Cortical thickness and related depressive symptoms in early abstinence from chronic methamphetamine use
Abstract
Methamphetamine use is surging globally as a cause of morbidity and mortality. Treatment is typically sought in early abstinence, when craving and depressive symptoms are intense, contributing to relapse and poor outcomes. To advance an understanding of this problem and identify therapeutic targets, we conducted a retrospective analysis of brain structure in 89 adults with Methamphetamine Use Disorder who were in early abstinence and 89 healthy controls. Unlike most prior research, the participants did not significantly differ in age, sex and recent use of alcohol and tobacco (p-values ≥ 0.400). We analysed thickness across the entire cerebral cortex by fitting a general linear model to identify differences between groups. Follow-up regressions were performed to determine whether cortical thickness in regions showing group differences was related to craving, measured on a visual analogue scale, or to the Beck Depression Inventory score. Participants in early methamphetamine abstinence (M ± SD = 22.1 ± 25.6 days) exhibited thinner cortex in clusters within bilateral frontal, parietal, temporal, insular, and right cingulate cortices relative to controls (p-values < 0.001, corrected for multiple comparisons). Unlike craving (β = 0.007, p = 0.947), depressive symptoms were positively correlated with cortical thickness across clusters (β = 0.239, p = 0.030) and with thickness in the anterior cingulate cluster (β = 0.246, p = 0.027) in the methamphetamine-dependent group. Inasmuch as anterior cingulate pathology predicts response to antidepressants for Major Depressive Disorder, cingulate structure may also identify patients with Methamphetamine Use Disorder who can benefit from antidepressant medication.
1 INTRODUCTION
Methamphetamine use and its role in overdose deaths are escalating.1-3 Because early relapse characterises Methamphetamine Use Disorder and thwarts therapeutic success,4 it is paramount to understand the biological bases of problems in early abstinence. Spontaneous craving for methamphetamine decreases during the first month,5, 6 but cue-induced craving rises before declining at 3 months of abstinence.5 Depressive symptoms pose further challenges as they predict low treatment attendance and continued methamphetamine use.7
The U.S. Food and Drug Administration has not approved any medication for Methamphetamine Use Disorder, and nonpharmacological interventions remain the mainstay of treatment. Cognitive behavioural therapy reduces craving, depressive symptoms and methamphetamine use,8, 9 as does exercise training, which also reduces stimulant use.8, 10-12 However, cognitive deficits interfere with therapy13-16 and predict poor treatment outcomes across substance use disorders.17
Novel approaches that consider problems in early abstinence, when patients typically seek treatment, are needed. Structural brain correlates of depressive symptoms promise to provide therapeutic targets and improve treatment matching inasmuch as numerous studies relate grey matter to the therapeutic response of patients with Major Depressive Disorder.18, 19 Anatomical neuroimaging studies, including some from our laboratory,20, 21 have compared participants with Methamphetamine Use Disorder and healthy controls. Participants ranging in abstinence from hours to several months had deficits relative to controls in grey matter volumes of orbitofrontal, dorsolateral prefrontal and superior temporal cortices22 and grey matter densities of the left middle frontal gyrus and bilateral insula.23 When the abstinence period lasted 4 to 7 days, participants with Methamphetamine Use Disorder exhibited smaller grey matter volumes in cingulate, limbic and paralimbic cortices of the right hemisphere21 and smaller grey matter volumes in the right inferior temporal, left superior temporal, right supramarginal and left precentral gyri.20
Cortical deficits appear to reverse and even show compensatory increases. Grey matter volume in the left superior temporal gyrus increased between 1 and 4 weeks of abstinence from methamphetamine,20 and volumes of orbitofrontal and parietal cortices were positively related to the duration of abstinence, which ranged from 12 to 400 days, in men.24 Participants who were abstinent from methamphetamine for 6 months or more exhibited higher grey matter density in the right middle frontal gyrus than those abstinent for shorter periods, but both groups had lower grey matter density in this region than healthy controls.25
Prior studies of early methamphetamine abstinence were of small sample sizes, and case and control groups were generally not matched on sex or use of substances other than methamphetamine. The largest study compared 61 participants with Methamphetamine Use Disorder (54 smoked tobacco) with 44 controls (17 smoked) and excluded individuals with an abstinence period of less than 2 weeks.23 This and another study22 grouped individuals over several months of abstinence from methamphetamine when compensatory changes in brain structure may occur.23-25 Moreover, studies measured cortical volume, a composite of thickness and surface area, which are independent and should be considered separately.26 Indeed, individuals ranging in methamphetamine abstinence from hours to decades exhibited smaller grey matter volumes in bilateral frontal and right insular cortices, and thickness deficits in bilateral frontal and rostral anterior cingulate cortices, but no abnormalities in surface areas compared with healthy controls.27
Here we studied 89 individuals with Methamphetamine Use Disorder in early abstinence (M ± SD = 22.1 ± 25.6 days) and 89 healthy controls who were similar in age, sex and recent use of alcohol and tobacco. Groups were compared on the entire cerebrum to identify regional differences in cortical thickness, a measure associated with lower false positive rates in surface-based analysis than volume and surface area.28 We hypothesised that thickness deficits in early methamphetamine abstinence would be related to craving and depressive symptoms, thereby revealing possible targets for intervention.
2 MATERIALS AND METHODS
2.1 Study design
This cross-sectional investigation pooled data from studies were approved by the institutional review board of the University of California at Los Angeles. Some of these data were used in other analyses.20, 29-36 Participants were recruited with online and print advertisements. They provided written informed consent after a detailed explanation of the respective studies and received monetary compensation. Eligibility was ascertained by interview, physical examination, and laboratory tests. Participants were excluded for major medical illnesses and psychiatric conditions warranting treatment other than Methamphetamine or Nicotine Dependence, which were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).37 Given the correspondence of DSM-IV Stimulant Dependence with DSM-5 Stimulant Use Disorder,38, 39 we refer to methamphetamine-dependent participants as having Methamphetamine Use Disorder. They had urine tests positive for methamphetamine at study entry but negative tests for methamphetamine, amphetamines, cocaine, opiates, benzodiazepines and alcohol at behavioural and neuroimaging assessments. Healthy controls tested negative for all substances listed above at baseline and times of assessment.
Participants were between the ages of 26 and 54. The lower end of the age range represents a time after cortical thinning starts decelerating in young adulthood.40, 41 T1-weighted brain images were collected using a magnetisation-prepared rapid acquisition gradient echo sequence on a 1.5-T Sonata (field of view: 256 × 256 mm2, 160 sagittal slices, resolution: 1 mm3), a 3-T Trio (field of view: 256 × 256 mm2, 176 sagittal slices, resolution: 1 mm3) or a 3-T Prisma scanner (field of view: 300 × 320 mm2, 208 sagittal slices, resolution: 0.8 mm3; all Siemens, Erlangen, Germany). We used the recon-all pipeline of FreeSurfer 6.0.0 (surfer.nmr.mgh.harvard.edu) and constructed 3D models of cortical surface to measure local cortical thickness.42 For group analysis, the data were resampled into a common space and smoothed with a 10-mm full-width half-maximum kernel.
Among 488 participants with brain images, data from 297 met FreeSurfer quality control criteria as determined by the automated tool Qoala-T.43 Cortical thickness data from 287 participants (89 with Methamphetamine Use Disorder and 198 healthy controls) could be harmonised using ComBat, a method that adjusts for differences in scanner type and scan protocol while preserving biological associations.44 ComBat thereby reduces spurious findings and increases the power and reproducibility of statistical tests.44 This also holds when participant characteristics are not randomly distributed across scanners and protocols as demonstrated by harmonising two datasets with different age groups to conduct a life-span study.44
We applied the case–control algorithm of SPSS 27 (IBM, Armonk, NY, USA) to match 89 of the 198 healthy controls to the 89 participants with Methamphetamine Use Disorder on sex, years of formal education, and whether they smoked cigarettes. Of the 178 participants for final analysis, a subsample of 32 was also matched on cannabis use status, depressive symptoms and scanner for image acquisition. Evaluation with the ENIGMA Cortical Quality Control Protocol 2.0 (enigma.usc.edu) revealed frontal or temporal pole underestimations in three participants from the Methamphetamine Use Disorder group and two from the control group. The analyses reported included these participants as their exclusion did not change any results.
2.2 Analytic strategy
Analyses of group differences in demographics and use of addictive substances other than methamphetamine were conducted in SPSS, using a significance level of 0.05 (two-tailed). Categorical variables were assessed with Pearson's chi-square and Fisher's exact tests. Continuous variables were examined with Mann–Whitney U and Kruskal–Wallis tests after determining that the data were not normally distributed by evaluating histograms, normal quantile-quantile plots and tests of normality (Kolmogorov–Smirnov and Shapiro–Wilk).
We conducted a general linear model vertex-wise analysis over the whole cerebrum, using FreeSurfer software to identify group differences in cortical thickness. Cohen's d was calculated from the age-adjusted model as a measure of effect size (0.2 = small, 0.5 = medium, 0.8 = large).45 Vertices were considered significant at p < 0.001, with a cluster correction set at p < 0.05 to account for multiple comparisons.28
Follow-up regressions were performed in SPSS, using a significance level of 0.05 (two-sided). Regressions were controlled for age40 in keeping with the vertex-wise analysis. The average thickness across clusters with significant group differences, weighted by cluster size, was used as the dependent variable. We first assessed whether this measure was related to participant characteristics exhibiting at least trend-level group differences. We then analysed how the weighted average thickness across clusters was related to sex, methamphetamine use (lifetime years, days in the past 30) and aspects of early abstinence (duration, craving, depressive symptoms). In case of significant effects, we conducted subsequent regressions on clusters in regions of interest. Correlation coefficients are reported to quantify the relationship between variables that were significantly linked to cortical thickness.
3 RESULTS
3.1 Description of the study sample (Table 1:)
| Healthy control | Methamphetamine use disorder | Group difference | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 34.9 ± 6.9 | 36.0 ± 7.5 | U = 4249.5, z = 0.842, p = 0.400 |
| Women/Men | 34/55 | 34/55 | None |
| Education (years) | 13.3 ± 1.8 | 12.4 ± 2.0 | U = 2800.5, z = −3.483, p < 0.001a |
| Employed | 53 | 15 | Χ2 (1) = 36.032, p < 0.001a |
| Ethnicity/race | Χ2 (4) = 26.256, p < 0.001a | ||
| African American | 22 | 6 | + |
| Asian/Pacific Islander | 6 | 5 | − |
| Caucasian | 44 | 31 | + |
| Hispanic | 12 | 27 | + |
| Other | 5 | 20 | + |
| Any use in past 30 days | |||
| Alcohol | 57 | 58 | Χ2 (1) = 0.069, p = 0.792 |
| Tobacco (cigarettes) | 73 | 73 | None |
| Cannabis | 26 | 38 | Χ2 (1) = 3.513, p = 0.061 |
| Methamphetamine use | |||
| Age of first use | 23.2 ± 7.8b | ||
| Lifetime years | 9.8 ± 6.6b | ||
| Days in the past 30 | 16.1 ± 10.7c | ||
| Preferred route of administrationd | |||
| Smoking | 57 | ||
| Intravenous | 18 | ||
| Intranasal | 11 | ||
| Oral | 1 | ||
| None | 1 | ||
| At brain scan | |||
| Days abstinent | 22.1 ± 25.6c | ||
| Craving | 21.4 ± 26.2b | ||
| BDI | 3.1 ± 5.5 | 8.7 ± 8.6b | U = 4993.0, z = 5.851, p < 0.001a |
| Scanner | Χ2 (2) = 29.469, p < 0.001a | ||
| 1.5-T Sonata | 23 | 18 | − |
| 3-T Trio | 9 | 41 | + |
| 3-T Prisma | 57 | 30 | + |
- Note: Values of variables are the number of participants in the relevant group or group M ± SD. Craving for methamphetamine was measured on a visual analogue scale from 0 (none) to 100 (extreme). The Beck Depression Inventory (BDI) distinguishes no or minimal (< 10), mild to moderate (10–18), moderate to severe (19–29) and severe depressive symptoms (30–63).61 U (Mann–Whitney U test), Χ2 (Pearsons chi-square test).
- a Significant group difference at p < 0.05, significant (+) or nonsignificant (−) at p < 0.05 in Bonferroni-adjusted post hoc analyses.
- b Nonsignificantly different at p < 0.05 across scanners in Kruskal–Wallis tests (specific p-values given in text).
- c Significantly different at p < 0.05 across scanners in Kruskal–Wallis tests (specific p-values given in text).
- d Nonsignificantly different across scanners in Fishers exact test (Χ2 (8) = 3.526, p = 0.981).
The sample consisted of 89 adults with Methamphetamine Use Disorder and 89 healthy controls who were matched 1:1 on sex and cigarette smoking status. Healthy controls were also selected based on educational attainment, but participants with Methamphetamine Use Disorder had fewer years of formal education and were more often unemployed than controls (p-values < 0.05). Hispanics were overrepresented, whereas African Americans and Caucasians were underrepresented in the Methamphetamine Use Disorder group (p-values < 0.05 in Bonferroni-adjusted pairwise comparisons). The groups were comparable in age and whether they consumed alcohol in the past 30 days (p-values ≥ 0.400). Cannabis used in the past 30 days was numerically more frequent in participants with Methamphetamine Use Disorder (p = 0.061), who reported more depressive symptoms than controls (p < 0.05). Other variables related to early abstinence and methamphetamine use are listed in Table 1.
The subsample consisted of 16 adults with Methamphetamine Use Disorder and 16 healthy controls who were matched 1:1 on image acquisition (per group: 4 scanned on 1.5-T Sonata, 6 on 3-T Trio, and 6 on 3-T Prisma), sex (4 women per group) and any tobacco or cannabis use in the past 30 days (13 and 4, respectively, per group). Years of education (Mann–Whitney U = 104.5, z = −1.022, p = 0.381) and the Beck Depression Inventory score (Mann–Whitney U = 149.5, z = 0.843, p = 0.423) were comparable across case (years: 12.2 ± 1.2, score: 2.8 ± 3.6) and control groups of the subsample (years: 12.4 ± 1.2, score: 2.4 ± 3.6).
3.2 Cortical thickness and participant characteristics
The vertex-wise analysis of thickness across the entire cerebral cortex revealed group differences for 17 clusters, all of which displayed less thickness in individuals with Methamphetamine Use Disorder relative to controls (p-values < 0.001, corrected for multiple comparisons; Table 2, Figure 1). These deficits were not attributable to group differences in image acquisition or participant characteristics as indicated by no main effects of scanning (p-values of dummy variables ≥ 0.275), ethnicity (p-values of dummy variables ≥ 0.192), education (p = 0.704), employment (p = 0.997), cannabis use (p = 0.553) or depressive symptoms (p = 0.248) on the weighted average thickness across the 17 clusters. Group (β = −0.548, p < 0.001) and age (β = −0.281, p < 0.001) showed main effects in this regression (R2 = 0.41, F (12, 148) = 8.48, p < 0.001), with the Methamphetamine Use Disorder group and older age being associated with reduced cortical thickness. Age and group status were not significantly correlated (rS = 0.063, p = 0.401).
| Size (mm2) | MNI305 (X, Y, Z) | HC (M ± SE) | MUD (M ± SE) | -lg(p) | Cohen's d | |
|---|---|---|---|---|---|---|
| Left hemisphere | ||||||
| Frontal lobe | ||||||
| Orbital gyri | 417 | −42.6, 37.3, −13.9 | 2.77 ± .015 | 2.64 ± .014 | 6.3568 | 0.79 |
| 140 | −16.9, 33.9, −21.7 | 2.55 ± .022 | 2.42 ± .019 | 4.8242 | 0.67 | |
| Frontomarginal g., s. | 805 | −27.0, 57.3, −8.9 | 2.71 ± .019 | 2.57 ± .016 | 6.7319 | 0.82 |
| Temporal lobe | ||||||
| Fusiform g. | 232 | −38.5, −62.5, −17.4 | 2.73 ± .019 | 2.61 ± .017 | 4.4877 | 0.64 |
| Insula | ||||||
| Inferior circular s. | 234 | −46.5, −5.9, −16.3 | 3.09 ± .021 | 2.93 ± .019 | 5.8533 | 0.75 |
| Subcentral G. and Sulci | 148 | −44.0, −20.4, 16.6 | 2.83 ± .023 | 2.69 ± .022 | 4.0309 | 0.60 |
| Right hemisphere | ||||||
| Frontal lobe | ||||||
| Orbital gyri | 454 | 15.4, 16.6, −22.5 | 2.79 ± .021 | 2.63 ± .016 | 7.1616 | 0.85 |
| 229 | 39.6, 25.4, −11.7 | 2.98 ± .021 | 2.83 ± .021 | 6.2791 | 0.78 | |
| Superior frontal g. | 397 | 9.3, 61.2, 6.7 | 2.70 ± .018 | 2.57 ± .015 | 4.2660 | 0.62 |
| Frontomarginal g., s. | 423 | 36.0, 55.1, −7.2 | 2.59 ± .018 | 2.48 ± .014 | 4.4753 | 0.64 |
| Limbic lobe | ||||||
| Anterior cingulate g., s. | 173 | 15.1, 40.4, 13.9 | 2.71 ± .015 | 2.59 ± .017 | 6.2876 | 0.78 |
| Middle-posterior cingulate g., s. | 258 | 5.3, −22.1, 39.6 | 2.85 ± .016 | 2.72 ± .014 | 7.0042 | 0.84 |
| Temporal lobe | ||||||
| Lateral-superior temporal g. | 693 | 63.0, −13.2, −2.0 | 2.91 ± .016 | 2.76 ± .016 | 6.6551 | 0.81 |
| Middle temporal g. | 145 | 64.2, −24.6, −12.7 | 3.07 ± .019 | 2.95 ± .017 | 5.6962 | 0.74 |
| Insula | ||||||
| Inferior circular s. | 147 | 46.0, −15.1, −8.7 | 2.75 ± .020 | 2.61 ± .019 | 4.3889 | 0.63 |
| Parietal lobe | ||||||
| Precuneus | 146 | 5.0, −61.6, 28.2 | 2.60 ± .016 | 2.51 ± .014 | 4.6405 | 0.65 |
| Occipital lobe | ||||||
| Occipital pole | 209 | 9.2, −90.7, 14.0 | 1.93 ± .017 | 1.83 ± .016 | 4.3997 | 0.63 |
- Note: The vertex-wise analysis of cortical thickness (listed in mm) over the whole cerebrum was controlled for age.40 Vertices were considered significant at p < 0.001, with a cluster correction set at p < 0.05 to account for multiple comparisons.28 Cohen's d was calculated as a measure of effect size (0.2 = small, 0.5 = medium, 0.8 = large).45
- Abbreviations: g, gyrus; HC, Healthy Control; MNI305, Montreal Neurological Institute coordinates; MUD, Methamphetamine Use Disorder; s, sulcus.
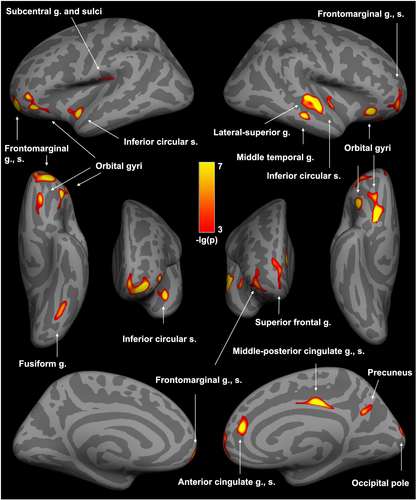
The main effect of group on the weighted average thickness across clusters (β = −0.543, p = 0.005) remained significant in the subsample analysis (R2 = 0.60, F (8, 23) = 4.27, p = 0.003), controlling for age (β = −0.296, p = 0.053), ethnicity (p-values of dummy variables ≥ 0.227), education (p = 0.236), employment (p = 0.959) and depressive symptoms (p = 0.438).
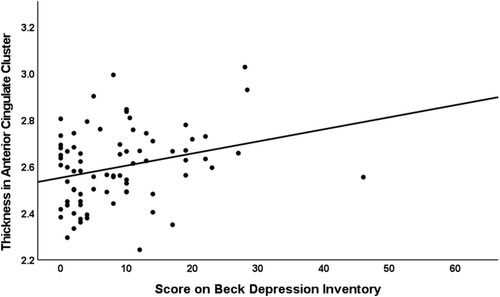
Depressive symptoms (β = 0.239, p = 0.030) and age (β = −0.270, p = 0.014) exerted main effects on the weighted average thickness across clusters in the Methamphetamine Use Disorder group (R2 = 0.15, F (2, 75) = 6.54, p = 0.002). Age and depressive symptoms were not significantly correlated in these participants (rS = −0.056, p = 0.625). We conducted a regression analysis on the cluster in the anterior cingulate because positron emission tomography and functional magnetic resonance imaging studies linked this region to depressive symptoms in Methamphetamine Use Disorder.46, 47 Depressive symptoms in early methamphetamine abstinence (β = 0.246, p = 0.027) were also related to cortical thickness in the anterior cingulate cluster (R2 = 0.12, F (2, 75) = 5.26, p = 0.007; Figure 2).
Further regressions on data from the Methamphetamine Use Disorder group revealed no main effects on the weighted average thickness across clusters of sex (β = −0.004, p = 0.966), years of methamphetamine use (β = −0.072, p = 0.500), days used in the past 30 (β = 0.068, p = 0.508), days abstinent (β = −0.116, p = 0.333) or craving (β = 0.007, p = 0.947). These variables were in part unevenly distributed across scanners: sex (Pearson's Χ2 (2) = 4.756, p = 0.093), years of methamphetamine use (Kruskal–Wallis H (2) = 1.303, p = 0.521), days used in the past 30 (Kruskal–Wallis H (2) = 23.855, p < 0.001), days abstinent (Kruskal–Wallis H (2) = 22.099, p < 0.001), craving (Kruskal–Wallis H (2) = 4.869, p = 0.088). Yet, separate regressions for each scanner subgroup did not reveal main effects on the weighted average thickness across clusters of sex (p-values ≥ 0.236), years of methamphetamine use (p-values ≥ 0.242), days used in the past 30 (p-values ≥ 0.210), days abstinent (p-values ≥ 0.190) or craving (p-values ≥ 0.332).
4 DISCUSSION
Reports on brain structure in early methamphetamine abstinence20-23 are inconsistent, probably due to small sample sizes, different volumetric measurements and insufficiently controlled confounders. We thus conducted the largest analysis to date of brain structure and the first of cortical thickness across the entire cerebrum in early-abstinent adults with Methamphetamine Use Disorder. Relative to healthy controls, who were comparable in age, sex, and recent use of alcohol and tobacco, they exhibited clusters of thinner cortex in bilateral frontal, parietal, temporal, insular and right cingulate regions.
Thickness across clusters and thickness of the anterior cingulate cluster were positively correlated with self-report of depressive symptoms in early abstinence, suggesting that grey matter loss accompanies processes leading to cortical thickening and depressive symptoms. In this regard, methamphetamine causes oxidative stress, excitotoxicity, inflammation, reactive gliosis and neuroadaptations.48-50 It is conceivable that neuronal loss translates into cortical thinning, whereas glial proliferation following neuronal damage leads to cortical enlargement.48 The proliferation of glial cells has a higher metabolic demand than that of neurons, and cortical enlargement may be related to a higher metabolic rate for glucose.48 Grey matter volumetric deficits were congruent with metabolic deficits in the right anterior cingulate in overlapping samples of individuals in early abstinence from chronic methamphetamine use,21, 47 and depressive symptoms covaried positively with metabolic activity in this region.47 These reports and our data suggest that depressive symptomatology in early abstinence is a behavioural expression of cortical enlargement in regions, such as the right anterior cingulate, where a relative grey matter deficit is apparent in people with Methamphetamine Use Disorder.
Major Depressive Disorder features grey matter deficits in the anterior cingulate cortex,51 where enlargements in early stages have also been associated with increased metabolism, inflammation and neurocompensatory processes.52, 53 Greater pretreatment anterior cingulate volume and increase in thickness early in treatment of Major Depressive Disorder predict response to antidepressants.18, 19 Leveraging the biomarker potential of the anterior cingulate may improve machine learning algorithms that use pretreatment whole-brain patterns and reach accuracies of 64%–89% for antidepressant response prediction in these patients.18 Antidepressants can reduce depressive symptoms and relapse in methamphetamine abstinence as shown for bupropion and mirtazapine in some individuals.54-56 Future studies may examine whether anterior cingulate thickness in those who respond to these medications differs from that in nonresponders. Results from such analysis should be interpreted with the understanding that a single medication will likely not be effective for the entire heterogeneous population of people with Methamphetamine Use Disorder.56
The multiple areas of thickness deficits across the cerebral cortex echo the range of cognitive deficits associated with methamphetamine use, including problems with language, attention, memory, psychomotor function, cognitive control and decision-making.13-16 Some impairments have been linked to structural and functional brain abnormalities in early methamphetamine abstinence.13, 14, 16 The most consistent correlations are reported for poor cognitive control and decision-making with attenuated activity in prefrontal and anterior cingulate cortices as measured by positron emission tomography and magnetic resonance imaging.16 Both regions exhibit structural deficits in the present work, suggesting that they may underlie these functional abnormalities. The prefrontal and anterior cingulate cortices are therefore promising therapeutic targets, particularly as poor self-control and decision-making have been related to treatment failures.14 Transcranial magnetic and transcranial direct current stimulations of the dorsolateral prefrontal cortex have produced positive effects on executive functions and withdrawal symptoms in people with Methamphetamine Use Disorder.8 The stimulation of the prefrontal cortex can modulate activity in the anterior cingulate, which may partly explain the beneficial effects.57 Technological advances allow for noninvasive direct stimulation of the anterior cingulate, promising more precise therapies of cognitive and affective dysfunctions than is possible by stimulating superficial areas.57
4.1 Limitations
The cross-sectional design of this study precludes causal claims regarding the effects of methamphetamine, abstinence from the drug and depressive symptoms on brain structure. Cortical thickness was not significantly associated with duration of use, recent use or days of abstinence but was inversely correlated with cumulative methamphetamine use in other studies,58, 59 which suggests a threshold effect for structural change. Longitudinal studies over longer periods of methamphetamine abstinence than those studied here suggest normalising effects of abstinence on cortical deficits,20, 23-25 with at least partial recovery following gray matter loss. Deficits may also predate Methamphetamine Use Disorder as less orbitofrontal cortical thickness in adolescents using methamphetamine was associated with a family history of drug use.58 They might also be influenced by differences in alcohol, tobacco and cannabis use20, 60 as quantity of use for these substances was not controlled although the groups did not differ in the percentages of participants who used them. Finally, we conducted a vertex-wise analysis over the whole cerebral cortex to identify methamphetamine-related alterations that might be accessible to available interventions (e.g., transcranial brain stimulation) and thus did not include subcortical structures.
4.2 Conclusion
Early abstinence from chronic methamphetamine use features widespread cortical thickness deficits. The severity of depressive symptoms is associated with these deficits, specifically within the right anterior cingulate cortex. Just as anterior cingulate pathology in Major Depressive Disorder predicts response to antidepressants, cingulate structure may also identify patients with Methamphetamine Use Disorder who can benefit from antidepressant medication. Transcranial stimulation of the right anterior cingulate offers another exciting avenue to reduce depressive symptoms and relapse to methamphetamine use. Future research may link the identified thickness deficits to specific cognitive impairments and thereby reveal more targets for improving brain function and therapeutic success.
ACKNOWLEDGEMENTS
This research was supported by grants from the National Institute on Drug Abuse (R01 DA027633) and the National Institutes of Health (K23 DA927734, R21 DA034928 [ACD]; R01 DA015179, R01 DA020726, P20 DA022539 [EDL]). Additional funding was provided by UCLA Clinical and Translational Science Institute (UL1TR000124), the Thomas P. and Katherine K. Pike Chair of Addiction Studies (EDL), the Marjorie M. Greene Trust (EDL) and a postdoctoral research grant from the Max Kade Foundation (JP). The funders were not involved in the conceptualisation of the study; collection, analysis and interpretation of data; preparation of the manuscript or decision to submit it for publication.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
EDL, JP, ACD, J-BP and DGG designed the study. ACD, DGG and J-BP contributed to data collection. JP and J-BP processed and analysed the data. EDL and ACD supervised the analyses. JP drafted the manuscript and finalised edits of the manuscript. EDL, ACD, RDLG, DGG and J-BP revised the manuscript. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




