Association of contextual cues with morphine reward increases neural and synaptic plasticity in the ventral hippocampus of rats
Abstract
Drug addiction is associated with aberrant memory and permanent functional changes in neural circuits. It is known that exposure to drugs like morphine is associated with positive emotional states and reward-related memory. However, the underlying mechanisms in terms of neural plasticity in the ventral hippocampus, a region involved in associative memory and emotional behaviors, are not fully understood. Therefore, we measured adult neurogenesis, dendritic spine density and brain-derived neurotrophic factor (BDNF) and TrkB mRNA expression as parameters for synaptic plasticity in the ventral hippocampus. Male Sprague Dawley rats were subjected to the CPP (conditioned place preference) paradigm and received 10 mg/kg morphine. Half of the rats were used to evaluate neurogenesis by immunohistochemical markers Ki67 and doublecortin (DCX). The other half was used for Golgi staining to measure spine density and real-time quantitative reverse transcription-polymerase chain reaction to assess BDNF/TrkB expression levels. We found that morphine-treated rats exhibited more place conditioning as compared with saline-treated rats and animals that were exposed to the CPP without any injections. Locomotor activity did not change significantly. Morphine-induced CPP significantly increased the number of Ki67 and DCX-labeled cells in the ventral dentate gyrus. Additionally, we found increased dendritic spine density in both CA1 and dentate gyrus and an enhancement of BDNF/TrkB mRNA levels in the whole ventral hippocampus. Ki67, DCX and spine density were significantly correlated with CPP scores. In conclusion, we show that morphine-induced reward-related memory is associated with neural and synaptic plasticity changes in the ventral hippocampus. Such neural changes could underlie context-induced drug relapse.
Introduction
Drug addiction can be conceptualized as a chronic relapsing disorder associated with long-lasting molecular, cellular and behavioral consequences (Nestler 2001; Koob & Volkow 2010). Repeated drug exposure is able to induce positive emotional states and reward-related learning and memory (Nesse & Berridge 1997; Hyman et al. 2006). A growing body of evidence suggests engagement of common neural circuitries and molecular mechanisms during drug addiction and memory (Kelley 2004). Morphine as an addictive substance may hijack brain mechanisms of learning and memory and induce aberrant memory formation (Eisch et al. 2000). In addition, contextual cues present at the time of an addictive drug administration can prime memories associated with the prior drug experience and strongly result in drug-seeking behavior (Mueller et al. 2002).
The conditioned place preference (CPP) paradigm is one of the most popular behavioral models for investigating the relation between context (compound conditioned stimulus) and rewarding effects of an addictive drug (unconditioned stimulus; Bardo & Bevins 2000). In this procedure, the conditioned rewarding properties of drugs are measured by introducing the animal to a drug-associated environment and an environment that is explicitly paired with a drug-free state. The greater the time an animal spends in the drug-associated environment, the higher the CPP is. Previous studies have suggested that chronic morphine administration significantly enhances the preference of animals for a morphine-paired environment (Harris & Aston-Jones 2003; Sun et al. 2015).
Prior studies proposed the hippocampus as a structure in the brain crucial for CPP. Indeed, the hippocampus is profoundly implicated in the formation of addictive memory and drug reward experience (Nestler 2001; Dong et al. 2006). For example, in morphine-induced CPP, the hippocampus plays a role in the arrangement of CPP behavior, and lesion of this region changes normal performance (Corrigall & Linseman 1988; Ferbinteanu & McDonald 2001). The hippocampus is functionally heterogeneous along its septotemporal extent. The dorsal hippocampus is particularly important for spatial memory (Moser et al. 1995), whereas the ventral portion is involved in associative memory and emotional behaviors through a neurocircuit connecting the nucleus accumbens, amygdala and prefrontal cortex (Henke 1990; Sahay & Hen 2007). The CA1 subregion as a critical output structure of the hippocampus is important for the formation of contextual memory (Daumas et al. 2005). The dentate gyrus (DG) of the hippocampus is one of the two major regions of the rodent brain in which new neurons are generated over lifespan (Epp et al. 2013). It is widely accepted that adult neurogenesis happens through various stages such as proliferation of progenitor cells, differentiation, maturation and survival (van Praag et al. 2002). This process has a profound role in maintaining hippocampal function and plasticity, which is affected by some internal factors such as growth factors and environmental factors such as learning, exercise and enrichment environment (Snyder et al. 2005; Lee & Son 2009). Interestingly, levels of adult-generated DG neurons are increased in response to hippocampus-dependent learning tasks (Epp et al. 2007).
Dendritic spines have been considered as essential components for neuronal connectivity and synaptic plasticity and neurochemical mechanisms for learning and memory. The morphology and number of these small postsynaptic membrane structures are extremely dynamic and variable (Yuste & Bonhoeffer 2001; Harris et al. 2003). Recent work suggests that morphine elicits structural and morphological alterations in hippocampal synapses and could upregulate the synaptic numbers and postsynaptic density (Heidari et al. 2013).
Brain-derived neurotrophic factor (BDNF), a member of the nerve growth factor family, is widely expressed in several areas of brain including subregions of the hippocampus and the adult forebrain (Schmidt-Kastner et al. 1996; Bramham & Messaoudi 2005). BDNF binds to the TrkB receptor–protein tyrosine kinase and involved in synaptic transmission and plasticity (Alonso et al. 2004). The correlation between BDNF mRNA expression and learning and memory processes has been reported (Yamada & Nabeshima 2003). In addition, BDNF contributes in neurogenesis and synaptic changes following drug exposure (Briones & Woods 2013). There is evidence demonstrating that BDNF could increase spine density of CA1 pyramidal neurons in the hippocampus (Alonso et al. 2004). In the context of drug addiction, BDNF is the potent candidate engaged in enhancing the neural and behavioral plasticity following drug exposure (Hall et al. 2003). In view of these studies, we hypothesized that morphine-induced reward-related memory is associated with changes in neurogenesis, spine density and BDNF and TrkB mRNA expression levels.
In the present study, we focused on ventral hippocampal mechanisms associated with morphine-induced associative and emotional memory. We hypothesize that morphine as a stimulus-inducing emotional state could entail lasting changes in ventral hippocampal neuroplasticity. To look into these issues, we examined how morphine-induced contextual memory affects adult neurogenesis in the DG of the ventral hippocampus. For this, the number of adult newborn granule cells was analyzed based on the immunohistochemical marker Ki67, a protein that is expressed during all active phases of cell division, and doublecortin (DCX), a microtubule-associated protein that is expressed in neuroblasts. Furthermore, the effect of morphine-induced CPP on dendritic spine density in the ventral hippocampus was studied.
Materials and Methods
Animals
Male Sprague Dawley rats 300–400 g of body weight were obtained from Charles River Laboratory and used as subjects. The rats were individually housed in cages with free access to food and water ad libitum on a regular 12/12-hour light/dark cycle (lights on at 8:00 am). All trials were performed during the light phase. The animals were allowed to habituate to the laboratory condition 1 week prior to the beginning of testing. During adaptation period, each animal handled daily to decrease injection stress. All experimental procedures were carried out in accordance with institutional guidelines approved by the Committee for Animal Experiments of the Radboud University Medical Centre, Nijmegen, the Netherlands.
Conditioned place preference
The conditioning apparatus was a wooden chamber and contained two equally sized compartments (31 × 30 × 30 cm) with distinctive visual and textual cues. One compartment had black and white striped walls with a smooth black floor, and the other was defined by checked walls and textural floor. Both compartments could be accessed by a white smaller intermediate part (11 × 30 × 30 cm) through removable walls.
In general, the CPP training consisted of three different phases: preconditioning (day 1), conditioning (day 2–9) and post conditioning (day 10). During preconditioning, we determined the initial preference for either compartment. The rats were individually placed in the intermediate compartment and allowed to move freely for 15 minutes in whole chamber. The movement of each animal was recorded by using a video camera on top of the box, and total time spent in each of the conditioning compartments was measured by using ethovision 3.1 software.
During conditioning (40 minutes per day), morphine CPP rats (n = 18) were subcutaneously injected with morphine (10 mg/kg) and immediately placed in the least preferred compartment. The next day, the CPP rats were treated with (0.9 percent saline) s.c. and then confined to the opposite compartment. This injection order was repeated for 8 consecutive days. Rats in the saline CPP group (n = 18) received saline during all conditioning days and placed in the preferred and non-preferred compartment alternatively. Morphine and saline homecage groups (n = 12) were separately administered either with morphine (10 mg/kg) or saline s.c. time matched with morphine or saline CPP groups in the experiment room. Morphine or saline homecage groups were included to control for the influence of morphine or saline injection alone on synaptic plasticity. A separate control group (n = 18) was exposed to the CPP paradigm without receiving any injection to evaluate the effect of exposure to CPP box.
At the post-conditioning (test) day, the walls between the compartments were removed and the rats were given free access to the entire chamber for 15 minutes without receiving any injection. Time spent in each compartment was measured. The preference score for each rat was calculated as total time spent in the morphine-paired compartment during the post-conditioning test day minus the total time spent in the non-preferred compartment during pre-conditioning. Locomotor activity for each animal was estimated by considering the total distance traveled (cm) on the test day. After the CPP test, we divided animals into two groups. Half of the rats were used for immunohistochemistry, and the other half was used for Golgi staining and real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
3,3′-Diaminobenzidine immunohistochemistry
One day after the CPP test, the rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with phosphate-buffered saline (PBS; 0.1 M, pH 7.3) followed by 4 percent paraformaldehyde. Subsequently, the brains were removed and post-fixed in 4 percent paraformaldehyde overnight at 4°C. The next day, they were transferred to 0.1 M PBS at 4°C. The brains were cut into coronal sections at a thickness of 40 μm in six parallel series on a freezing microtome (Microm HM 450, Waldorf, Germany) and stored in 0.1 M PBS containing 0.1 percent sodium azide. 3,3′-Diaminobenzidine (DAB) immunohistochemistry was carried out on free floating slices. The tissue sections were rinsed three times in 0.1 M PBS, followed by incubation with 30 percent H2O2 for 30 minutes to remove any endogenous peroxidase activity. We used blocking buffer (0.1 M PBS with 0.1 percent bovine serum albumin and 0.5 percent Triton X-100) to block non-specific binding for 30 minutes in room temperature. Subsequently, the slides were incubated overnight at room temperature with one of the following primary antibodies: goat anti-DCX, C-18 terminal, 1:8000 (Santa Cruz Biotechnology Inc, Santa Cruz, California, USA) and rabbit anti-Ki67 1:8000 (Abcam, Cambridge, UK; Cat # ab1558). As negative control, we did not add the primary antibodies. The next day, the procedure was continued by incubation in donkey antigoat and donkey antirabbit IgG Biotin SP-conjugated 1:1500 (Jackson Immuno Research Laboratories, West Grove, Pennsylvania, USA) as secondary antibodies. Stained cells were visualized by the avidin biotinylated enzyme complex (Vector ABC-Elite kit PK 6100) and DAB as the chromogen.
Quantification of cells
The images of Ki67 DAB-stained brain slices containing the granular (GCL), SGZ and hilar region of the ventral DG were captured with a Zeiss Axioplan light microscope in neurolucida 11.0 software (MBF Biosciences) by using a 10× objective. Measurements were performed within a stereotaxic range from bregma −6.00 to −6.36 mm (Paxinos 2005) on 3 to 4 sections/animal (n = 6) by using the 40× objective lens. The quantitative assessment was completed by applying imagej software, which could isolate ki67-labeled cells from background staining as the threshold.
Doublecortin-labeled cells in the subgranular cell (SGZ) layer of ventral DG were counted blindly with the Optical Fractionator applying stereoinvestigator software (Micro-Brightfield Inc, Williston, Vermont, USA) connected with a Zeiss Axioplan light microscope. The number of DCX-labeled cells was quantified manually within a counting frame of 50 × 50 μm in a 250 × 250 μm counting grid. DCX-labeled cells were sampled from 3 to 4 sections/animal (n = 6) from bregma −6.00 to −6.36 mm (Paxinos 2005).
Immunofluorescence
To obtain anatomical pictures for visualization purposes only, the rats were perfused transcardially and brains were removed. The brains were cut into coronal sections (40 μm) by using freezing microtome and stored at PBS as described in the preceding texts. For immunofluorescence, double-labeling free-floating sections were washed in PBS and pre-incubated in pre-incubation buffer including PBS, 0.1 percent BSA and 0.1 percent Triton X-100 for 30 minutes in room temperature. Then, they incubated with primary antibodies overnight, washed following day, incubated with appropriate fluorophore-conjugated secondary antibodies (1:100) and diluted in PBS-BT for 3 hours at room temperature. As primary antibodies, we used goat anti-DCX, C-18 terminal, 1:4000 (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) and rabbit anti-Ki67 1:1000 (Abcam, Cambridge, UK; Cat # ab1558). After that, sections were mounted on coated slides and dried for 24 hours. Then, slides were washed three times with PBS and cover slipped. Images were obtained by using the fluorescence Imager A1 microscope (Zeiss) by the 10× and 40× objective lenses.
Golgi staining
xFor measuring spine density, Golgi staining was done by using FD Rapid GolgiStain Kit (FD Neuro Technologies, Inc). Deeply anesthetized rats were decapitated, and fresh brains were removed and one hemisphere of the brains rinsed in Milli-Q water. The other brain hemisphere was used for qRT-PCR. The brains were cut into 1mmthick blocks and immersed in the impregnation solution including equal volume of solution A and B for 2 weeks in the dark and room temperature. Afterward, the brains were transferred to solution C and stored for 72 hours at room temperature and dark place. Following this procedure, tissues were sectioned at 180 μm thickness in coronal orientation by using a freezing microtome (Microm HM 450, Waldorf, Germany) at the level of the ventral hippocampus. The sections were mounted on gelatin-coated microscope slides with Solution C and left to dry naturally for 2 days. Then, after dehydration, the slides were coverslipped with Paramount.
Analysis of dendritic spine density
Spine counting was performed on apical dendrites of CA1 pyramidal neurons and DG granule cells in the ventral hippocampus. Cells were sampled for DG in sections from bregma −5.52 to −6.24 mm and CA1 in bregma −5.28 to −6.12 mm (Paxinos 2005). Five individual neurons were reconstructed for each animal in all groups (n = 4) by using computer-based neuron tracing system (NeuroLucida 11.0, MBF Biosciences) at a 40× objective. For each of neuron, five segments 120–160 μm away from the soma were analyzed by using a 100× oil immersion lens.
Dendritic spine density was counted by dividing the number of spines by the length of the dendrite, and data were shown as number of spines/10 μm dendrite. Neurons with the following properties were selected to trace in each of experimental group: (1) location within the outer layer of the ventral CA1 or DG, (2) isolated from neighboring stained cells and (3) entire and dark staining and impregnation. All data were collected in a blind fashion.
qRT-PCR
After sacrificing the animals, the ventral part of hippocampus was removed, frozen in dry ice and stored at −80°C. We performed qRT-PCR to measure BDNF and TrkB mRNA levels in the ventral hippocampus. Total RNA was isolated from each tissue sample by using RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. RNA quantity and quantify were determined with a NanoDrop ND2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Then, single strand cDNA was synthesized from total RNA by using the SensiFast cDNA Synthesis Kit (Bioline, Germany). The cDNA was diluted 10-fold and used to perform RT-PCR by using SensiFAST SYBR No-ROX Kit (Bioline, Germany). The sequences of forward and reverse primers were BDNF (forward: TCACAGCGGCAGATAAAAAG; reverse: TGGGATTACACTTGGTCTCG), TrkB (forward: TTCCAAGTTTGGCATGAAAG; reverse: GAAGAAGACGGAGTGTTGCTC) and tubulin-α (forward: CAGGGCTTCTTGGTTTTC, reverse: TCCATCAGCAGGGAGGTG). The amounts of BDNF and TrkB cDNA were normalized against housekeeping gene tubulin-α cDNA in the corresponding samples, and analysis was performed by using the 2-ΔΔCT method.
Statistical analyses
All analyses were done by using graphpad prism 6 software. Experimental data for neurogenesis, spine density and BDNF and Trkb mRNA levels were analyzed by using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. Data are presented as mean ± SEM. Pearson correlation analyses were used to determine the relationship among CPP preference scores, number of Ki67 and DCX labeled cells, dendritic spine density in CA1 and DG, and BDNF and TrkB mRNA levels. Values of P < 0.05 are assumed significant in all experiments.
Results
Morphine induces conditioned place preference but fails to increase locomotor activity
The time spent in the morphine and saline-paired compartment for CPP morphine, CPP saline and CPP without injection groups is illustrated in Fig. 1a. On the post-conditioning test day, statistical analysis using one-way ANOVA showed significant differences between groups (F(2,39) = 18.66, P < 0.0001), generally. Tukey's post hoc analysis indicated that the morphine-treated animals (morphine-CPP) displayed a significantly enhanced place preference for the drug paired compartment relative to saline-treated rats (saline-CPP, P < 0.001) and rats that were exposed to the CPP paradigm without any injections (CPP without injection, P < 0.0001). There was no significant difference between saline-CPP and CPP without injection groups (P > 0.05).
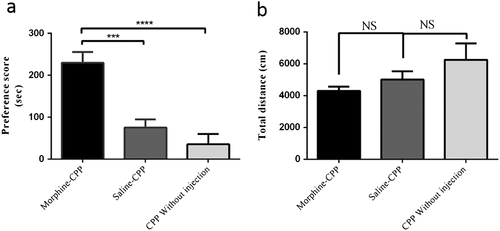
Locomotor activity was evaluated to exclude possible influences of drugs on the motivation of the animals. As shown in Fig. 1b, morphine administration did not affect locomotor activity and there were no significant differences among three treatments groups including morphine-CPP, Saline-CPP and CPP without injection groups (F(2,41) = 2.09, P = 0.137).
Morphine-induced conditioned place preference increases Ki67-labeled cells in the ventral DG
Ki67 is a cell cycle protein marker that is expressed during all active phases of cell division. Visualization of proliferated neurons from progenitor cells using Ki67 immunoreactivity in the GCL, SGZ and hilus is illustrated in Fig 2. Comparing the number of Ki67-labeled cells in the ventral DG across all groups revealed significant differences between groups generally (F(4,25) = 5.18, P = 0.003). Quantification of the absolute number of ki67-labeled cells showed that the number of labeled cells in the morphine-CPP group was significantly greater than those of saline-CPP (P < 0.05) and CPP without injection (P < 0.01) groups. Further statistical analysis revealed an increase in the number of Ki67-labeled cells in the morphine-CPP group as compared with the group given repeated morphine time matched with the CPP (P < 0.05). Also, there was an insignificant reduction in the number of ki67-labeled cells in the repeated morphine-treated group time matched with the CPP as compared with the repeated saline-treated group time matched with the CPP (P > 0.05).
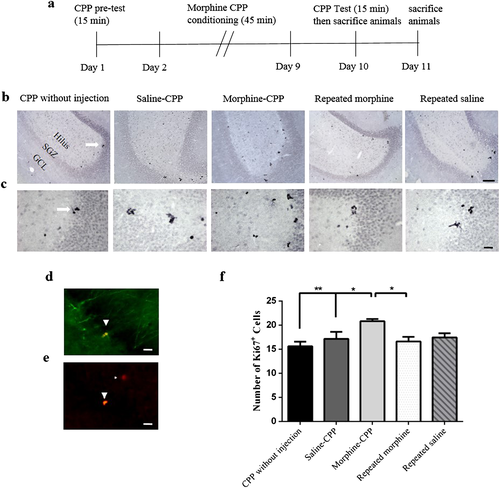
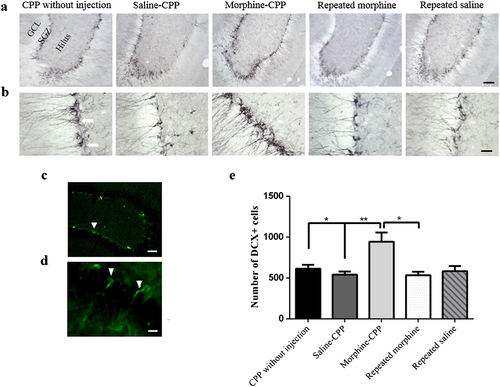
Morphine-induced conditioned place preference increases the number of DCX-labeled neurons in the ventral DG
Doublecortin immunoreactivity was measured in the SGZ of the ventral DG. DCX is a protein engaged in the stabilization of microtubules and important in the migration and differentiation of developing neurons (Lee et al. 1990; Deuel et al. 2006). Interestingly, the number of DCX positive neurons was changed significantly across all groups (F(4,27) = 5.79, P = 0.002) generally. Fig 3 shows the number of DCX-expressing newborn neurons in the ventral DG in five different groups (n = 6). Further data analysis indicated that the number of DCX positive neurons in the ventral DG increased significantly in the CPP-morphine group as compared with the CPP-saline group (P < 0.01). Furthermore, we found that the number of DCX-labeled cells in the morphine-CPP group was significantly higher than that of the CPP-without injection group (P < 0.05). Additionally, a significant increase in the number of DCX positive cells was observed in the morphine-CPP group when compared with the groups of rats that were treated with repeated morphine time matched with the morphine-CPP group (P < 0.05).
Morphine-induced conditioned place preference enhances the dendritic spine density of hippocampal CA1 pyramidal cells
Golgi staining was used to examine the relationship between morphine-induced CPP and synaptic plasticity in pyramidal neurons of the ventral hippocampal CA1 region. Apparent differences were observed in dendritic spine density among different groups (F(4,14) = 8.83, P = 0.001; Fig 4), generally. We found a significant increase in the spine density of hippocampal CA1 pyramidal neurons in the morphine-CPP group as compared with the saline-CPP (P < 0.05) and CPP without injection (P < 0.01) groups. Comparing the dendritic spine density between the morphine-CPP group and repeated morphine time matched with CPP (P < 0.001) showed a significant increase in the dendritic spine density due to morphine-induced CPP.
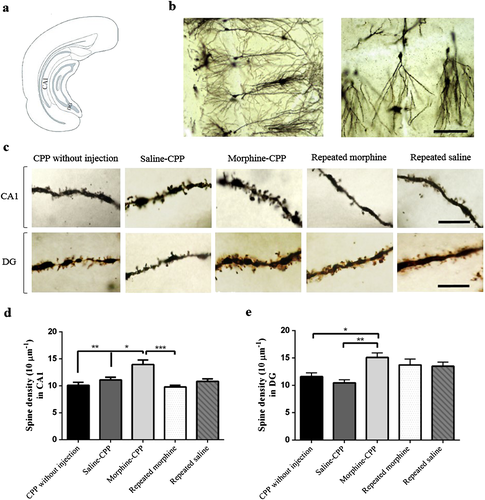
Morphine-induced conditioned place preference increases dendritic spine density in the DG granule cells
Using one-way ANOVA analysis, we compared dendritic spine density level in five experimental groups and we observed significant differences in spine density (F(4,13) = 6.05, P < 0.006; Fig 4). The morphine-CPP group displayed an increased spine density as compared with the saline-CPP (P < 0.01) and CPP without injection (P < 0.05) groups. No significant change was found in the repeated morphine and repeated saline groups timed matched with CPP compared with any other group.
Morphine-induced conditioned place preference increases BDNF and TrkB mRNA levels in the ventral hippocampus
One-way ANOVA analysis indicated significant differences in levels of BDNF mRNA between groups (F(4,15) = 16.34, P < 0.0001), generally. As shown in Fig. 5a, BDNF mRNA levels were increased in the morphine-CPP group as compared with the saline-CPP group (P < 0.05), as well as the repeated morphine-injected groups time matched with CPP (P < 0.001). In addition, BDNF mRNA levels were upregulated in the saline-CPP group as compared with the repeated saline CPP time-matched injected group (P < 0.05).
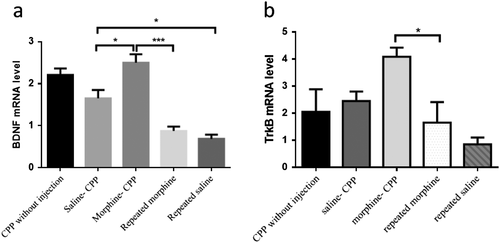
Comparing all groups revealed a significant alteration in TrkB mRNA levels (F(4,14) = 6.46, P = 0.004). TrkB mRNA levels were significantly elevated in the morphine-CPP group compared with the repeated morphine time-matched injected with CPP group (P < 0.05). There was an insignificant increase in TrkB mRNA levels of morphine-CPP group as compared with saline-CPP and CPP without injection groups (P > 0.05; Fig .5b).
Conditioned place preference scores correlate with neurogenesis and dendritic spine density markers
Using Pearson correlation analyses, we determined the correlations among CPP preference scores of the morphine-CPP, saline-CPP and CPP without injection groups and the neuroplasticity markers. Number of Ki67 and DCX-labeled cells in DG and dendritic spine density in CA1 were significantly correlated with CPP preference scores (Table 1). There were no significant correlations between CPP scores and DG dendritic spine density and BDNF and TrkB mRNA levels.
| Ki67-labeled cells | r = 0.491 | P = 0.033* |
|---|---|---|
| DCX-labeled cells | r = 0.500 | P = 0.021* |
| CA1 spine density | r = 0.665 | P = 0.025* |
| DG spine density | r = 0.426 | P = 0.167 |
| BDNF mRNA levels | r = −0.011 | P = 0.978 |
| TrkB mRNA levels | r = −0.083 | P = 0.832 |
- Preference score of individual rats exposed to the conditioned place preference test correlates positively with the number of Ki67/doublecortin (DCX)-labeled cells in dentate gyrus (DG) and dendritic spine density in CA1 of the ventral hippocampus of the same animal. R and significance levels are shown.
- * P < 0.05 is statistically significant.
In addition, we calculated the correlations among all neuroplasticity parameters. The results are presented in Table 2.
| TrkB mRNA levels with BDNF mRNA levels | r = 0.588 | P = 0.021* |
|---|---|---|
| TrkB mRNA levels with Ki67-labeled cells | r = 0.599 | P = 0.018* |
| DG spine density with Ki67-labeled cells | r = 0.555 | P = 0.049* |
| DG spine density with CA1 spine density | r = 0.725 | P = 0.005** |
| DCX-labeled cells with DG spine density | r = 0.746 | P = 0.003** |
| DCX-labeled cells with Ki67-labeled cells | r = 0.462 | P = 0.035* |
- BDNF, brain-derived neurotrophic factor; DCX, doublecortin; DG, dentate gyrus.
- * P < 0.05 is statistically significant.
- ** P < 0.01 is statistically significant.
Discussion
The results of the present investigation confirm our hypothesis and illustrate that morphine-induced CPP is associated with changes in adult hippocampal neurogenesis, spine density and BDNF/TrkB mRNA expression.
Our findings of increased adult neurogenesis following morphine-induced CPP in the ventral DG are consistent with previous studies reporting that repeated exposure to high reward conditions enhances adult neurogenesis (Glasper et al. 2012). Considering the relationship between repeated morphine (CPP time matched) injections and adulthood neurogenesis, we found that the number of newborn neurons was reduced as compared with the repeated saline-injected animals. Others showed that chronic exposure to morphine via subcutaneous pellet implantation also negatively influenced adult neurogenesis in mice (Arguello et al. 2008). This suggests that the increase in neurogenesis as we found in the morphine CPP groups is related to the morphine-induced reward-related memory, and not to the rewarding effects of morphine alone. Sensibly, the effect of exposure to a learning test during morphine administration is different from injecting morphine alone. A large number of studies have indicated that newly generated neurons in the adult hippocampus can make synaptic connections and modulate memory formation and cognitive functions (Winocur et al. 2006). A disruption in memory formation (Deng et al. 2009) as well as hippocampal dependent conditioning (Shors et al. 2001) due to a remarkable reduction in the number of adult born DG cells was reported. Other studies showed that cell proliferation in the hippocampus increased during the learning process and newly born cells differentiated to new neurons (Döbrössy et al. 2003). All together, these studies suggest that learning and memory processes could enhance adult hippocampal neurogenesis, in line with our observation. Our finding that CPP preference scores and neurogenesis are significantly correlated further support this.
We also assessed structural changes of apical dendrites of CA1 pyramidal neurons and DG granular neurons in the ventral hippocampus during morphine-induced emotional memory. The spine density of apical dendrites in the hippocampus of morphine-conditioned rats was higher than that of saline-CPP, repeated morphine without CPP treatment and CPP without injection groups. These findings are consistent with previous experiments, in which morphine-induced CPP increased dendritic number and length in the nucleus accumbens core (Kobrin et al. 2015). A wealth of experimental evidence suggests that drug-induced instrumental (response-reinforcement) learning increases spine density in the hippocampus (Robinson & Kolb 2004). The dendritic spines in the hippocampus are the post-synaptic targets for excitatory synapses, and changes in their morphology are considered as a measure of synaptic plasticity (Matsuzaki et al. 2004). A large number of studies have attempted to assess the correlation between learning and memory processes and structural changes of neurons, and they confirmed that different kinds of learning and memory tasks enhance spine density. For example, a study on structural plasticity in the CA1 area of the hippocampus demonstrated high spine density after spatial learning and eye-blink conditioning (Andersen & Trommald 1995). An increase in spine density of the piriform cortex following olfactory learning has been reported (Knafo et al. 2001). These studies were in line with our results—a significant correlation between CPP preference score and spine density in CA1—showing that morphine-induced reward-related memory is associated with increased spine density in the ventral hippocampus. When we evaluated the rewarding effect of repeated morphine administration in the absence of CPP on spine density, we found that repeated morphine increased spine density in the ventral DG and decreased it in the CA1. These changes were, however, not statistically significant. Our data are in line with previous studies showing that repeated morphine administration reduced spine density in the medial frontal cortex and the CA1 pyramidal cells and increased it in the orbital frontal cortex (Robinson et al. 2002; Robinson & Kolb 2004). These studies show that the effect of morphine on spine density does not obey a unified pattern across different parts of the brain.
Here, we show that morphine-induced CPP, as compared with saline-induced CPP and repeated morphine administration, significantly upregulates BDNF and TrkB mRNA levels in the ventral hippocampus. Consistent with our study, previous research showed that chronic morphine administration elevated BDNF mRNA levels in the cortex (Zhang et al. 2006). Investigations in rodent models in vivo showed that BDNF has an essential role in learning and memory. For example, expression of BDNF mRNA increased in the hippocampus of animals that were trained in the contextual fear conditioning tests (Hall et al. 2000). Based on these lines of evidence, we examined whether gene expression was altered in the CPP groups. We found that BDNF and TrkB mRNA levels in the CPP morphine were higher than that of the repeated morphine-injected CPP time-matched groups. It has been reported that TrkB is involved in the regulation of hippocampal neurogenesis and that lack of TrkB in the DG neurons impairs proliferation and neurogenesis (Li et al. 2008). We found here a positive correlation between TrkB mRNA levels and the number of Ki67-labeled cells, confirming that TrkB has an important role in neurogenesis. However, there was no significant correlation between the total CPP score and BDNF/TrkB mRNA levels (Table 1). A possible explanation is that, when considering Figure 5, BDNF mRNA levels were not different between the morphine-CPP and CPP without injection groups. Furthermore, TrkB mRNA levels were not different morphine-CPP and saline-CPP/CPP without injection groups. While the BDNF mRNA differences between the morphine-CPP and saline-CPP groups suggest that BDNF is implicated in drug-induced memory formation, TrkB signaling may be more strongly related to neurogenesis than to drug-induced contextual memory.
This study has some limitations that are important to mention. First, we measured BDNF and TrkB mRNA levels, but not protein levels. Because mRNA levels do not always translate 1:1 to protein levels, it is plausible that the correlation between BDNF–TrkB and neuroplasticity measures is either weaker or stronger at protein level. Second, neuroplasticity markers were assessed at one time, and therefore, the maintenance of the changes found here over time remains to be tested. Third, while we show that reward-related memory is significantly correlated with ventral hippocampal neuroplasticity markers, we do not provide causal evidence for these correlations. Interestingly, we previously demonstrated that a brain-penetrant Trk-B receptor antagonist was able to reduce cocaine self-administration behavior (Verheij et al. 2016), implying that the BDNF–TrkB pathway is a common denominator for the action of several drugs of abuse. It would be of interest to test in future studies whether this pharmacological approach prevents the occurrence of morphine-induced and other drug-induced reward-related memories and thereby could advance the treatment of drug addicts.
In conclusion, our findings support the idea that morphine-induced reward-related memory is associated with neural and synaptic plasticity changes, specifically neurogenesis and spine density, in the ventral hippocampus. Such neural changes could underlie contextual cue-induced drug relapse in morphine exposure subjects. Therefore, the hippocampus is suggested to be a relevant area for future drug-related research.
Acknowledgements
A part of this study was supported by grant from the Cognitive Science and Technologies Council, Iran (grant number: 2042). We thank Anthonieke Middelman for excellent technical assistance and Dirk Schubert who helped in Golgi staining analysis.
Authors Contribution
JH and YF designed the study. MSA and MB performed the experiments and collected the data. MSA analyzed the data and wrote the manuscript. JH and YF edited the manuscript. All authors critically reviewed the content and approved the final version for publication.




