Auricular transcutaneous vagus nerve stimulation for alcohol use disorder: A chance to improve treatment?
Anyla Konjusha and Lorenza Colzato contributed equally.
Abstract
Alcohol use disorder (AUD) is a relapsing–remitting condition characterized by excessive and/or continued alcohol consumption despite harmful consequences. New adjuvant tools, such as noninvasive brain stimulation techniques, might be helpful additions to conventional treatment approaches or even provide an alternative option for patients who fail to respond adequately to other treatment options. Here, we discuss the potential use of auricular transcutaneous vagus nerve stimulation (atVNS) as an ADD-ON intervention in AUD. Compared with other techniques, atVNS has the advantage of directly stimulating nuclei that synthesize GABA and catecholamines, both of which are functionally altered by alcohol intake in AUD patients. Pharmacological options targeting those neurotransmitters are widely available, but have relatively limited beneficial effects on cognition, even though restoring normal cognitive functioning, especially cognitive control, is key to maintaining abstinence. Against this background, atVNS could be a particularly useful add-on because there is substantial meta-analytic evidence based on studies in healthy individuals that atVNS can enhance cognitive control processes that are crucial to regaining control over drug intake. We discuss essential future research on using atVNS as an ADD-ON intervention in AUD to enhance clinical and cognitive outcomes by providing a translational application. Given that this novel technique can be worn like an earpiece and can be employed without medical supervision/outside the clinical settings, atVNS could be well integratable into the daily life of the patients, where the task of regaining control over drug intake is most challenging.
1 INTRODUCTION
Alcohol use disorder (AUD) is an often chronic relapsing–remitting condition characterized by excessive and/or continued alcohol consumption despite harmful consequences.1 The common symptoms include urges or cravings to drink, consuming alcohol in greater quantities, inability to control/reduce alcohol use, tolerance development and/or experiencing withdrawal symptoms when alcohol use is stopped,2 and health, economic, or social problems due to excessive drinking.2 According to the World Health Organization, European countries have the highest alcohol consumption per capita and consequently the highest proportion of compromised health and premature death mainly caused by alcohol.
To treat AUD, new adjuvant tools, such as nonpharmacological, noninvasive brain stimulation techniques, might be beneficial additions to conventional treatment approaches or maybe even provide an alternative option for patients who fail to adequately respond to other treatment options.3 So far, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) have been the main focus of previous research on brain stimulation approaches in relation to AUD.3 Although further research is needed, several studies have found that rTMS and tDCS induce beneficial effects in AUD patients.4-6 For a more comprehensive review, please Ghin et al.3 rTMS and tDCS are used to target specific cortical regions, which immediately affect the chosen brain region and may thereby modulate complex neuronal networks. While both immediate and longer lasting effects of these approaches (especially rTMS) have been reported, they do not allow to specifically target neurotransmitter systems that are implicated in AUD, such as γ-aminobutyric acid (GABA) or noradrenaline (NA). Furthermore, rTMS (but not necessarily tDCS) usually needs to be applied by specialists/in a clinical setting. In contrast, auricular transcutaneous vagus nerve stimulation (atVNS) is not applied to predefined brain regions but to specific neurotransmitter systems. It is thought to stimulate the nuclei synthetizing GABA and catecholamines, which are some of the primary neurotransmitters involved in AUD. Notably, atVNS can be worn like an earpiece in everyday life, making this novel technique a possibly ideal translational application to magnify clinical outcomes in AUD on demand (i.e., specifically in situations when such support is most needed). Due to those fundamental differences in underlying mechanisms and everyday applicability, atVNS could provide alternative and/or additional options to previously discussed brain stimulation approaches in AUD. Against this background, our article aims to discuss the potential benefits and applications of tVNS in AUD. In the following, we will discuss the neurobiology of AUD, followed by the neurobiological effects of atVNS. Second, we will outline the effects of AUD on cognitive control. Lastly, we will consider future implications and insights, including atVNS as a possible ADD-ON intervention in AUD and how atVNS might affect clinical outcomes in AUD.
2 THE NEUROBIOLOGY OF AUD IN RELATION TO THE NEUROBIOLOGICAL ATVNS EFFECT PROFILE
Alcohol targets various neurotransmitter systems and many different brain structures.3 Given that the focus of this article will be on atVNS as a potentially new ADD-ON intervention in AUD, we decided only to describe aspects modulated by both acute or repeated alcohol exposure and by atVNS in this section. Most importantly, alterations in GABA and catecholamines strongly contribute to cognitive control impairments, and behavioral deficits reported in AUD and are thus of utmost importance to regain control over drug intake.7 As those transmitter systems may be modulated using atVNS, it is conceivable to assume that this stimulation technique could help alleviate the cognitive symptoms of AUD.
2.1 GABA and glutamate
Alcohol affects the neural excitability equilibrium by interfering with GABA, the primary inhibitory neurotransmitter, and by interfering with glutamate, the primary excitatory neurotransmitter of the brain.8 GABA and glutamate play an essential role in the central nervous system, with the former being involved in learning and control processes, and the latter modulates synaptic strength in learning and memory functions.9, 10 Furthermore, the glutamatergic and GABAergic systems are considered to be modulators of response inhibition, response selection and conflict monitoring.11, 12 In line with this, GABA levels, as measured by magnetic resonance spectroscopy, have been found to modulate the efficacy of response inhibition processes and action control.11-13 Glutamate and GABA are modulated by both acute and chronic alcohol use.14 Acute alcohol intoxication hampers glutamatergic transmission by reducing the efficiency of N-methyl-D-aspartate (NMDA)15 and increasing the release of GABA.16 This leads to an overall reduction in neuronal excitability and prevalent intoxication symptoms, like higher sedation, lower anxiety, and impairments of memory, learning17 and cognitive control processes.18, 19 Other work has shown that acute alcohol exposure potentiates GABAA and reduces excitatory neurotransmission.20, 21 Along the same lines, human studies have shown that acute alcohol exposure reduces GABA levels.22 In individuals with prolonged/chronic alcohol use, the homeostatic counter-regulation underlying tolerance development results in the functional upregulation of the glutamatergic NMDA receptor23, 24 and the functional downregulation of GABAA receptors.20, 23 This ultimately causes neuronal hyper-excitability, which drives withdrawal- and craving-associated clinical symptoms such as anxiety, irritation, and increased risk of seizures.25 Furthermore, in response to widespread GABAergic overstimulation, chronic alcohol use leads to GABAA receptor downregulation.26 Animal studies have supported this, which have indicated long-lasting alterations in the down-regulation of GABA-related central inhibition.27 In sum, GABAA receptors are implicated in both the short and long term effects of alcohol in the central nervous system.28 Considering this, chronic alcohol use might impact the GABAA receptor functions, subcellular localization and expression- all factors that contribute to alcohol dependence and withdrawal.29
2.2 Catecholamines
Several lines of evidence suggest that alterations in the catecholaminergic (i.e., in the dopaminergic and the noradrenergic) system occur in AUD patients.30, 31 NA plays a crucial role in facilitating response selection processes and task-related decisions.32-36 While not all evidence seems to point in the same direction, it can be assumed that NA levels are increased during acute intoxication and withdrawal but decreased after chronic/long-term alcohol use (for summary, please refer to detailed reviews37, 38). Interestingly, the level of activation of the locus coeruleus (LC; noradrenergic center of the brain) has been shown to predict the tendency to drink alcohol in rats.39 Further, the local administration of ethanol (1–2 g/kg) directly into the LC induces suppression of its neuronal firing rate.40 NA levels have been reported to be elevated in mice41 and the cerebrospinal fluid42-44 and the plasma37, 45 of alcohol-dependent patients. In contrast, Berggren and colleagues46 showed a long-lasting (up to 6 months) reduced α2 adrenoceptor function in abstinent alcohol-dependent individuals. In line with these findings, chronic alcohol use also decreases NA concentrations in the brain.47, 48 In sum, the assumption of AUD-associated changes in NA is supported by studies investigating alcohol exposure, alcohol withdrawal and chronic use. Specifically, as hypothesized by Fitzgerald,37 enhanced noradrenergic signalling is involved in the aetiology of alcohol abuse, while long-term use also impairs this neurotransmitter system by inhibiting it. Whereas (chronic) alcohol use suppresses noradrenergic signalling, a tolerance-associated rebound of the noradrenergic system occurs during acute withdrawal, resulting in enhanced NA release.37
Dopamine (DA) is considered important in alcoholism because the midbrain dopaminergic pathway, which projects to the prefrontal cortex (PFC) and nucleus accumbens (NAc),30 is involved in reward, associative learning and the salience of incentives.49 Like other drugs acting on the reward system, alcohol intoxication elicits DA release from the VTA to subcortical regions such as NAc and enhances reward-seeking behaviour. Consequently, alcohol intoxication enhances the release of DA in the PFC, known to be implicated in addiction-associated reward processing, decision-making and cognitive control.50
Acute alcohol use (but not chronic) and withdrawal have been found to decrease NA levels in rats' brains.48 Another study, including healthy participants, found that acute alcohol intake caused plasma NA to increase 30 min after consumption lasting approximately 4 h.51 Acute alcohol significantly affects catecholamine dynamics, implying that continuous alcohol exposure might cause profound modulations in both DA and NA brain systems.52 Animal experiments have shown that long-term alcohol intake alters dorsal striatal dopamine release and uptake and the regulation of DA release by D2/3 dopamine autoreceptors in male but not female rhesus macaques.53 Another study assessing DA levels in rats fed an alcohol diet for a year found decreased DA levels in both dorsal striatum and ventral striatum.54 These findings suggest that continuous alcohol exposure eventually leads to long term neuroplasticity changes, such as over-compensatory regulation of normal neurotransmitter activity and neurotoxicity. In sum, consistent with the idea that DA has a critical role in driving cognitive control by regulating the balance between PFC and basal ganglia,55, 56 chronic alcohol intake is suspected of causing deficits in DA signaling30 as well as in behavioral flexibility,57 various facets of cognitive control, learning and motor processes.30
2.3 Neurobiological effects of (at)VNS
Auricular tVNS stimulates the vagus nerve via a special earplug electrode applied to the outer ear pinna, where the innervation of the auricular branch of the vagus nerve is.58-60 This is particularly relevant considering that chronic alcohol consumption has significantly reduced the density of myelinated fibres in the vagal nerve.61 AtVNS stimulates the afferent (i.e., the thick-myelinated Aβ) fibres. From there, the afferent signal propagates to nuclei in the brainstem, such as the nucleus of the solitary tract (NTS) and the LC, and, ultimately, to higher structures such as the hippocampus, the insula, the motor cortex, and the PFC58 (see Figure 1). This is especially important when considering the aforementioned AUD-associated functional changes.
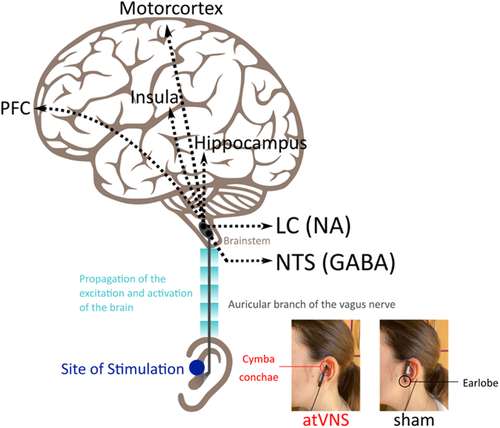
Even though many neurotransmitter systems have been implied in (at)VNS effects,59 various functional magnetic resonance imaging (fMRI) studies demonstrated that atVNS activates afferent fibres and causes increased activation in brainstem regions, including the NTS and the LC.62-68 These nuclei are the brain's GABAergic and noradrenergic “centres,” as they are the origins of the main GABAergic and noradrenergic neurotransmitter pathways.69, 70 Regarding indirect markers, the short-interval intracortical inhibition, a TMS paradigm indicative of GABAA activity, has been significantly enhanced in the right motor cortex following atVNS.71 Both animal and human studies have linked the stimulation of vagal afferent fibres to GABA, mainly due to the activation of NTS. The concentration of GABA in the cerebrospinal fluid of epileptic patients (epileptic seizures thought to be caused due to GABA imbalance) and depressed patients after iVNS was elevated.72, 73 In healthy participants, short term (60 min) atVNS altered cortical excitability74 and automatic motor inhibition,75 which are commonly linked with GABA activity.12 Regarding the long-term effects, one study showed that GABAA receptor density is increased in epileptic patients receiving continuous invasive VNS for a year.76 Nevertheless, more longitudinal research is needed to understand the long term effects of VNS on GABA and other neurotransmitters' activity. Further studies have found that atVNS can cause pupil dilation (depending on the stimulation protocol used),77-79 which is known to mirror the activity of the LC-NA system.80, 81 Animal studies have also found that VNS increases NA levels in the hippocampus and cortex.82 Long-term effects of invasive VNS are mainly investigated in clinical studies, where the stimulation duration has been applied in 3 months,83 6 months,73 and 12 months,84 with the majority of them reporting improvement in the patients' symptoms. This suggests that continuous stimulation of the vagal afferent fibres might be beneficial for treating many conditions. Further, only two imaging studies showed that atVNS activates the NAc, besides the activation of the LC.64, 85 The NAc is part of the mesolimbic reward system and is innervated by DA.86 To date, no direct or indirect DA markers have been investigated following atVNS. Nevertheless, research has indicated that atVNS might increase reward-seeking behaviour,87 which implicates possible dopamine activity. Vagal afferent activation is thought to regulate critical brain circuits indirectly involved in reward. After concurrent atVNS, neuroimaging studies utilizing fMRI revealed increased activity in the NTS and other motivation-related areas such as the dopaminergic midbrain and striatum.64, 88As a result, dopamine might be a possible target of atVNS, although additional research is needed to make more conclusive remarks. Taken together, these findings evidence that atVNS modulates GABA and catecholamines levels, and thus important neurotransmitters involved in AUD. The NTS has prominent projections to the LC, which is why NTS-LC structures are likely primarily targeted by vagus nerve stimulation.89 Based on this specificity in targeted structures, it has been argued that atVNS is more precise and, therefore, may have a better potential as a therapeutical means to target the NA system than pharmacological interventions trying to modulate the NA (catecholaminergic) system.60 In the same manner, atVNS also allows to specifically target the GABAergic system. This is of particular relevance in the context of AUD for two main reasons:
First, the GABAergic and the catecholaminergic systems are already targeted in current pharmacological treatment approaches for AUD. Second and crucially, GABAergic and catecholaminergic signalling are essential for cognitive control processes, known to be predictive for the clinical outcome in AUD treatments90 and relevant to regaining control over drug intake.7 There is substantial meta-analytic evidence based on studies in healthy individuals that atVNS can enhance cognitive control processes91 and thus essential processes relevant to regaining control over drug intake. Below, we briefly delineate alterations in cognitive control processes in AUD and current pharmacological treatments. Based thereon, we provide a possible roadmap for research necessary to establish atVNS as a possible ADD-ON in treatments of AUD.
3 COGNITIVE CONTROL PROCESSES IN AUD
Cognitive control refers to (mainly) top-down processes necessary for goal-directed thought and action and mental and behavioural flexibility.92 There are related but dissociable domains of cognitive control. These encompass inhibition, which is the ability to suppress unwanted or inappropriate thoughts and responses; working memory (WM), which is involved in immediate conscious perceptual processing of current information, including monitoring and updating; and mental flexibility, which refers to the capability of repressing old or irrelevant tasks and instead rely on new or more appropriate ones.92 A more detailed discussion on cognitive control deficits, and related neurobiological correlates, can be found elsewhere.3, 7
Deficits in top-down cognitive functions characterize AUD, frequently encompassing the domains of memory and executive functions.93 For instance, AUD patients showed poor performance in neuropsychological tests assessing WM.94 Similarly, compared with control subjects, AUD patients scored lower in neuropsychological assessments of semantic cued recall, free recall, and recognition memory.95 Along the same lines, mental shifting and flexibility are affected by AUD, with patients performing worse than the control group.96 Inhibition is considered one of the most affected domains of alcohol use disorder.3 It can further be subdivided into behavioral inhibition, which refers to the ability to withhold automatic response tendencies, habits and interference control which indicates the ability to suppress interfering information or inappropriate thoughts/actions. Indeed, the ability to inhibit an action/habit is key to controlling alcohol intake, especially after it has become habitual.3 Therefore, impairments in inhibition processes may, in turn, foster alcohol abuse.3 Evidence indicates that both acute and chronic alcohol consumption have detrimental effects on response inhibition.97, 98 Impaired performance in tasks assessing response inhibition has been shown in AUD patients compared with healthy control groups.90, 99 Additionally, AUD patients demonstrated higher errors of commission than the control group in continuous performance tasks (measuring response inhibition), further evidencing impaired inhibitory control and increased impulsivity.100 Notably, a study training response inhibition showed that repeatedly stopping prepotent responses toward alcohol-related stimuli successfully reduces excessive alcohol use,101 suggesting a bidirectional link between response inhibition and alcohol intake. Regarding interference control, impairments have been demonstrated both as a consequence of acute alcohol intoxication102, 103 and in AUD patients.104 Indeed, acute alcohol intoxication has been linked to deficits in conflict monitoring and error detection.105 In line with this finding, intoxicated participants showed an increased Stroop interference effect than sober controls, which indicates difficulties in resolving the conflict between (incorrect) prepotent response tendencies and incompatible alternative (correct) responses.106 However, it should be noted that some studies failed to find any differences in terms of interference control in intoxicated (compared with sober) subjects.107, 108
In sum, AUD is broadly compromising the different facets of cognitive control. Most importantly, cognitive control inhibits or modifies habitual and automatic responses associated with drinking behavior.3 There is typically a shift from controlled to habitual alcohol use in individuals who develop AUD, while the cognitive capabilities required to keep the increasingly habitual use in check gradually weaken. Together, these two detrimental developments substantially reduce the chances of reaching and maintaining abstinence.109 Therefore, the proper functioning of cognitive control processes is indisputably key to the successful treatment of AUD. Given that GABA and catecholamines modulate different facets of cognitive control,32, 110, 111 disturbances in these neurotransmitters systems caused by AUD likely strongly contribute to cognitive control impairments described above. Hence, the above paragraphs suggest that changes in cognitive control characterize AUD and that the catecholaminergic and the GABAergic systems play a role. Modulating catecholamines and GABA levels via atVNS may prove useful for regaining cognitive control in AUD, given that atVNS has been quantitatively found to exert beneficial effects on cognitive control.91
4 CURRENT PHARMACOLOGICAL TREATMENTS IN AUD
Pharmacological treatments primarily aim to reduce cravings and withdrawal symptoms. Although some medications have proven helpful in this respect, they are not directly targeted at the neurobiological mechanisms underlying the imbalance between automatic and controlled behaviour that can be considered a characteristic causal feature of AUD.112 Acamprosate is an anticraving medication113 thought to normalize the tolerance-induced shifts in glutamatergic and GABAergic activity, eventually reducing neuronal excitability and potentially helping to support sobriety in abstinent AUD patients.114 So far, acamprosate has shown no conclusive effects on cognitive control.115 Benzodiazepines which are selective GABAA agonists are also used in treating AUD113 to reduce alcohol-associated withdrawal symptoms, such as anxiety and hyperactivity. The effects of benzodiazepines on cognitive control functions remain inconclusive.116 Regarding noradrenergic targets, as nicely reviewed by Haass-Koffler et al.,31 prazosin and doxazosin (drugs that that block post-synaptic alpha-1 receptors), propranolol (a drug that blocks post-synaptic beta receptors), as well as clonidine and guanfacine (drugs that act on presynaptic alpha-2 auto-receptors) have been demonstrated to have a beneficial effects in reducing alcohol consumption. However, there is, unfortunately, only one ongoing clinical trial with guanfacine (NCT03137082), which is specifically investigating the effect of this drug on cognitive control in women with AUD.
While medical treatment is regarded as a valuable tool in improving clinical outcomes (relapse rate, abstinence, harm reduction, cognitive impairments) for some substance disorders, effect sizes are still questionable for AUD treatment.117, 118 To cope with this issue, new adjuvant tools that can be combined with current intervention strategies to treat AUD might be helpful additions to conventional treatment approaches or maybe even provide an alternative option for patients who fail to adequately respond to other treatment options. Further, there is currently no pharmaceutical approach that has noteworthy, let alone satisfactory, effects on restoring the balance between cognitive control and automatic behaviour3—considering the complexity of AUD, many different aspects that contribute to the drinking patterns of the individual need to be taken into account. The establishment of a treatment plan tackling the difficulties of each individual patient by combining different treatment strategies might be most efficacious. Accordingly, recent developments in AUD research have shifted their focus towards brain stimulation techniques.3, 113 In the next section, we will outline how atVNS may serve as an ADD-ON to existing pharmacotherapeutic approaches in AUD and detail what steps are essential to take in future research.
5 ATVNS AS A POSSIBLE ADD-ON TREATMENT IN AUD?
AUD is a complex condition, so optimal (clinical) treatment outcomes are probably most likely when different types of therapy are combined. A combination of new adjuvant tools such as atVNS with current pharmacotherapy might be useful for those who fail to sufficiently respond to pharmacotherapy alone.3 Below we summarize the most important literature about vagus nerve stimulation (VNS), addiction and cognitive control, outline suggestions for future research and the efficiency and practicality of atVNS.
5.1 (at)VNS, addiction, and cognitive control
Several animal studies have been conducted to examine the effects of VNS on addiction. First, a sham-controlled study aimed to evaluate the effect of VNS on heroin seeking behaviour found a decline in relapse rates during daily extinction sessions in rats.119 This suggests that VNS may suppress heroin- or heroin cue-induced relapse, likely via an NA-induced activation from the LC to the NAc via the hippocampus.120 Along the same lines, another animal study showed that cocaine-addicted lab rats reduced their drug-seeking behaviour after VNS intervention compared with sham stimulation.121, 122 This suggests that VNS may facilitate the extinction of strong drug cue/reward associations across many substances, including heroin119 and cocaine.121, 122 Besides animal studies, one study in humans using atVNS (the NSS-2 Bridge device) demonstrated a reduction of symptoms associated with opioid withdrawal, including heroin and methadone, as indexed by the clinical opioid withdrawal scale.123 Along the same lines, the administration of the NSS-2 Bridge device for 30 min revealed, in at least 30% of the cases, the mitigation of autonomic symptoms linked to opioid withdrawal.124 This suggests that this kind of stimulation might effectively alleviate withdrawal opioid symptoms.125 Specifically, regarding alcohol, one atVNS study in AUD patients showed reduced depression symptoms and improved sleep quality after withdrawal in the treatment group (receiving active stimulation) compared with the control group (receiving no stimulation).126 Further, recent work by Grünberger and colleagues127 found that after receiving atVNS, AUD patients displayed a decline in alcohol craving and an increased parasympathetic activity as measured by pupil dilation, which is known to mirror the activity of the LC-NA system.80, 81 Even though no correlation between alcohol cravings and pupil diameter was reported and no control group was included, this study provides promising results regarding the potential use of atVNS in treating AUD patients. Further, literature reviews and meta-analyses proposed atVNS as a reliable tool to enhance cognitive control in healthy humans.58, 91 That is, atVNS, increasing NA and GABA levels promotes NA and GABA-related cognitive performance.58 Specifically, Ridgewell et al.91 showed that atVNS had significant beneficial effects on cognitive control, even though stimulation parameters may differentially affect the size of the obtained cognitive effects.
In sum, VNS shows promising results, especially in the extinction of strong drug cue/reward associations across a wide range of substances. These results are particularly relevant given that the formation of drug cue/reward associations tends to carry on long after drug-taking termination and exacerbate the long-term risk of relapse. Next, atVNS seems to be an effective tool in enhancing processes related to WM, response inhibition and conflict monitoring, all of which are relevant to regaining control over drug intake.
5.2 Future research: atVNS as an adjuvant treatment to pharmacotherapy
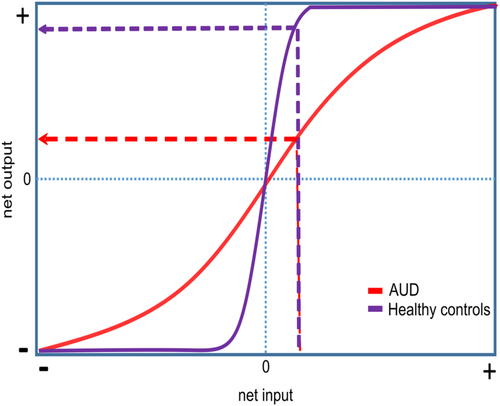
It has been suggested that atVNS causes a concomitant stimulation of the NA and GABAergic system.128 Notably, these two neurotransmitter systems interact with each other.129 The activation of alpha-1 receptors decreases GABAergic synaptic transmission, whereas activation of beta receptors enhances GABAergic synaptic transmission.129 Regarding cognitive processes, the stimulation of alpha-1 receptors and lower affinity beta-receptors has been shown to cause a detrimental effect on WM, while the activation of alpha-2 receptors seems to enhance it.130, 131 Keeping in mind that atVNS has been reported to only modulate inhibitory control when WM is involved, it is reasonable to assume that atVNS affects the NA system through alpha-2 receptors.128 Recently, we suggested that the concomitant effect of NA and GABA promotes cognition by enhancing the signal-to-noise ratio (SNR).58 The SNR is robustly modulated by the catecholaminergic system32, 132, 133 and GABA,134-136 and this modulation has been hypothesized to mirror neuronal gain control mechanisms.133, 137, 138 In a nutshell, augmenting gain control can be framed in terms of increasing the slope of the sigmoid input–output function so that the modulated neurons are getting better at distinguishing between relevant input and irrelevant neuronal noise.139 Hence, high gain control is associated with better or more stable cognitive performance across various cognitive domains.139-141
Notably, NA and GABA are known to decline as a consequence of long-term alcohol abuse,25, 37 and the SNR is modulated by the catecholaminergic system32, 132, 133 and GABA.134-136 As decreased levels of these neurotransmitters lower the SNR, there should be more noise and a consequently hampered distinction between relevant and irrelevant information.32, 132, 133 Accordingly, we hypothesize AUD (flatter slope of the sigmoidal function, red curve) to be associated with lower/less efficient gain control than healthy controls (purple curve); see Figure 2. On the behavioural level, this should manifest as impairments in cognitive control. In order to test this idea, future pharmacological studies should combine targeting of the different NA receptor subtypes with atVNS. If our hypothesis that atVNS modulates the NA system via alpha-2 receptors is correct,128 we expect the combination of atVNS and clonidine or the combination of atVNS and guanfacine (drugs that act on presynaptic alpha-2 auto-receptors and have been demonstrated to have beneficial effects in reducing alcohol consumption)31 to boost clinical outcomes in AUD. Given that currently used medications have been found to have limited beneficial effects on cognition in AUD,3 the combination of atVNS and pharmacotherapy could prove to be helpful—mainly because there is substantial meta-analytic evidence based on studies in healthy individuals that atVNS can enhance cognitive control processes91 which are crucial to regaining control over drug intake. In sum, we suggest that atVNS may work as potential ADD-ON therapy to pharmacotherapy to magnify both clinical and cognitive outcomes that allow regaining control in AUD.
5.3 Future research: Stimulation parameters of atVNS
Stimulation parameters of atVNS are crucial to attaining specific goals.58, 59, 142 Several parameters should be considered and optimized for effective use of atVNS in AUD. This concerns the stimulation protocol, including the ear site (tragus vs. cymba), the settings of the device (current intensity and pulse width), and the side of stimulation (left ear vs. right ear). However, an essential parameter seems to be the type of stimulation (event-related vs. intermittent following ON–OFF cycles vs. continuous). The most reliable effects on LC-NA system activity and indirect correlations have been found using phasic or event-related stimulation.77, 142 Recently, Villani et al.79 argued that some of the current atVNS protocols are not effective because they are not considering the two different activity modes of the LC, that is, phasic and tonic.32, 69 According to Aston-Jones and Cohen,32 intrinsic alertness is supported by tonic activity in the LC-NA system. While low tonic activity in the LC-NA system (i.e., low alertness) is associated with low susceptibility to incentives and leads to sedation, high tonic activity (i.e., high alertness) is associated with high sensitivity to incentives and leading to exploration. An average level of tonic activity (i.e., average alertness) leads to task engagement by supporting phasic activity in the LC-NA system (in response to task-relevant information), which enhances the SNR and related gain control. Hence, this model proposes that, following an inverted-U curve, the phasic activity is reduced if the tonic activity is either too low or too high in conditions of low or high intrinsic alertness. According to Villani et al.,79 it is reasonable to assume that when atVNS is administered intermittently (i.e., following ON–OFF cycles) tonic LC firing is likely to differ between stimulation conditions and this might, in turn, affects the phasic LC-NA activity.79 Along the same line, it is difficult to predict how a continuous stimulation with atVNS might alter tonic activity and, in turn, phasic firing.79 To address the issue of the best stimulation type to activate the LC-NA system, Villani et al.79 proposed a new stimulation approach called “event-related” atVNS, where short bouts of atVNS (i.e., a couple of seconds) are synchronized (i.e., temporally coordinated) with the presentation of stimuli. This approach has been demonstrated to be successful in modulating the tonic and phasic modes of the LC-NA system as well as cognitive processes. Accordingly, we suggest that this new protocol might be ideal when using atVNS in AUD. The fact that this protocol considers the distinction between phasic and tonic mode LC-NA activity is a big advantage compared with pharmacotherapy targeting the noradrenergic system which is assumed to modulate only the tonic mode. A possible translation to real-life situations could be to encourage AUD patients to switch on the atVNS whenever they encounter or anticipate any triggers related to alcohol (e.g., when passing a street with several bars). In so doing, they will receive a phasic stimulation that should help them to more successfully resist the temptation (by transiently boosting cognitive control abilities). Next to “event-related” atVNS, future research should lead towards closed-circuit atVNS as already explored by respiratory-gated atVNS143 and by close loop cervical tVNS (NCT02992899). That is, closed-circuit techniques can be based not only on stimuli (e.g., bars) but also on physiological arousal (e.g., increased respiratory rate). Indeed, as arousal may increase during craving in AUD, particularly in those with poor cognitive control and/or stress disorders (which is a large portion of those with substance use disorders more broadly), closed-circuit techniques can be very helpful in copying with autonomic symptoms during withdrawal.
5.4 atVNS efficiency and practicality
atVNS is a U.S. Food and Drug Administration (FDA) approved therapy currently used to treat conditions such as pharmacoresistant depression and epilepsy.142 In terms of practicality, atVNS is small and portable and does not require any surgical intervention or set up by health professionals.144 Moreover, most atVNS devices are battery-powered control units, making it a convenient and easy to use the tool. The device and its settings can be easily operated after minimal training, and its' earphone-like electrodes can be used in everyday life, such as going to the grocery store, watching TV, or working in front of a computer screen. The potential widespread accessibility and applicability of the atVNS technology further add to its' therapeutic advantages. Other noninvasive brain stimulation techniques such as rTMS (and, to a certain degree, tDCS) are confined to clinical settings where professionals can only be operated by professionals.145 Additionally, atVNS is a relatively inexpensive tool that can be easily purchased and customized in duration, current intensity and stimulation frequency.144 Notably, atVNS could also be combined with cognitive-behavioural treatments (CBT) for alcoholism. Once the most potent cues for drinking have been identified via CBT, atVNS can be implemented to strengthen strategies for reducing the impact of these drinking cues. AUD patients could switch on the atVNS whenever they face or foresee any triggers related to alcohol (e.g., when passing the shelves for alcoholic drinks at the grocery store). Combining CBT and atVNS could reduce craving and the risk of relapse, and its effectiveness could be evaluated by well-established craving and relapse measurements in alcoholism, such as the Obsessive–Compulsive Drinking Scale and the Lübeck Craving Scale.146 In sum, atVNS is a functional and easy to operate the device, making this novel tool an ideal translational brain stimulation application to magnify clinical outcomes in AUD.
6 CONCLUSION
A dysregulated function of the vagus nerve has been proposed to be causally involved in AUD. The present review introduces the potential use of atVNS as an ADD-ON intervention in AUD. In contrast to other noninvasive brain stimulation techniques, this novel method directly stimulates the nuclei synthetizing GABA and catecholamines, which are some of the primary neurotransmitters involved in AUD. In order to test the effectiveness of atVNS in AUD, we strongly recommend that future studies examine the combination of atVNS with pharmacological agents targeting the alpha-2 receptors. Further, we encourage using well-defined atVNS experiments using the new “event-related” atVNS stimulation protocol whenever possible. Given that atVNS can be worn like an earpiece, this novel technique might become an ideal translational application to magnify clinical and cognitive outcomes in AUD. Because atVNS can be applied without medical supervision and is not confined to clinical settings, this treatment is well practicable in the daily life of the patients, where the task of regaining control over drug intake is most challenging.
ACKNOWLEDGEMENTS
This work was supported by the SFB TRR 265 granted by the Deutsche Forschungsgemeinschaft (DFG). Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTION
Conceptualization: CB, LC, FG, AKS. Writing of the initial draft: AK, LC. Critical review: all authors. Final approval: all authors.
Open Research
DATA AVAILABILITY STATEMENT
This publication contains no data.




