Development of sensitization to methamphetamine in offspring prenatally exposed to morphine, methadone and buprenorphine
Abstract
Heroin use among young women of reproductive age has drawn much attention around the world. However, there is lack of information on the long-term effects of prenatal exposure to opioids on their offspring. Our previous study demonstrated that prenatally buprenorphine-exposed offspring showed a marked change in the cross-tolerance to morphine compared with other groups. In the current study, this animal model was used to study effects of methamphetamine (METH)-induced behavioral sensitization in the offspring at their adulthood. The results showed no differences in either basal or acute METH-induced locomotor activity in any of the groups of animals tested. When male offspring received METH injections of 2 mg/kg, i.p., once a day for 5 days, behavioral sensitization was induced, as determined by motor activity. Furthermore, the distance and rate of development (slope) of locomotor activity and conditioned place preference induced by METH were significantly increased in the prenatally buprenorphine-exposed animals compared with those in other groups. The dopamine D1R in the nucleus accumbens of the prenatally buprenorphine-exposed offspring had lower mRNA expression; but no significant changes in the μ-, κ-opioid, nociceptin, D2R and D3R receptors were noted. Furthermore, significant alterations were observed in the basal level of cAMP and the D1R agonist enhanced adenylyl cyclase activity in the prenatally buprenorphine-exposed group. Overall, the study demonstrates that D1R and its downregulated cAMP signals are involved in enhancing METH-induced behavioral sensitization in prenatally buprenorphine-exposed offspring. The study reveals that prenatal exposure to buprenorphine caused long-term effects on offspring and affected the dopaminergic system-related reward mechanism.
Introduction
According to the US National Survey on Drug Use and Health, 4.4% of pregnant women ages 15–44 used illicit drugs in 2009–2010 (SAMHSA 2011). This report also showed that the rate of current illicit drug use in the combined 2009–2010 data was 16.2% among pregnant women ages 15–17, 7.4% for ages 18–25 and 1.9% for ages 26–44. The US study indicates that illicit drug use during pregnancy and among younger women are urgent and important issues.
Continual use of opioids results in addiction and the development of tolerance to and dependence on these drugs. In the case of opioid abuse, children born from heroin- or morphine-addicted mothers have been known to suffer from higher mortality and problems with the central nervous system (Ostrea, Ostrea & Simpson 1997; Yanai et al. 2003), such as dysfunction in intellectual ability or in emotional control (Wilson et al. 1979; Ornoy 2003). These findings suggest that investigating effects of prenatal opioid exposure upon offspring is important.
Methadone and buprenorphine are two maintenance agents for heroin addicts. Methadone is a synthetic mu- (μ-) opioid receptor agonist and used for heroin patients (including pregnant women) on maintenance for over 30 years. It has been shown to be effective in reducing not only withdrawal symptoms but also the impulsive continuing injection of heroin (Joseph, Stancliff & Langrod 2000). However, chronic use of methadone has also led to addictive liability and respiratory depression in some subjects (Lobmaier et al. 2010). Studies by Dashe et al. (2002) have shown that maternal methadone dosage is associated with neonatal abstinence syndrome (NAS) score on the percentage of treatment for withdrawal and the duration of neonatal hospitalization. These findings indicate that methadone in high doses would not be a good therapeutic agent for pregnant women.
Recently, high doses (2–32 mg) of buprenorphine have also been used to treat opioid dependence in human. Buprenorphine is an opioid mixed agonist and antagonist; it can act on several opioid receptors as a partial agonist for the μ-opioid receptor, an antagonist for the kappa- (κ-) opioid receptor and a full/partial agonist for the nociceptin (NOP) receptor (Bloms-Funke et al. 2000; Lutfy & Cowan 2004). Buprenorphine is better than methadone for maintenance purposes because it is less addictive and less likely to produce respiratory depression (Raisch et al. 2002). In neonatal studies, buprenorphine has been shown to have more beneficial effects on mortality rate and NAS than methadone in human (Jones et al. 2010) or animals (Hutchings et al. 1995). However, some clinical and animal studies have provided different results (Robinson & Wallace 2001; Kahila et al. 2007; Chiang et al. 2010). A higher dose (3 mg/kg) of buprenorphine increased the number of stillbirths and raised the mortality index in the animal model (Robinson & Wallace 2001; Chiang et al. 2010). A clinical study also showed that 57% of newborns of mothers who received buprenorphine during pregnancy presented severe NAS and needed therapy; this was also associated with a higher number of sudden infant deaths (Kahila et al. 2007). These findings suggest that higher doses of buprenorphine may induce complex effects or serious systemic toxicities in the offspring, and that more extensive investigations are needed.
Other evidence also indicates interactions between opioidergic and dopaminergic systems (Tien & Ho 2011). Microinjection of μ-opioid receptor agonist DAMGO caused an increase in the extracellular level of dopamine (DA) and its metabolites in the ventral striatum of rats (Devine et al. 1993). Acute morphine administration also increased the level of extracellular DA in the nucleus accumbens (NAc) and the striatum of mice (Fadda et al. 2005). Blockade of the μ-opioid receptor attenuated the development of METH-induced sensitization (Chiu, Ma & Ho 2005; Shen et al. 2010), which is associated with changes in the extracellular levels of DA and its metabolites (Lan et al. 2008). However, the prenatal effects of opioids on dopaminergic system are unclear and need further investigation.
Methamphetamine (METH) is a psychostimulant that increases DA levels in the mesolimbocortical dopaminergic pathways from the ventral tegmental area (VTA) to the NAc, and is well known to associate with rewarding circuitry and sensitization (Segal & Kuczenski 1997). Chronic exposure to METH induces behavioral sensitization, which refers to the progressive enhancement of species-specific behavioral responses upon repetitive drug exposure, and provides a useful model for investigating sensitization of drug craving (Wolf 2002).
In our previous study, cross-tolerance to morphine was observed in prenatally opioid-exposed offspring. This finding may indicate that exposure to opioid during the prenatal stage causes long-term cellular changes in the central nervous system of the offspring. Behaviors of animals were also changed by re-exposure to morphine at their adulthood. In this study, prenatal effects of opioids on the dopaminergic system-related behaviors and cellular signals were evaluated in the prenatally opioid-exposed offspring using METH to perturb dopaminergic systems. For this purpose, we aimed to investigate if prenatal administration of opioids altered behavioral and cellular events induced by postnatal systemic METH administration.
Materials and Methods
Animals
Pregnant Sprague-Dawley (SD) rats (BioLASCO Taiwan Co.) and their male offspring were used in the experiments. The pregnant female rats (at E2) were shipped from animal breeding company. After arrival, the dams were acclimatized to a room with controlled temperature (25°C), humidity (50 ± 10%) and a 12-hour day–night cycle (light on 7:00 am–7:00 pm) for 24 hours before experimentation. Pregnant rats were kept individually in separate cages, and their offspring were housed 2–3 per cage after weaning. All animals were provided with food (Western Lab 7001, Orange, CA, USA) and water ad libitum. The ethical guidelines provided by Laboratory Animal Center of the National Health Research Institutes were followed throughout the study.
Drugs
Morphine (NBCD, Taipei, Taiwan), methadone (USP, Maryland, MD, USA) and buprenorphine (Sigma Aldrich, St. Louis, MO, USA) were dissolved in distilled water and were administrated subcutaneously (s.c.) in a volume of 1.0 ml/kg body weight. METH (NBCD) was dissolved in saline and was administrated intraperitoneally (i.p.) in a volume of 1.0 ml/kg body weight. Heroin is a major drug of abuse by addicts; however, it is rapidly converted to morphine after crossing the blood–brain barrier into the central nervous system. Accordingly, we used morphine directly as a test agent in this study.
Prenatal treatments
Pregnant SD female rats, 10–12 weeks old and weighing 200–250 g, were randomly assigned to different groups and were s.c. injected with opioids or vehicle during the gestational period (E3 to E20). The experiments were conducted on four groups of animals. Group 1 (vehicle control) rats received distilled water 1 ml/kg, s.c., twice a day. Group 2 (morphine) rats received morphine, 2–4 mg/kg (initial to final dose), s.c., twice a day; the dose was increased by 1 mg/kg every week. Group 3 (methadone) rats received methadone, 7 mg/kg, s.c., twice a day (5 mg/kg began at E3). Group 4 (buprenorphine) rats received buprenorphine, 3 mg/kg, s.c., once a day. The offspring were weaned at postnatal day 28; experiments were performed upon them at 8–12 weeks old with body weight between 250 and 350 g. The number of offspring per litter and the weight of offspring at birth and 8–12 weeks were not different among all the groups tested. The male offspring from the same dam were randomly assigned to different experiments to avoid the litter effect (the female offspring were not used in this study).
Locomotor activity test
To examine the locomotor activity of exploratory stage and behavioral sensitization induced by METH, all groups of rats were removed from their home cage and placed into a locomotor testing box (45 × 45 × 30 cm). Basal locomotor activities were measured for 30 minutes; then the rats received 2 mg/kg, i.p., of METH to induce hyperlocomotor activities for 2 hours. The locomotor activities were monitored and recorded (500 ms for tracing time intervals) in an acoustically insulated room by video tracer software (Trace Mouse II, SINGA, Taipei, Taiwan). Water was used to clean the inner surface of the apparatus between every test. All experiments were performed during the light phase (7:00 am–7:00 pm).
Conditioned place preference (CPP) test
The CPP apparatus used was a three-compartment acrylic plastic box. A narrower compartment (10 × 25 × 25 cm) set in the center to separate two equal-size compartments (30 × 25 × 25 cm), one with black on the four walls and floor as a visual cue, the other all in white. Subjects were first placed into the central compartment of the apparatus and given free access to the entire box for 15 minutes to measure the pre-drug preference. During the conditioning, METH (0.5 mg/kg, i.p.) was paired with the non-preferred white compartment, while the vehicle (saline) was paired with the black compartment. Animals were kept for 1 hour in the corresponding compartment with the connection doors closed. There were two drug-paired and two vehicle-paired conditioning trials before the postdrug test. The postdrug place preference was examined at day 6 and conducted for 15 minutes. Behaviors of the animal were recorded by video tracer software.
Isolation of total RNA and real-time reverse-transcription polymerase chain reaction (RT-PCR)
The NAc region was dissected from a fresh brain and frozen in liquid nitrogen. Total RNA was isolated from the NAc using the Trisure reagent (Bioline, Taunton, MA, USA) according to the manufacturer's protocol. The mRNA levels of opioid and DA receptors were measured by real-time quantitative RT-PCR using the Bio-Rad iQ5 (Bio-Rad, Philadelphia, PA, USA) sequence detection instrument. The fluorogenic probe of SYBR Green (ABI, New York, NY, USA) was used to determine the threshold cycle (Ct), which correlated inversely with the target mRNA level (cycle 6–10). The mRNA levels of the μ-, κ-opioid, NOP and DA receptors (D1R, D2R, D3R) were normalized with GAPDH mRNA. The sequences of the forward and reverse primers were designed by Primer Express 3 software (ABI).
Adenylyl cyclase activity assay
For adenylyl cyclase assay, tissues of the NAc were homogenized in a Tris-HCl buffer (in mM: Tris-HCl 50, NaCl 120, KCl 5, CaCl2 2 and MgCl2 1 containing proteinase inhibitors PMSF 0.5, p-tosyl-arginine methyl ester 1; pH 7.6). The homogenate was first centrifuged at 1000 × g in 4°C for 5 minutes. The supernatant was centrifuged again at 34 000 × g in 4°C for 30 minutes. Afterwards, the resulting pellets were resuspended in TE buffer (50 mM Tris–HCl and 1 mM CaCl2, pH 7.4). The assay was carried out in a volume of 100 μl at 30°C for 10 minutes. Duplicate membrane samples (5 μg protein) were incubated in the reaction buffer (in mM: Tris-HCl 50, MgCl2 5, ATP 2, creatine phosphate 20, 3-isobutyl-1-methylxanthine 0.5, 50 IU/ml creatine kinase and 2 mg/ml bovine serum albumin) in the presence or absence of the DA D1R agonist SKF 38393 (10, 100 nM). After incubation, the reaction was stopped by adding 200 μl of ice-cold stop buffer (50 mM Tris-HCl and 4 mM EDTA; pH 7.4). The assay mixture was boiled for 3 minutes and centrifuged at 13 750 × g for 10 minutes at 4°C. The supernatants were diluted 1:20 to apply to a Cyclic AMP EIA kit (Cayman, Ann Arbor, MI, USA) for determining the amount of cAMP produced, details as described in the manufacturer's instructions.
Data analyses and statistics
All data were analyzed using GraphPad Prism (GraphPad, La Jolla, CA, USA) software. Results were expressed as mean ± standard error of the mean (SEM). The results were tested by analysis of variance (ANOVA) with the post hoc Bonferroni's correction. A P value < 0.05 was considered significant.
Results
Effects of METH on locomotor activity and behavioral sensitization in prenatally opioid-exposed offspring
There was no significant difference observed in locomotor activities of basal or acute METH administration among prenatally vehicle-, morphine-, methadone- and buprenorphine-exposed offspring at their adult age (data not shown). Effects of chronic METH administration on behavioral sensitization in prenatally opioid exposed offspring were then assessed. As shown in Fig. 1, daily systemic METH injections induced behavioral sensitization in all groups of animals as compared with the saline control [(F(4,210) = 38.86, P < 0.0001), Vehicle-METH (F(1,70) = 103.04, P < 0.0001), M-METH (F(1,83) = 98.83, P < 0.0001), Me-METH (F(1,95) = 241.7, P < 0.0001), Bu-METH (F(1,82) = 150.38, P < 0.0001) as compared with Vehicle-Sa group]. The locomotor activity induced by METH showed significant differences in the prenatally buprenorphine-exposed group when compared with other groups tested (F(3,170) = 5.4, P < 0.001). Moreover, the buprenorphine prenatally exposed animals had a significantly higher degree of sensitization to METH than the saline, morphine and methadone prenatally treated animals [Vehicle-METH (F(1,72) = 8.65, P < 0.01), M-METH (F(1,85) = 5.42, P < 0.05), Me-METH (F(1,97) = 16.48, P < 0.0001)]. As calculated by linear regression, the slope of behavioral sensitization development to METH in prenatally vehicle-, morphine-, methadone- and buprenorphine-exposed offspring showed a significant difference (F(3, 12) = 5.42, P < 0.01) in METH-induced behavioral sensitization. A significant difference was also observed in prenatally buprenorphine-exposed rats as compared with prenatally vehicle- (F(1, 6) = 6.03, P < 0.05), morphine- (F(1, 6) = 5.9, P < 0.05) and methadone- (F(1, 6) = 7.67, P < 0.05) exposed rats. These results showed that although basal and acute METH-induced locomotor activity was not significantly different among all groups tested, prenatally buprenorphine-exposed animals developed behavioral sensitization to chronic systemic METH administration was more quickly than those in other groups.
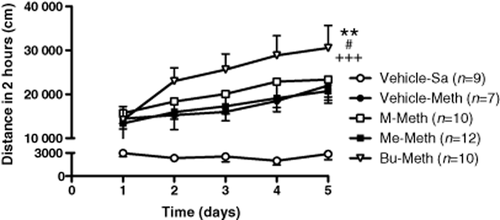
Behavioral sensitization development to METH in prenatally vehicle-, morphine-, methadone- and buprenorphine-exposed offspring. Animals were injected METH, 2 mg/kg, i.p., and placed in a locomotor test box to measure locomotor activities. The X-Y plot graphs illustrate the cumulative distances of locomotor activity for a 2-hour observation period. All data are expressed as mean ± SEM (n = 7–12 per group). **P < 0.01 as compared with the prenatally vehicle-exposed group, #P < 0.05 as compared with the prenatally morphine-exposed group and +++P < 0.001 as compared with the prenatally methadone-exposed group with methamphetamine-treatment (two-way ANOVA)
Effects of low dose of METH on CPP in prenatally opioid-exposed offspring
Prenatal exposure to opioids did not change the basal place preference of rats (saline/saline) (Fig. 2). However, in the case of two conditioned pairs with a lower dose (0.5 mg/kg) of METH, prenatally buprenorphine-exposed offspring showed significant changes in METH-induced CPP as compared with the no-drug (saline) treated prenatally buprenorphine-exposed group and the METH treated prenatally vehicle-, morphine- and methadone-exposed groups. These results indicate that prenatally buprenorphine-exposed offspring exhibited more sensitivity to a lower dose of METH in the CPP test.
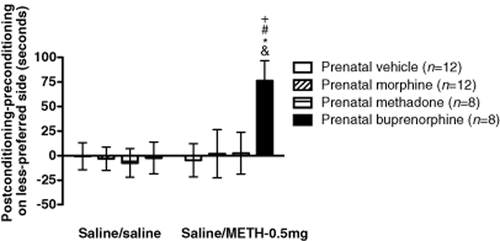
Effects of METH on CPP in prenatally vehicle-, morphine-, methadone- and buprenorphine-exposed offspring. Time scores show differences between post-conditioning and preconditioning time spent in the saline or 0.5 mg/kg METH-paired environment in the prenatally buprenorphine-exposed group. All data are expressed as mean ± SEM (n = 8–12 per group). *P < 0.05 as compared with the METH-treated prenatally vehicle-exposed group. #P < 0.05 as compared with the METH-treated prenatally morphine-exposed group, +P < 0.05 as compared with the METH-treated prenatally methadone-exposed group and P < 0.05 as compared with the prenatally buprenorphine-exposed control group (one-way ANOVA)
Effects of prenatal exposure to opioids on the mRNA levels of opioid and DA receptors in the NAc
To determine if the transcription levels of opioid receptors were affected by prenatal exposure to opioids, the mRNA levels of the μ-, κ-opioid and NOP receptors in the NAc were measured in all groups of animals tested. Results from real-time quantitative PCR analysis showed no significant difference in the mRNA levels of the μ-, κ-opioid and NOP receptors in the NAc of prenatally opioid-exposed rats as compared with the control (data not shown). However, results showed a significant 23% decrease (P < 0.05) in mRNA levels of D1R in the NAc of prenatally buprenorphine-exposed rats as compared with the control group (Fig. 3a), but no difference in D2R (Fig. 3b) or D3R (Fig. 3c) was observed in any group of animals tested. These results demonstrate that the mRNA expression of the μ-, κ-opioid and NOP receptors in the NAc was not significantly changed by prenatal exposure to morphine, methadone or buprenorphine. However, the dopaminergic system, especially the mRNA levels of D1R, was affected by prenatal exposure to a higher dose of buprenorphine.
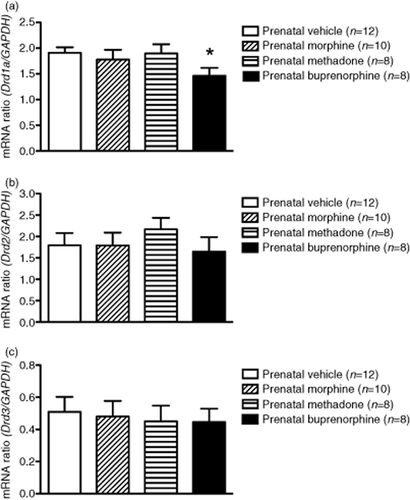
The mRNA levels of dopamine receptor expressions in the NAc of rats prenatally exposed to vehicle, morphine, methadone or buprenorphine. Amounts of mRNA were measured by real-time quantitative RT-PCR using SYBR Green as a probe and adjusted with GAPDH mRNA from the same sample. The mRNA levels of (a) D1R, (b) D2R, and (c) D3R expressed in the NAc. Data are expressed as mean ± SEM (n = 8–12 per group). *P < 0.05 as compared with the prenatally vehicle-exposed group (one-way ANOVA)
Effects of prenatal exposure to opioids on the cAMP levels in the NAc
Because of the finding that D1R receptor mRNA was altered in the NAc of rats prenatally exposed to buprenorphine, the basal levels of cAMP in the NAc were examined. Results obtained showed that adenylyl cyclase activity was decreased by 23.9% (P < 0.05) in prenatally buprenorphine-exposed rats, but no difference was observed in the prenatally morphine- or methadone-exposed groups (Fig. 4). This result implicates that prenatal exposure to a higher dose of buprenorphine not only decreased DA D1R mRNA expression but also reduced the downstream cAMP production at the adulthood of the offspring.
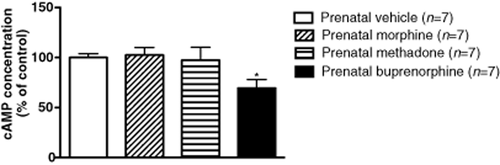
Basal levels of cAMP in the NAc of rats prenatally exposed to vehicle, morphine, methadone and buprenorphine. Amounts of cAMP were measured by cAMP ELISA kit for testing the basal adenylyl cyclase activities of the NAc. The data were adjusted to the prenatally vehicle-exposed group (control group). Data are expressed as mean ± SEM (n = 7 per group). *P < 0.05 as compared with prenatally vehicle-exposed group (one-way ANOVA)
Effects of D1R agonist SKF 38393 on the cAMP levels in the NAc of prenatally opioid-exposed offspring
In order to test the hypothesis that METH-induced behavioral sensitization is due to altered sensitivity of D1R-mediated signaling in the NAc, effects of D1R agonist SKF 38393 on the level of cAMP was conducted. As shown in Fig. 5, SKF 38393 (10 and 100 nM) significantly enhanced adenylyl cyclase activity in the prenatally buprenorphine-exposed group as compared with the groups prenatally exposed to vehicle, morphine and methadone. This suggests that D1R-mediated cAMP production was more sensitive in the prenatally buprenorphine-exposed group when the D1R was activated.
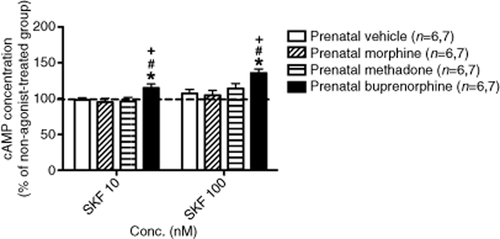
Effect of dopamine D1R agonist, SKF 38393, on the levels of cAMP accumulation in the NAc of rats prenatally exposed to vehicle, morphine, methadone and buprenorphine. The data of each group were adjusted with the basal (non-agonist treated) group. Data are expressed as mean ± SEM. (Numbers of samples in the 10 nM and 100 nM are 6 and 7, respectively). *P < 0.05 as compared with the prenatally vehicle-exposed group, #P < 0.05 as compared to the prenatally morphine-exposed group, +P < 0.05 as compared with the prenatally methadone-exposed group (one-way ANOVA)
Discussion
The goal of this study is to evaluate effects of prenatal exposure to morphine, methadone and buprenorphine on the offspring when they were exposed to METH at adulthood. Prenatal exposure to opioids did not affect the basal locomotor activities in offspring at their adulthood. This suggests that the neuroadaption occurred after chronic prenatal exposure to opioids; therefore, behavioral outcomes appeared to be similar to controls. However, a significant increase in METH-induced behavioral sensitization (the distance and rate of development), as assessed by motor activity, was observed in the prenatally buprenorphine-exposed offspring as compared with other groups. A marked and significant effect on the CPP, which was induced by low-dose (0.5 mg/kg) and short-term (two times) METH administration, was also observed in the prenatally buprenorphine-exposed offspring. The expression of DA D1R and its downstream cAMP signaling were reduced by prenatal exposure to buprenorphine. The significant increase in D1R-mediated cAMP production was also observed in the prenatally buprenorphine-exposed group. These studies demonstrate that prenatal administration of buprenorphine brought about a significant cellular change in the dopaminergic system and altered the METH-induced behavioral sensitization and CPP. The possible mechanisms may be due to changes in the expression and sensitivity of DA D1R and its downstream cAMP signaling in the NAc.
Early study has shown that rats prenatally exposed to morphine (5–10 mg/kg/twice a day) during embryonic 11–18 period (E11–18), the levels and turnover of norepinephrine (NE) were decreased and increased, respectively, dependent on the brain regions and gender. However, no changes were observed in basal levels or in the turnover of DA in any brain regions (hypothalamus, frontal cortex, striatum and cerebellum) tested (Vathy et al. 1994). In both adult male and female rats receiving morphine 5–10 mg/kg prenatally, the lever-pressing behavior for cocaine reward was not altered (Vathy, Slamberova & Liu 2007). The METH-induced behavioral sensitization and CPP were also not changed in male rats prenatally exposed to morphine (2–4 mg/kg) in this study. These findings suggest that dopaminergic-related signals, which were activated by postnatal treatment with cocaine or METH, did not differ in rats prenatally exposed to morphine or vehicle at their adult age.
It has been reported that the levels of DA and NE in the forebrain significantly decreased in prenatally methadone-exposed (1–6 mg/kg) offspring, when they were compared with other tested groups at postnatal days 1 and 20 (McGinty & Ford 1980). However, the level of NE had returned to normal on day 40, while the contents of DA remained significantly lower in the stratum. These data demonstrate that prenatal methadone exposure may retard catecholaminergic axonal growth in the forebrain of rats. However, in prenatally methadone-exposed offspring, no basal or METH-induced behavioral differences with their control mates were observed in this study. The possible explanations may be due to that the level of DA was not different from that of the control at 8–12 weeks because of the whole striatum buffered the changes in the NAc or by an unknown compensative mechanism.
The present study showed that notable changes in METH-induced behaviors and the dopaminergic system were observed only in the prenatally buprenorphine-exposed group, but not in morphine or methadone prenatally exposed offspring. Results obtained from biochemical studies also indicate that there are no differences in the expression of the DA receptor, its activity or its downstream cAMP signaling in morphine or methadone prenatally treated animals. A possible explanation for these findings is the pharmacological difference of buprenorphine from morphine and methadone. Both morphine and methadone are pure μ-opioid receptor agonists, while buprenorphine is a partial μ-opioid and full/partial NOP receptor agonist, as well as a κ-opioid receptor antagonist. More complex receptor–receptor interactions may occur during prenatal exposure to buprenorphine than to expose to morphine or methadone. Prenatal exposure to buprenorphine has been reported to transient change in the expression of opioid receptors. Prenatal exposure to buprenorphine (0.5–1 mg/kg) caused a reduction in the expression of μ-opioid receptors and an increase in the expression of κ-opioid receptors in the brain of postnatal day 1 (P1) offspring, but returned to normal at P7 (Belcheva et al. 1994). Our study also showed that the mRNA levels of the μ-, κ-opioid and NOP receptors did not differ in the NAc of adult offspring in any of the groups measured. However, activation of the NOP receptor has been reported to alter the dopaminergic system. The in vivo study has shown that activation of the NOP receptor, by treating it with N/OFQ, inhibited the release of DA in the striatum (Flau et al. 2002). N/OFQ also inhibited tyrosine hydroxylase, DA synthesis, D1R-mediated cAMP formation, and N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor phosphorylation in the NAc slices and primary cells by the activation of presynaptic and postsynaptic NOP receptor (Olianas et al. 2008). Additionally, intracerebral ventricular (i.c.v.) administration of N/OFQ-induced locomotor activity could be reversed by DA D1R antagonist (Florin et al. 1996). The NOP receptor has also been reported to be expressed early at embryonic 12 (E12) in the cortical plate, basal forebrain, brainstem and spinal cord (Neal, Akil & Watson 2001). These findings may imply that the NOP receptor is involved in regulating the dopaminergic system in prenatal stages. However, there still lacks the study of effects of buprenorphine on the release of catecholamines and glutamate in the NAc of the offspring prenatally exposed to buprenorphine. According to the literatures described above, prenatal exposure to morphine or methadone reduced the level of DA in the striatum and NAc, and activation of NOP receptor also decreased the level of DA. This indicates that prenatal buprenorphine may cause a synergistic reducing effect on the release of DA by both activation of μ-opioid and NOP receptor. In the study of Parkinson's disease, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice lacked presynaptic DA, but increased postsynaptic DA receptor sensitivity to apomorphine (Spooren et al. 1998). This finding may explain the mechanism of the development of D1R supersensitivity in the prenatally buprenorphine-exposed offspring, which may due to prolonged presynaptic loss of the neurotransmitter. More detailed study on effects of signals mediated by the activation of μ-opioid and NOP receptors on the dopaminergic and glutamatergic systems, especially the level of neurotransmitters, during prenatal exposure to buprenorphine is warranted.
Another possible mechanism for the METH-induced alterations in the prenatally buprenorphine-exposed offspring may be the dose effect of opioids on pregnant rats. In our study, the final doses of opioids used were 4 mg/kg morphine, 7 mg/kg methadone and 3 mg/kg buprenorphine, respectively. The limitation of this study was to use a single higher dose of buprenorphine, which almost the highest dose for human maintenance (∼32 mg for 70 kg human, converted based on body surface areas). The higher dose of buprenorphine would activate both μ-opioid and NOP receptors. Whether low dose of buprenorphine, which only activates μ-opioid receptor, causes similar effects like morphine or methadone, require further studies. Although, the dose of buprenorphine used was higher than the other drugs tested, high dose of buprenorphine may not cause more μ-opioid receptor-induced actions due to its partial agonistic properties, but the NOP-mediated signalings were also activated at this time. According to the pharmacologic profile and antinociceptive studies, both morphine and methadone are full μ-opioid receptor agonists. Additionally, compared with morphine, methadone showed 1–2.5-fold greater efficacy of antinociception (s.c. injection) in rats (He et al. 2009; Taracha et al. 2009). Most recently, a study showed a negative genetic correlation between METH consumption and sensitivity to the opioid-induced effects in the selectively bred mouse lines with different level of METH intake (Eastwood & Phillips 2012). This study indicates that the μ-opioid receptor system is involved in the intake of METH. Although the dose of methadone used in this study was also higher than that of morphine, there was no significant dosing effect on the level of μ-opioid receptor and METH-induced behavioral changes between these two groups. This may imply that significant effects of METH on behaviors and cellular signaling in prenatally buprenorphine-exposed offspring than other groups may not only be due to the direct agonistic effects on μ-opioid receptor in pregnant rats; a NOP receptor-mediated mechanism may also be involved.
Previous studies showed that rats prenatally exposed to buprenorphine exhibited more antinociceptive resistance and tolerance development to morphine in 4-day postnatal pups (Robinson & Wallace 2001) and adult pups (Chiang et al. 2010), but failed to obtain changes in the threshold of pain sensitive and acute morphine-induced antinociception. Robinson & Wallace (2001) also found that morphine ED25 values were significantly increased in pups prenatally exposed to buprenorphine, as compared with prenatal methadone after morphine challenge. Pups exposed to buprenorphine either prenatally, postnatally or both pre- and postnatally were more resistant to the antinociceptive response to morphine. These findings suggest that buprenorphine appears to have a greater ability than methadone to induce tolerance to morphine. Additionally, our previous findings provided direct evidence to indicate that prenatal exposure to buprenorphine caused faster development of tolerance to morphine than prenatally saline-, morphine- and methadone-exposed rats at adulthood (Chiang et al. 2010). Given the results of previous findings and the current study, it is suggested that prenatal exposure to buprenorphine does not alter the basal behavioral outcomes of offspring, which may be due to the adaptation of homeostasis. However, the balances are disrupted after re-exposure to the opioid or METH at the offspring's adulthood.
It is well known that the DA D1R is involved in the development of METH-induced behavioral sensitization in the induction and expression stages. Early studies have shown that systemic or intra-VTA administration of D1R antagonist during a period of repeated amphetamine (AMPH) treatment blocked the induction of behavioral sensitization (Bjijou et al. 1996; Vezina 1996). In addition, repeated intra-VTA administration of D1R agonist SKF 38393 produced behavioral sensitization to AMPH and cocaine (Pierce et al. 1996). Recently, a study of two-injection protocol for the induction of sensitization, which is a simple animal model to explore the sensitization mechanisms, demonstrated that the D1R, NMDA receptor and cAMP-dependent pathway were required for induction stage of psychostimulants-induced sensitization (Valjent et al. 2010). Although, a study showed that locomotor-activating effects and sensitization of AMPH were reduced, but not fully blocked, in D1R-deficient mice (Xu et al. 2000), sensitized behavioral responses to D1R agonists in psychostimulant pre-treated animals have not been observed following either systemic or intra-NAc administration (Vanderschuren & Kalivas 2000). These findings suggested that D1R and its mediated signaling are essential for the induction of abuse drug-induced sensitization but may not be for the expression stage. Beutler et al. (2011) showed that the NMDA receptor, which was removed by the conditional knockout method on the D1R-expressed medium spiny neurons, significantly attenuated AMPH-induced behavioral sensitization in mice. This indicates that METH-induced behavioral sensitization may do so through altering other relevant receptors (such as NMDA), enzymes (such as DARPP-32) or genes (such as Fos) in the NAc (Nestler 2004; Chen, Chen & Chiang 2009). Exposure to METH, cocaine or morphine upregulated the cAMP-protein kinase A signaling pathway in the NAc, which also contributed to the behavioral changes induced by cocaine and METH (Self 2004; Chiang & Chen 2007). In the current study, the D1R-mediated cAMP signaling showed more sensitive to D1R agonist in the prenatally buprenorphine-exposed group than other groups tested. The increased sensitivity to METH in the prenatally buprenorphine-exposed offspring may be due to the D1R-mediated cAMP pathway.
The balance of the DA D1R and D2-like (D2R and D3R) receptors also plays a critical role in the development of addiction (Vanderschuren & Kalivas 2000; Chen et al. 2009). The anatomical and physiological evidence showed that the D1R and D2R are co-localized in the same neurons of the NAc but influence different downstream brain areas (Self 2004). The D2-like receptor is believed to function in a manner opposite of D1R for development of addiction in behaviors and signaling (Self 2004; Chen et al. 2009; Zhang et al. 2012). The level of D1R but not D2-like (D2R and D3R) receptor was altered by prenatal exposure to buprenorphine in this study. Park et al. (2001) has shown that the mRNA level of D1R increased more than that of D2R in the NAc of μ-opioid receptor knockout mice. This may indicate that the expression of D1R was more sensitive to the changes of opioid system and also provide a possible mechanism to explain why the expression of D1R, not D2R, was significantly altered in this study.
In summary, we compared three kinds of opioids in METH-induced behavioral changes to verify effects of prenatal exposure to opioids on the dopaminergic system of the offspring at their adulthood. Although buprenorphine is considered a newer and safer therapeutic agent than methadone for treating heroin addicts, the current study shows that prenatal exposure to buprenorphine can induce faster behavioral sensitization and CPP development to METH. This study further provides evidence to show that prenatal effects of buprenorphine in the dopaminergic system exhibit long-term changes in the expression and sensitivity of DA D1R and its downstream cAMP signaling in the NAc, even at the adulthood. This cellular evidence provides a possible mechanism for the marked changes of METH-induced behaviors in prenatally buprenorphine-exposed offspring. Several previous studies have suggested that changes of opioid receptors in prenatal exposure to opioids may be the primary factor in behaviors. Although differences in the mRNA levels of the μ-, κ-opioid and NOP receptors were not observed in the NAc in any of the groups tested, details of the changes in the opioid receptors in other brain areas—especially in the roles of the NOP receptor for higher dose buprenorphine administration during pregnancy—are still unclear. For this reason, we intend to investigate the roles of the NOP receptor in the NAc of rats in prenatal buprenorphine exposure. Finally, prenatal exposure to buprenorphine caused more notable effects on METH-induced behaviors and the dopaminergic system than the other opioids tested in the current study. This raises the question of whether higher buprenorphine is an ideal maintenance medication for treating pregnant women. This animal study could provide an important reference for clinical usage of buprenorphine in treating pregnant women who are heroin addicts.
Acknowledgements
We thank Dr. M. Swofford for English editing before submission of the paper. The work was supported by the National Health Research Institutes (NHRI-102A1-PDCO-1312141 and NHRI-EX102-10224NC) and the China Medical University Hospital (DMR-101–117) in Taiwan. The authors declare no conflict of interest to report.
Authors Contribution
YCC designed and performed the experiments, analyzed the data, and drafted the manuscript. TWH co-performed the experiments. YCC and IKH conceived the study and revised the final manuscript. All authors have critically reviewed the content and approved the final version for publication.




