to work for food in an operant self-administration paradigm.5, 6 Our current findings add another crucial parameter to this list of aberrant eating behaviours, namely, eating despite known negative consequences. This was evidenced by the fact that DIO animals failed to reduce their kcal intake or latency to start eating in the presence of aversive signal.
Taken together, these data demonstrate that the propensity for obesity is associated with compulsive-like behaviour towards HFHS, thus providing support for a common endophenotype underlying some forms of diet-indued obesity and drug addiction. Moreover, our data provide further evidence of the utility of the Levin outbred DIO model. Robust preclinical models of addictive eating will facilitate the development of effective and comprehensive obesity treatments which should go beyond weight loss to reduce food craving, promoting the maintenance of healthy eating habits.
4.1 Increased GluA1 expression in DIO rats is similar to cocaine-induced changes in the nucleus accumbens
Accumbal glutamatergic dysfunction has been demonstrated in animal models of cocaine,14 nicotine,21 alcohol30 and heroin31 addiction. More recently, accumbal glutamatergic dysfunction (viz., an increase in AMPA/NMDA currents in parallel with a deficit in LTD) was also reported in DIO animals,5 suggesting dysregulated accumbal glutamate signalling in DIO. To investigate this further, assessed GluA1 expression in the NAc and dorsal striatum, finding that 8 weeks of HFHS diet were sufficient to increase expression of GluA1-containing AMPA receptors selectively in the NAc of DIO animals. Not only is this consistent with the increased ratio of AMPA receptor mediated currents as compared to NMDA previously observed5 but a body of literature exists demonstrating a role for NAc GluA2-lacking (presumably GluA1-containing) AMPAR in incubation of drug craving and enduring vulnerability to relapse.9, 16, 32 Furthermore, it seems that AMPA receptors lacking the GluA2 subunit (presumably GluA1-AMPAR) are responsible for the increased sensitivity of NAc neurons to drug associated cues.13, 16 For example, a study of rats undergoing prolonged withdrawal from cocaine using whole-cell patch-clamp recording of medium spiny neurons in the NAc core showed an enhanced responsiveness of those neurons to excitatory inputs, largely relying on GluA2-lacking AMPA receptors.16 Plus, in the same study, they showed that intra-NAc administration of 1-naphthylacetylspermine (Naspm), a selective antagonist of GluA2-lacking AMPA receptors, reduced cue-induced cocaine-seeking.
Our findings are also consistent with work demonstrating exposure to HFHS selectively increases the function of calcium permeable AMPAR in the NAc of DIO rats, but not their DR counterparts.33 Moreover, enhanced cue triggered approach behaviour (as measured by Pavlovian Instrumental Transfer) in DIO rats is mediated by calcium-permeable AMPAR in NAc core,9 providing a possible mechanism by which enhanced cue triggered motivation could drive compulsive-like behaviour towards HFHS (see also Oginsky et al.33 and Alonso-Caraballo et al.34). Thus, it has previously been established that, as with cocaine, AMPAR plasticity in NAc occurs in response to food rewards. We extend this research by showing a specific association between the extent of GluA1 expression in NAc and the level of compulsive-like behaviour towards HFHS food. This provides a link between glutamatergic dysfunction in NAc and compulsive-like behaviour towards ‘junk food’ in obesity. One possible mechanism by which HFHS food is increasing GluA1 in DIO rats is via the generation of de novo silent synapses,34 as observed following cocaine use.35
4.2 DIO rats display xCT upregulation in the nucleus accumbens but no change in GLT1
We also observed changes at the level of xCT, a catalytic chain subunit of the cystine/glutamate antiporter (also known as the xc− system or Slc7a11). This system transports cystine into neurons in exchange for glutamate at a ratio of 1:1. It plays an important role in glutamate homeostasis in many parts of the brain, and in the NAc, this system is responsible for 60% of extracellular glutamate.36 In drug addiction studies, the xc− system is thought to contribute to pathological glutamate signalling linked to addiction.37 In these studies, downregulation of xCT is typically observed in NAc after a period of withdrawal.38-40 The directionality of these findings is in contrast with our data where we observe an increase in xCT expression in NAc in the DIO rats displaying addictive-like behaviour towards HFHS. However, importantly, in the current study, the NAc tissue used was obtained from rats that were not subject to withdrawal from HFHS food. Indeed, increased levels of xCT are observed in ethanol-dependent rats, and the opposite is observed once rats are placed into withdrawal.39 xCT upregulation in the NAc is also associated with an increased risk for relapse to alcohol seeking in mice.41 Thus, it would appear that upregulation of accumbal xCT levels promotes vulnerability to addiction-like behaviour. In contrast to xCT, no changes were observed in NAc expression of GLT1. Simplistically, this suggests that xCT may play a bigger role in glutamate-mediated changes in DIO rats than GLT1. However, it should be noted that one recent study suggests that in NAc membrane GLT1 expression is activity dependent and can undergo rapid transition from upregulation to downregulation.42
In summary, we have observed that the predisposition to obesity on a ‘junk food’ diet is associated with differences in the xCT system in NAc, providing support for the proposal that glutamatergic dysfunction in NAc underlies compulsive eating in obesity. Future studies could specifically examine changes in behaviour and xCT/GLT1 expression induced by withdrawal from ‘junk food’. It would be of interest to observe whether prolonged withdrawal causes incubation of ‘craving’ in DIO animals and concomitant HFHS food-seeking behaviour which correlates with upregulation of xCT expression in NAc. These data support a common mechanism between substance abuse and aberrant eating behaviour.
4.3 No significant differences observed between DIO and diet resistant rats with respect to dorsal striatum levels of GluA1, xCT, GluN2B and GLT1
A potentially intriguing finding is the lack of observed differences in the dorsal striatum levels of any of the glutamatergic markers observed. Given the compulsive nature of the behaviour assessed and the known role for dorsal striatum in the transition to compulsive drug use,22 in addiction, we would potentially hypothesize that glutamatergic dysfunction in dorsal, not ventral, striatum would underlie the differences observed. However, this was found not to be the case. Considering the established involvement of the dorsal striatum in the later stages of addiction,22 it may be that our protocol was simply not long/extensive enough to recruit this brain region. Another possibility could be due to food being a natural reward, as opposed to a pharmacological agent, and, as such, not providing as strong a stimulus to induce these changes. However, binge-like consumption of palatable food has been shown to accelerate habitual control of behaviour in a dorsolateral striatum-dependent manner,43 providing direct evidence to the contrary. Thus, given the distinct role for the dorsolateral subregion of the striatum compared to the dorsomedial in the transition from goal-directed to more habitual behaviour, it may be that had we tested protein levels from these distinct striatal subregions that we may have observed an effect. Future studies should explore these possibilities.
4.4 Weight gain on the HFHS diet and nucleus accumbens GluA1 and xCT expression are positively associated with addiction scores
While compulsive overeating is mostly reported in people suffering from eating disorders and some people with obesity, milder variations of this behaviour are also observed in healthy individuals (e.g., stress-related overeating, emotional eating).44 It is unclear what precipitates the shift from occasional overeating of junk food to more maladaptive patterns of consumption. To generate effective treatment and prevention strategies, we need appropriate preclinical models. Eating is essential for life and in that regard is very different from drug use. Yet, as is the case with drug use, most individuals who occasionally overindulge in junk food are generally in control of their behaviour and do not develop compulsive habits. Similarly, in our model, only a small proportion of outbred DIO rats developed ‘severe’ addiction-like behaviour (15% positive for all 3 addiction criteria) after chronic exposure to the HFHS diet. Importantly, weight gain on the HFHS diet was predictive of propensity to develop addiction-like behaviour (animals positive for all three addictive criteria were only found in the DIO group, i.e., top gainers). Strikingly, we observed an association between both the development of addiction-like behaviour and changes in brain reward circuits. We hypothesize that a propensity for overeating and thus weight gain when exposed to HFHS food is an early indication of increased risk for developing compulsive eating.
In this regard, compounds acting on glutamate homeostasis may show promise for the treatment of compulsive eating in diet-induced obesity. As we described above, DIO rats with compulsive-like eating display dysregulation of glutamate homeostasis in NAc.5 In several psychostimulant studies, dysregulated glutamate homeostasis in NAc and, thus, relapse-like behaviour is restored by N-acetylcysteine, a cysteine pro-drug.20, 45-48 We recently showed that sub-chronic administration of N-acetylcysteine reduced escalation of operant responding for HFHS pellets and addictive-like behaviour towards junk food in DIO rats.6 Moreover, N-acetylcysteine has been shown to reduce binge-like eating49 and cue-induced reinstatement of food-seeking in rats.46 In light of the present results showing increased xCT expression in this model, it is possible that N-acetycysteine reduces binge eating/addictive eating due to its well-established effects on increasing basal glutamate, reducing the motivation to overeat to increase xCT expression. Collectively, these data support a common mechanism between compulsive drug taking and compulsive eating behaviour and highlights the therapeutic potential of glutamate homeostasis restoring treatments.
Limitations of this study deserve note. First, the western blot analyses were performed on tissue obtained from animals that were subjected to the conditioned suppression protocol, which could potentially affect results. However, this allowed us to examine the putative molecular basis of behavioural differences observed and perform correlations between protein expression and behavioural endpoints. Second, studies suggest that there are sex-differences in addictive-like behaviours and preclinical evidence suggests that there could be sex-differences in the glutamate system related to drug-exposure.50 Moreover, we know that women are at a higher risk for stress-induced HFHS eating, and preclinical studies show sex-differences in binge-like eating.44 Therefore, it is important that in future studies addiction-like behaviour and glutamatergic changes are assessed in female rats too. In conclusion, DIO rats exhibit compulsive-like behaviour towards HFHS junk food accompanied with increased GluA1 and xCT expression in the NAc. Further, the extent of glutamatergic dysregulation observed predicted the extent of addiction-like behaviour. These data suggest addiction-like glutamatergic dysfunction underlies compulsive eating of HFHS foods in diet-induced obesity.
ACKNOWLEDGEMENTS
We thank David Pow (RMIT University, Australia) via Tony Hannan (Florey) for the gift of the GLT1 antibody. This research was funded by National Health and Medical Research Council project grant 1108092. RMB is supported by RMB is supported by Australian Research Council (ARC) DECRA (DE190101244). PS is supported by a National Health and Medical Research Council Investigator Grant (1178482), and AJL is supported by a National Health and Medical Research Council Principal Research Fellowship (1116930). We acknowledge the Victorian State Government Operational Infrastructure Scheme. Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
RMB designed the experiments with input from AJL. DB ran behavioural testing under supervision of RMB and AJL. LL, NK, HT and TL optimized and ran western blots under supervision of RMB and JN, with input from LK. DS analysed data and wrote the manuscript with input from PS, RMB and AJL.
Open Research
DATA AVAILABILITY STATEMENT
The data from this manuscript are available upon request.




