Differential expression of miR-1249-3p and miR-34b-5p between vulnerable and resilient phenotypes of cocaine addiction
Laura Domingo-Rodriguez and Judit Cabana-Domínguez contributed equally.
Bru Cormand, Elena Martín-García and Rafael Maldonado equally supervised this work.
Abstract
Cocaine addiction is a complex brain disorder involving long-term alterations that lead to loss of control over drug seeking. The transition from recreational use to pathological consumption is different in each individual, depending on the interaction between environmental and genetic factors. Epigenetic mechanisms are ideal candidates to study psychiatric disorders triggered by these interactions, maintaining persistent malfunctions in specific brain regions. Here we aim to study brain-region-specific epigenetic signatures following exposure to cocaine in a mouse model of addiction to this drug. Extreme subpopulations of vulnerable and resilient phenotypes were selected to identify miRNA signatures for differential vulnerability to cocaine addiction. We used an operant model of intravenous cocaine self-administration to evaluate addictive-like behaviour in rodents based on the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition criteria to diagnose substance use disorders. After cocaine self-administration, we performed miRNA profiling to compare two extreme subpopulations of mice classified as resilient and vulnerable to cocaine addiction. We found that mmu-miR-34b-5p was downregulated in the nucleus accumbens of vulnerable mice with high motivation for cocaine. On the other hand, mmu-miR-1249-3p was downregulated on vulnerable mice with high levels of motor disinhibition. The elucidation of the epigenetic profile related to vulnerability to cocaine addiction is expected to help find novel biomarkers that could facilitate the interventions to battle this devastating disorder.
1 INTRODUCTION
Cocaine addiction results from a multistep process in which the initial controlled recreational use leads to a compulsive need to seek and consume the drug accompanied by a loss of control over the amount of drug consumed and an emergence of a negative emotional state when access to the drug is prevented.1, 2 This transition to addiction only occurs in a fraction of those individuals exposed to the drug,3 indicating a differential interindividual susceptibility triggered by the interaction between a vulnerable phenotype or personality defined by genetics and the environment.4 This complex interplay between genetics and environment suggests an essential role of epigenetic mechanisms that remodel the brain circuitries involved in motivational, emotional and behavioural control underlying addiction.5
Cocaine interacts with the mesolimbic pathway in the brain's reward system resulting in an enhancement of dopamine acting in the nucleus accumbens (NAc)-ventral tegmental area (VTA) pathway.6, 7 Cocaine inhibits dopamine reuptake in those projections and increases extracellular dopamine levels in the NAc.8 At the same time, the NAc receives glutamatergic projections from the medial prefrontal cortex (mPFC), an area implicated in the executive functions, producing a top-down control over drug seeking.9 Prolonged drug intake induces neuroplastic adaptations in mesolimbic and cortical networks prompting incentive saliency to the drug and, ultimately, behavioural inflexibility in vulnerable individuals, leading to compulsive drug consumption.10 Understanding the neurobiological mechanisms that predispose some individuals to develop cocaine addiction is crucial to identify biomarkers and design efficient therapeutic strategies to battle the disorder. Hence, preclinical addiction models have been developed to evaluate this disease's behavioural hallmarks in rodents.11-13 In this study, we used a drug self-administration procedure to evaluate two addiction-like criteria that resemble those included in the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5), used to diagnose substance use disorders in humans. MicroRNAs (miRNAs) have recently emerged as potential epigenetic biomarkers based on their capacity to be extracellularly secreted, reaching the systemic circulation.14-16 Thus, miRNAs may open new promising therapeutic tools based on altering their expression.
MiRNAs are endogenous small non-coding RNAs (~22 bp) that act as post-transcriptional regulators of gene expression by binding to target messenger RNAs (mRNAs) to inhibit translation or promote mRNA degradation. Each miRNA can regulate the expression of hundreds of different mRNAs, and each mRNA can be targeted by several miRNAs, creating a complex and dynamic system that allows cells to fine-tune gene expression.17-19 In addition to the canonical cytoplasmic function, increasing evidence suggests that miRNAs located in the nucleus can regulate mRNA stability in the nucleolus and modulate alternative splicing, as well as activate or inhibit the transcription of target genes.20, 21 MiRNAs are abundant in the central nervous system and play essential roles in neuronal development, differentiation and survival.22-24 MiRNAs also play an essential role in different processes related to addiction, such as reward, synaptic plasticity, learning, memory, withdrawal and relapse.25, 26 Moreover, animal studies have demonstrated that cocaine induces robust alterations in the expression of a wide range of miRNAs in the brain.27 In rats, experimenter-administered cocaine reduced the expression of miR-495 in the NAc,28 miR-124 and let-7d in the dorsal striatum, and enhanced miR-181a expression in the NAc, PFC and hippocampus.29 On the other hand, cocaine self-administration increased the expression of miR-212 in the dorsal striatum of rats with extended daily access to cocaine.26, 30, 31 These findings point at miRNAs as potential biological markers for cocaine addiction. However, previous studies have only reported the effects of contingent and non-contingent cocaine administration on miRNA expression without considering the individual vulnerability to develop addiction. Only a minority of individuals exposed to drug abuse develop addiction,2 and there is an urgent need to understand the neurobiological mechanisms underlying the individual vulnerability to develop this disease.
We have investigated miRNA signatures of vulnerability and resilience to cocaine addiction using an operant conditioning mouse model of cocaine self-administration based on DSM-5 criteria. MiRNA profiling was used to compare two extreme subpopulations of mice classified as resilient and vulnerable to cocaine addiction, considering two criteria for cocaine addiction (persistence to response and motivation) and one phenotypic trait, motor disinhibition, considered as a risk factor for addiction.
2 MATERIALS AND METHODS
2.1 Animals
Male wild-type mice, JAX™ C57BL/6J (C57BL/6J), aged 8 weeks, were purchased from Charles River (France) and were housed individually in temperature- and humidity-controlled laboratory conditions (21 ± 1°C, 55 ± 10%) maintained with food and water ad libitum. Mice were tested during the dark phase of a reverse light cycle (lights off at 8:00 AM and on at 8:00 PM). Animal procedures were conducted in strict accordance with the guidelines of the European Communities Council Directive 2010/63/EU and approved by the local ethical committee (Comitè Ètic d'Experimentació Animal-Parc de Recerca Biomèdica de Barcelona, CEEA-PRBB, agreement N°9213). In agreement, maximal efforts were made to reduce the suffering and the number of mice used.
2.2 Surgery and drugs
The surgery of the intravenous catheter implantation was performed as previously detailed32 (see supporting information Materials and Methods for more details).
2.3 Operant self-administration apparatus
Mouse operant chambers (Model ENV-307A-CT; Med Associates Inc., Georgia, VT, USA) were equipped with two holes, one selected as the active hole and the other as the inactive hole. Pump noise and stimuli lights (cues), one located inside the active hole and the other above it, were paired contingently with the delivery of the cocaine infusion. Cocaine was infused in 23.5 μL over 2 s (0.5 mg/kg per injection, intravenously; see supporting information Materials and Methods for more details).
2.4 Experimental design
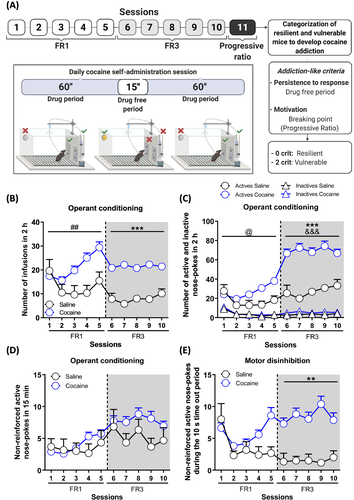
Mice were trained in operant boxes during 2.15-h daily sessions to self-administer cocaine (n = 72) or saline (n = 6). Each daily session began with 3 s of turning on the house light accompanied by a drug's priming injection (see supporting information Materials and Methods for more details; Figure 1A).
2.5 Behavioural statistical analyses
All behavioural statistical analyses were performed using Statistical Package for Social Science program SPSS® 25.0 (SPSS Inc, Chicago, USA). Repeated measures analysis of variance (ANOVA) considering two or three factors were used to test the evolution of the number of infusions in 2 h, the number of active and inactive nose pokes in 2 h, non-reinforced active nose pokes in 15 min, and non-reinforced active nose pokes during the time-outs (10 s) over the 10 self-administration sessions. The session variable was used as a within-subject factor, and the drug (cocaine or saline) and the nose-poke (active or inactive) variables were used as between-subject factors. Two-way ANOVA and subsequent post-hoc analysis (Fisher's least significant difference) were used for multiple group comparison. The Kruskal-Wallis followed by U Mann-Whitney test for multiple group comparison was used when the sample population did not follow the normal distribution analysed by the Shapiro-Wilk or Kolmogorov-Smirnov normality tests. See supporting information Materials and Methods for more details. The observed power analysis was calculated, and the criterion for significance (alpha) was set at 0.05. With the sample size of 16 mice (2–6 per group), our studies achieved a power between 70 and 100%, as obtained before.2 Supporting information tables (supporting information Tables S13–S16) provided a complete report of the statistical results for the data described in the figures.
2.6 RNA isolation and smallRNA sequencing
Animals from the cocaine group were euthanised by decapitation, and total RNA with miRNAs was isolated from mPFC and NAc using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen Düsseldorf, Germany) according to the manufacturer's protocol and stored at −80°C. SmallRNA sequencing (smallRNA-seq) was performed by the Centre de Regulació Genòmica (CRG, Barcelona, Spain). The analysis of smallRNA-seq data was carried out through the OASIS2 pipeline (http://oasis.dzne.de/).33 The differential expression analysis was done by DESeq2,34 considering the two criteria for cocaine addiction (persistence to response and motivation) and one phenotypic trait considered as a risk factor to develop an addiction (motor disinhibition). Results were corrected for multiple testing using a 5% false discovery rate (FDR; see supporting information Materials and Methods for more details).
2.7 Functional analysis of smallRNA-seq results
For each differentially expressed miRNA, we obtained a list of the miRNAs target genes both validated (miRTarBase; http://mirtarbase.mbc.nctu.edu.tw/php/index.php)35 and predicted (miRSystem; http://mirsystem.cgm.ntu.edu.tw/),36 and performed an analysis on Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways using WebGestalt 2019 (http://www.webgestalt.org/)37 considering all catalogued targets for each miRNA. Finally, we inspected whether those target genes had previously been associated at a gene-based level to related phenotypes in humans using genome-wide association studies (GWAS) on (1) cocaine dependence38 and (2) impulsivity and risk-taking phenotypes.39, 40
3 RESULTS
3.1 Establishment of cocaine addiction-like behaviour in inbred mice exposed to a cocaine self-administration paradigm
Mice were trained to self-administer cocaine (n = 72) or saline (n = 6) in operant chambers during five continuous daily sessions under an FR1 schedule of reinforcement followed by five continuous sessions under the FR3 schedule. Each daily session included two 1-h “drug periods” in which active responses resulted in a cocaine infusion paired contingently with a cue-light separated by a 15-min “drug-free period”, in which active responses were not reinforced as signalled by the entire operant box illumination (Figure 1A). Mice that were negative in the catheter's patency evaluation by thiopental sodium (n = 19) or did not achieve the acquisition criteria (n = 5) in the cocaine group were excluded from the study, ending up with n = 48 for the cocaine group and n = 6 for saline. Analysis of the operant conditioning by repeated measures ANOVA revealed that mice receiving cocaine had an upward evolution during the FR1 period, progressively increasing the number of achieved infusions across sessions, whilst mice receiving saline remain stable (the interaction between sessions x drug, p < 0.01, Figure 1B). Depending on the drug trained, a significant difference was preserved in FR3 sessions, showing higher infusions in mice trained with cocaine compared to mice trained with saline (main effect of the drug, p < 0.001, Figure 1B). These differences were replicated when the number of responses obtained in the 2-h active periods were considered. Notably, both groups showed good discrimination between active and inactive nose pokes (interaction between sessions x drug x nose poke, p < 0.05 in FR1 sessions; the main effect of drug and nose poke, p < 0.001 in FR3 sessions, Figure 1C). During the 15-min drug-free periods, no significant differences in the number of non-reinforced active nose pokes were revealed between the FR1 and FR3 periods (Figure 1D). Motor disinhibition was evaluated considering the time-out periods, 10 s after each cocaine infusion in which the cue-light was off, and no drug was provided after responding on the active nose poke. Mice trained with cocaine showed enhanced active responses during the time-out periods across the entire FR3 period, suggesting an increased motor disinhibition due to the inability to stop the behaviour once initiated (main effect of the drug, p < 0.01, Figure 1E).
3.2 Categorisation of two extreme subpopulations of mice vulnerable or resilient to cocaine addiction-like behaviour
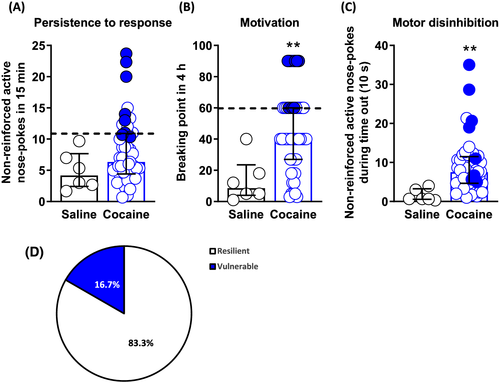
Two addiction-like criteria were considered to categorise mice as vulnerable or resilient to develop addiction-like behaviour towards cocaine: (1) persistence to response and (2) motivation for cocaine. Persistence to response was evaluated by the mean of non-reinforced active responses performed during the drug-free period of the last three self-administration sessions (8th, 9th, and 10th), and the motivation was tested by the breaking point achieved in the 4-h progressive ratio session performed continuously in the 11th day of self-administration. Animals that achieved a score value equal to or above the 75th percentile of the cocaine group distribution were considered positive for each specific criterion. Reaching two criteria was necessary to consider a mouse vulnerable to develop addiction-like behaviour, whilst mice that did not accomplish any of these criteria were classified as resilient. Mice trained with cocaine showed enhanced persistence to response (U Mann-Whitney, p = n.s, Figure 2A) and motivation (U Mann-Whitney, p < 0.01, Figure 2B) than mice trained with saline. As expected, the cocaine group was not homogeneous, with animals displaying extreme values in both criteria. Accordingly, only 16.7% (n = 8) of mice trained with cocaine were categorised as vulnerable animals to develop cocaine addiction-like behaviour (Figure 2D). Furthermore, mice trained with cocaine showed an enhanced motor disinhibition compared to mice trained with saline (U Mann-Whitney, p < 0.01, Figure 2C), a phenotypic trait considered as a factor of vulnerability to addiction.
3.3 Brain miRNA profiling in mice vulnerable or resilient to cocaine addiction-like behaviour
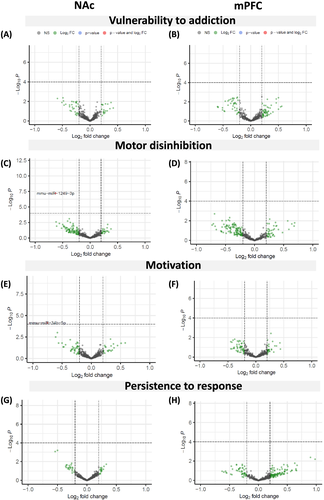
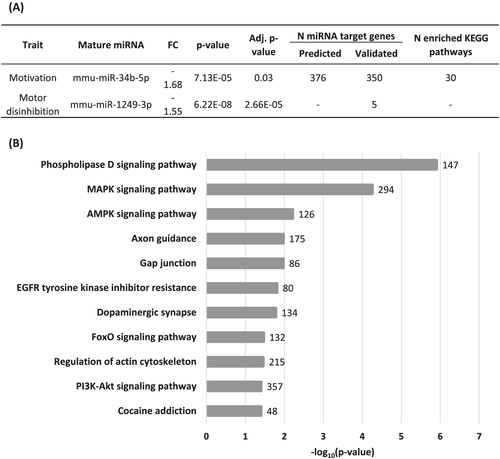
To identify miRNAs associated with the vulnerability to develop cocaine addiction in mice, we analysed the miRNA expression profiles in the NAc and mPFC, two areas critically engaged in rewarding and compulsive drug seeking,1 only in cocaine-experienced mice with a similar number of cocaine infusions. First, we compared two selected extreme subpopulations, the eight mice categorised as vulnerable and eight mice categorised as resilient (0 criteria), showing the lowest values in each criterion. These subgroups showed significant differences in persistence and motivation to seek the drug and motor disinhibition (t test, p < 0.001; t test, p < 0.001 and U Mann-Whitney, p < 00.1, Figure S1a–c). In contrast, non-significant differences were observed in the mean number of cocaine infusions suggesting that any transcriptomic change identified would be related to the addiction phenotype and not to differential exposure to cocaine (Figure S1d). When comparing these vulnerable and resilient mice, we could not identify any miRNA differentially expressed in the NAc or mPFC that overcomes 5% FDR correction (Figure 3 and Tables S1 and S2). Considering that vulnerability to addiction could be plural due to several subpopulations within the addict group,41 we examined our vulnerable subpopulation in more detail. The cluster analysis by the Ward method revealed that this group could be divided into two statistically different main clusters in each cocaine addiction-like criteria and motor disinhibition phenotypic trait (Figure S1e–g). Interestingly, the vulnerable high motivated and vulnerable high persistent mice or vulnerable high motor disinhibited were different (Figure S1a–c), and only one mouse overlapped in the three groups. The behavioural phenotype of the cluster of high persistence, high motivation and high motor disinhibition showed statistically higher values than the other clusters of vulnerable or resilient mice, as revealed by two-way ANOVA or Kruskal-Wallis (p < 0.01, Figure 4A–C and Table S15). The analysis of smallRNA-seq in the NAc revealed that among 431 mature miRNAs analysed, two miRNAs were differentially expressed, considering the 5% FDR correction. Mmu-miR-34b-5p was downregulated in vulnerable animals with high motivation (Wald test, padj = 0.03; FC = −1.68; Figure 4D and Table S3), and mmu-miR-1249-3p was downregulated in vulnerable mice with high motor disinhibition scores compared to all resilient mice (Wald test, padj = 2.6E-05; FC = −1.54; Figure 4E and Table S4). We could not identify any differentially expressed miRNA overcoming FDR correction related to high persistence of response, probably related to the limited sample size of this group (n = 3, Table S5). Furthermore, we performed the cluster analysis in the resilient group, and we obtained that this group could also be divided into two different main clusters in each cocaine addiction-like criteria and motor disinhibition phenotypic trait with one mouse overlapped in the three groups (Figure S1h–j). Thus, next, we compared the behavioural phenotype of four groups by two-way ANOVA or Kruskal-Wallis depending on the Shapiro-Wilk normality test. The behavioural phenotype of the cluster of low persistence, low motivation and low motor disinhibition showed statistically lower values than the other clusters of vulnerable or resilient mice, as revealed by two-way ANOVA or Kruskal-Wallis (p < 0.01, Figure 4A–C and Table S15). In subsequent analysis, we did not identify any miRNA differentially expressed in the vulnerable or resilient mice with low motivation, low motor disinhibition or low persistence, supporting that the downregulation of mmu-miR-34b-5p and mmu-miR-1249-3p was specific to animals of the cluster of high motivation and high motor disinhibition (Figure 4A–E).
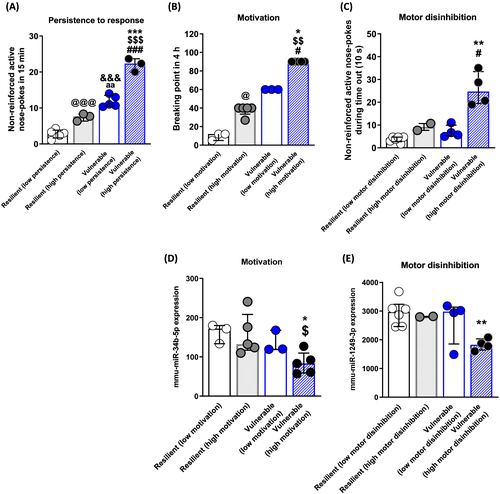
In addition, we performed smallRNA-seq analyses to compare the extreme subpopulations of resilient mice low motivated/persistent/motor disinhibited versus vulnerable mice high motivated/persistent/motor disinhibited. We found that mmu-miR-34b-5p and mmu-miR-1249-3p previously associated with high motivated and high motor disinhibited mice, respectively, remained associated, and the first one overcame significant after multiple testing corrections despite the reduced sample size. Finally, considering that the behavioural phenotype obtained in motivation and persistence to response showed a continuous pattern among the four subgroups, we also performed a wider analysis to identify miRNAs whose expression could correlate with them. We could not identify any miRNA that overcame multiple testing corrections. However, as previously described, mmu-miR-34b-5p was the miRNA that showed the most significant association with motivation (Figure S2a). In addition, we found two miRNAs that showed a high negative correlation with both traits, mmu-miR-532-3p and mmu-miR-700-3p (Figure S2b–e).
Regarding mPFC, we could not identify any differentially expressed miRNA that overcomes FDR corrections in any comparison; however, several miRNAs showed nominal associations (Tables S6–8).
3.4 Analysis of miRNA targets
We obtained a list of predicted and validated miRNA target genes for each differentially expressed miRNA and performed functional group enrichment. We identified 350 validated and 376 predicted target genes for mmu-miR-34b-5p (578 unique target genes in total), associated with high motivation for cocaine (Tables S9 and S10). Considering all of them, we found enrichment on several KEGG pathways relevant for the addictive process, including cocaine addiction, dopaminergic synapse, axon guidance and MAPK signaling pathway (Figures 5 and 6 and Table S11). Furthermore, 42 of these genes were found nominally associated with cocaine dependence in humans,38 and TAB2 overcomes the Bonferroni correction for multiple testing in our study (p = 0.05/578 target genes = 8.6E-05; Tables S9 and S10). We only found five validated target genes for mmu-miR-1249-3p, associated with motor disinhibition in the group of vulnerable mice (Tables S12). As expected, given the small number of genes, we could not find enrichment in any KEGG pathway. However, several of these genes were found previously associated with impulsive behaviour in humans in a GWAS: UGGT2 was associated with attentional impulsivity and TNFSF1 with drug experimentation (Table S12).40, 41 In addition, UGGT2 and AUTS2 were previously associated with risk tolerance, a trait also related to impulsive behaviours.39
4 DISCUSSION
In the present work, we identified an epigenetic signature involved in the vulnerability to develop cocaine addiction in mice. Specifically, we revealed two miRNAs, mmu-miR-34b-5p and −1249-3p, downregulated in the NAc in extreme subpopulations of vulnerable or resilient animals to cocaine addiction-like behaviour. We used an operant conditioning model of cocaine self-administration to differentiate mice vulnerable to developing an addiction from resilient ones. This categorisation was performed by considering the individual score obtained in two addiction criteria based on DSM-5, which was successfully applied in previous models developed in rats11, 42 and mice.43-45 Indeed, we mimicked two hallmarks of substance use disorders in humans, the difficulty of stopping drug use measured by perseverant drug seeking during a period signalled by the non-availability of cocaine and the high motivation for the drug measured by the maximal effort exerted to obtain a single drug infusion in a progressive ratio test. We used an inbred strain of mice to control the genetic factors, thus focussing on epigenetic mechanisms caused by the interaction between genetic factors and environmental experiences.46
The cocaine group showed higher scores in persistence to response and motivation than the saline group. However, high variability within the cocaine group was revealed, and only some cocaine-exposed mice (16.7%) reached the two addiction criteria and were consequently categorised as animals vulnerable to developing the disease. This percentage was similar to the number of animals considered addicted in the outbred strain of rats and mice after a long cocaine self-administration protocol,11, 13 possibly indicating that the addictive-like behaviours at early stages could inform the vulnerability to develop the disorder. This was also supported by human data showing that the estimated risk of developing cocaine dependence after the initial cocaine use in the first 12–24 months is 16%.47, 48
The operant conditioning model also examines other phenotypic traits related to addiction-like behaviour, such as impulsivity considered as motor disinhibition.49 Impulsivity is a complex construct that involves two components: motor impulsivity and choice impulsivity.50 We have recorded the non-reinforced active responses in the time-out periods to assess the motor impulsivity defined as a motor disinhibition.51 Mice receiving cocaine displayed higher motor disinhibition than mice receiving saline, and cocaine-exposed mice classified as vulnerable were more impulsive than resilient mice. In accordance, it has been shown that a high motor disinhibition trait in the rat predicted the transition to compulsive drug intake.52, 53 Consistent with these preclinical data, cocaine users show more significant impulsive behaviour than non-users.54-56 Thus, the impulsivity personality trait has been strongly associated with cocaine addiction, and finding epigenetic marks underlying this phenotypic trait could provide potential biomarkers and/or therapeutic targets for cocaine addiction.
MiRNA profiling was performed after cocaine self-administration to compare the two extreme subpopulations of mice classified as resilient and vulnerable to cocaine addiction. The number of cocaine infusions received by animals of both groups was equivalent. Thus, the differential miRNA expression found between groups was not due to a differential pharmacological effect of cocaine and was therefore related to the different behavioural phenotypes of both groups. Indeed, it is already known that passive cocaine administration in the NAc of mice induces changes in the expression levels of several miRNAs compared with saline-injected mice revealing an intrinsic cocaine pharmacological effect that has been discarded considering the experimental conditions chosen in our study.57 Indeed, the putative expression changes observed when comparing the saline group versus cocaine mice could be driven by the direct pharmacological effect of cocaine in the brain and not necessarily by the molecular adaptations that underlie resilience or vulnerability. Differentially expressed miRNAs were not identified in the first global comparison between mice resilient and vulnerable to cocaine addiction. Considering that several subpopulations should exist within the drug-addicts group, a cluster analysis was performed. We found that the vulnerable subpopulation could be divided into two main clusters, statistically different, considering each cocaine addiction-like criteria (high persistence to response and high motivation) and motor disinhibition phenotypic trait. Thus, we compared the resilient mice with the different subpopulations of vulnerable mice. This allows us to identify miRNAs specifically involved in vulnerability subphenotypes contrasting more homogeneous subgroups of vulnerable mice with the resilient ones. However, we have reduced sample size in these subgroups that could impact our ability to consider the differential miRNAs detected as potential biomarkers of addiction. So further studies would be needed to confirm our results. We first found that mmu-miR-34b-5p was downregulated in the NAc of vulnerable mice with high motivation for cocaine. This miRNA is a member of the miR-34/449 family, highly conserved in mammalian,58 that participates in several neuronal developmental processes like neuron differentiation, neurite outgrowth and spinal morphology,59-61 and seems to induce the differentiation of dopaminergic neurons.62
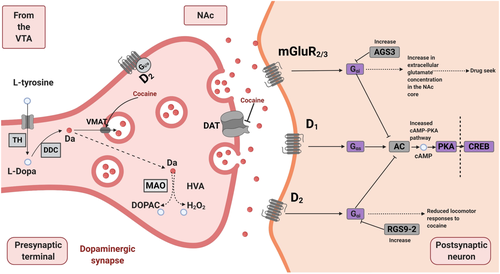
Interestingly, we found that miR-34b-5p target genes were enriched in several crucial pathways related to the addictive process, such as the cocaine addiction pathway and dopaminergic synapse (Figures 5 and 6 and Table S11). Dopaminergic neurons that project from the VTA to the NAc and other forebrain regions play an essential role in reinforcement for both natural rewards and addictive drugs.63 Cocaine blocks the dopaminergic transporter, responsible for the dopamine reuptake in the dopaminergic neurons. This effect increases the extracellular concentrations of dopamine that produce the characteristic cocaine “high”. Through the dopamine D1 receptor, dopamine activates the cAMP-PKA signalling pathway in the NAc, which phosphorylates CREB and induces the expression of immediate-early genes (e.g., Bdnf, ΔFosB and Jun) that mediate several behavioural responses to cocaine (Figure 6).64 Repeated cocaine use induces neuroadaptations that underlie craving and hedonic dysregulation. miR-34b-5p seems to regulate several genes from these pathways (like Bdnf, Creb1 and Prkacb, among others), suggesting an essential role in controlling addiction vulnerability. Interestingly, this miRNA has previously been related to psychiatric disorders that are highly comorbid with drug addiction in humans, such as attention-deficit/hyperactivity disorder, depression and anxiety.65-68
On the other hand, mmu-miR-1249-3p was downregulated in the most motor disinhibited vulnerable mice. We found five validated target genes of this miRNA: Auts2, Foxl1, Tnfsf10, Uggt2 and Zfp941. Some of them have been associated at the gene level with humans' impulsivity traits (Tables S9 and S10).40 TNFSF1 (tumor necrosis factor ligand superfamily member 10) was associated with drug experimentation, a scale that quantifies the number of 11 different classes of drugs an individual has tried in the lifetime. Drug experimentation is a necessary step for drug abuse and constitutes one of the first stages at which an individual's genotype can influence the risk of developing addiction. UGGT2 (UDP-glucose glycoprotein glucosyltransferase 2) was associated with attentional impulsivity from BIS-11 (Barrat impulsiveness scale), defined as the inability to concentrate or focus attention. Furthermore, this gene and AUTS2 (activator of transcription and developmental regulator) were previously associated with risk tolerance, defined as the willingness to take risks to obtain some reward, highly related to impulsive behaviours.39 Interestingly, the AUTS2 gene was upregulated postmortem in NAc of male human cocaine addicts69 and downregulated in lymphoblastoid cell lines in heroin-dependent individuals.70 Several studies have demonstrated that a particular single-nucleotide polymorphism (SNP) (rs6943555) in this gene might increase susceptibility to heroin and alcohol dependence.71-73
Our work demonstrated that the analysis of extreme subpopulations and vulnerable or resilient mice to develop cocaine addiction allows identifying relevant miRNA for these phenotypes. Interestingly, after clustering vulnerable individuals based on different phenotype criteria, significant differential signatures of miRNA expression emerged that might be associated with vulnerability to addiction. Thus, the model allowed the detection of epigenetic marks that could predict the vulnerability to addiction and may guide future research on therapeutical targets for these vulnerable phenotypes.
FUNDING AND DISCLOSURES
This work was supported by the Spanish “Ministerio de Economía y Competitividad-MINECO” (#SAF2017-84060-R-AEI/FEDER-UE), the Spanish “Instituto de Salud Carlos III, RETICS-RTA” (#RD12/0028/0023), the “Generalitat de Catalunya, AGAUR” (#2017 SGR-669), “ICREA-Acadèmia” (#2015) and the Spanish “Ministerio de Sanidad, Servicios Sociales e Igualdad”, “Plan Nacional Sobre Drogas of the Spanish Ministry of Health” (#PNSD-2017I068) to RM; “Fundació La Marató-TV3” (#2016/20-30), “Plan Nacional Sobre Drogas of the Spanish Ministry of Health” (#PNSD-2019I006) and NEURON-ERA-NET: MCIN/AEI/UE - PCI2021-122073-2A to E.M-G.; Spanish “Ministerio de Ciencia, Innovación y Universidades” (#RTI2018–100968-B-100), Spanish “Ministerio de Ciencia e Innovación (#PID2021-1277760B-100), “AGAUR-Generalitat de Catalunya” (#2017-SGR-738), “Plan Nacional Sobre Drogas of the Spanish Ministry of Health” (#PNSD-2017I050) and ICREA Acadèmia 2021 to BC and “Plan Nacional Sobre Drogas of the Spanish Ministry of Health” (#PNSD-2020I042) to N.F-C. The research leading to these results was also supported by the European Union H2020 Program [H2020/2014–2020] under grant agreements no 667302 (CoCA) and 728018 (Eat2beNICE) and by the “ECNP network on ADHD across the lifespan” to BC. JC-D was supported by the H2020 CoCA and Eat2beNICE projects and NF-C by “Centro de Investigación Biomédica en Red de Enfermedades Raras” (CIBERER).
ACKNOWLEDGEMENTS
We are grateful to the Genomics Unit at the CRG for assistance with the smallRNA-seq analysis. We thank M. Linares, R. Martín, D. Real and F. Porrón for their technical support. Figures with drawings are created with BioRender.com.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHORS CONTRIBUTIONS
E.M.-G., L.D.-R. and R.M. conceived and designed the behavioural studies with input from J.C.-D, B.C. and N. F.-C; E.M.-G. performed the surgery for i.v. catheterisation and the operant conditioning maintained by cocaine; L.D.-R. performed the behavioural graphs and statistical analyses with the supervision of E.M.-G. and R.M; J.C.-D. performed the RNA extractions, smallRNA sequencing and bioinformatics, and statistical analyses supervised by B.C. and N.F.-C.; E.M.-G. performed the KEEG pathway picture; L.D.-R., J.C.-D. and E.M.-G. wrote the manuscript and prepared the figures and tables, and N.F.C., R.M. and B.C. provided a critical review of the manuscript with all the other authors' inputs.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are available from the corresponding author upon reasonable request.




