GDNF is a novel ethanol-responsive gene in the VTA: implications for the development and persistence of excessive drinking
Abstract
Glial cell line-derived neurotrophic factor (GDNF) is a potent inhibitor of ethanol consumption and relapse, and GDNF heterozygous knockout mice display increased reward sensitivity to ethanol and consume more ethanol after a period of abstinence than their wild-type littermates. Here, we tested whether ethanol alters GDNF expression in the ventral tegmental area (VTA; GDNF's site of action) and/or the nucleus accumbens (NAc; the main source of GDNF), and if so, determine the role of the endogenous growth factor in the regulation of ethanol consumption. Systemic administration of ethanol increased GDNF expression and protein levels in the VTA, but not the NAc. Additionally, GDNF levels were elevated after an ethanol-drinking session in rats that consumed ethanol in the intermittent-access two-bottle choice procedure for 1 week, but not 7 weeks. Deprivation following 7 weeks of excessive ethanol intake reduced GDNF levels, while a short ethanol binge drinking period following deprivation upregulated GDNF expression. Importantly, knockdown of GDNF within the VTA using adenovirus expressing short hairpin RNA facilitated the escalation of ethanol drinking by ethanol-naïve rats, but not by rats with a history of excessive ethanol consumption. These results suggest that during initial ethanol-drinking experiences, GDNF in the VTA is increased and protects against the development of excessive ethanol intake. However, the growth factor's protective response to ethanol breaks down after protracted excessive ethanol intake and withdrawal, resulting in persistent, excessive ethanol consumption.
Introduction
Glial cell line-derived neurotrophic factor (GDNF) is a secreted growth factor, essential for the development, differentiation, proliferation and function of midbrain dopaminergic neurons (Airaksinen & Saarma 2002). The GDNF signaling pathway is initiated by the binding of GDNF to its co-receptor, GDNF family receptor-α 1, which then recruits and promotes the activation of the Ret receptor tyrosine kinase, leading to increases in gene expression as well as acute non-genomic events, such as increasing the firing activity of dopaminergic neurons (Yang et al. 2001; Wang et al. 2010). GDNF in the adult brain is highly expressed in the nucleus accumbens (NAc; Barroso-Chinea et al. 2005), while Ret is largely restricted to the ventral tegmental area (VTA; Treanor et al. 1996; Trupp et al. 1997). GDNF synthesized in the NAc is retrogradely transported to the VTA, where it binds to its receptors and activates its signaling pathway (Wang et al. 2010). The dopaminergic projection from the VTA to the NAc is a major component of the mesolimbic dopamine system, a main target of drugs of abuse, including ethanol (Nestler 2001).
Several studies documented the inhibitory effect of GDNF on the rewarding properties of various drugs of abuse (Reviewed in: Carnicella & Ron 2009; Ghitza et al. 2010). GDNF is also a potent negative regulator of ethanol intake. Specifically, we previously showed that infusion of recombinant GDNF into the rat VTA rapidly attenuates operant self-administration of ethanol, ethanol-seeking behaviors and relapse (Carnicella et al. 2008), and blocks excessive ethanol intake in the intermittent-access two-bottle choice paradigm (Carnicella, Amamoto & Ron 2009c). More recently, we found that intra-VTA infusion of recombinant GDNF reverses the ethanol withdrawal-associated allostatic deficiency of the mesolimbic system by restoring dopamine levels to normal in the NAc (Barak et al. 2011b). The inhibitory effect of GDNF on ethanol consumption is also long lasting owing to the ability of the growth factor to upregulate its own expression (Barak et al. 2011a). Additionally, we found that GDNF heterozygous knockout mice display increased conditioned place preference for ethanol, and consume more ethanol than their wild-type littermates following a period of abstinence (Carnicella et al. 2009b). These latter results imply that endogenous GDNF plays an important role in the regulation of ethanol drinking. Thus, we asked whether endogenous GDNF expression levels in the mesolimbic system are altered by cycles of excessive ethanol intake and withdrawal, and if so, what role does the growth factor plays in the regulation of ethanol consumption.
Materials and Methods
Reagents
TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). Deoxyribonuclease (DNAse) and ethidium bromide were purchased from Sigma (St. Louis, MO, USA). The Reverse Transcription System and 2X PCR master mix were purchased from Promega (Madison, WI, USA). The Taqman gene expression 2X master mix and the primer/probe kits were purchased from Applied Biosystems, Inc. (Foster City, CA, USA). The protease inhibitor mini tablets were purchased from Roche (Indianapolis, IN, USA). The BCA protein assay kit was purchased from Pierce Biotechnology (Rockford, IL, USA). Pre-cast NuPAGE 4–12% Bis-Tris gels were obtained from Invitrogen. The anti-GDNF antibodies were obtained from R&D Systems (Minneapolis, MN, USA). Antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The enhanced chemiluminescence (ECL) detection reagents were purchased from Fisher Scientific (Pittsburgh, PA, USA). The pRNAT-H1.1/Shuttle vector was obtained from the GenScript Corporation (Piscataway, NJ, USA). The Adeno-X vector, Expression System and Purification and Rapid Titer kits were purchased from Clontech (Mountain View, CA, USA).
Animals
Adult male Long-Evans rats (250–300 g at the beginning of experiments) were purchased from Harlan (Indianapolis, IN). Animals were individually housed with food and water available ad libitum, constant temperature (23°C) and humidity (50%), and a 12-hour light-dark cycle (lights on at 7:00 am). All experimental protocols were approved by the Ernest Gallo Research Center Institutional Animal Care and Use Committee, and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996.
Intermittent access 20% ethanol two-bottle choice
Following a 1-week habituation, rats underwent an intermittent-access two-bottle choice drinking paradigm as previously described (Carnicella et al. 2009c). Briefly, rats were given a bottle containing an ethanol solution (20% v/v in tap water) every other day for a 24-hour period. Control subjects were given an additional bottle of water in place of the ethanol solution. Rats were killed and the VTA and NAc were collected 30 minutes after the beginning (binge drinking), immediately following or 24 hours after (deprivation) the end of the last ethanol-drinking session as noted.
Reverse Transcription and quantitative Real-Time (qRT) Polymerase Chain Reaction (PCR)
Following dissection, tissue samples were immediately snap-frozen in liquid nitrogen and stored at −80°C until use. Frozen tissues were mechanically homogenized in TRIzol reagent and total RNA was isolated from each sample according to the manufacturer's recommended protocol. Total RNA was treated with DNAse. Messenger RNA (mRNA) was selectively reverse transcribed using the Reverse Transcription System and with oligo(dT) primers. PCRs for GDNF and the housekeeping gene, GAPDH, were conducted with the resulting complementary DNA (cDNA) and the following primers: GDNF upstream: 5′- GAC GTC ATG GAT TTT ATT CAA GCC ACC -3′; GDNF downstream: 5′- CTG GCC TAC TTT GTC ACT TGT TAG CCT -3′; GAPDH upstream: 5′- TGA AGG TCG GTG TCA ACG GAT TTG GC -3′; GAPDH downstream: 5′- CAT GTA GGC CAT GAG GTC CAC CAC -3′. For GDNF, 33–35 amplification cycles were used, while 27 cycles were used for GAPDH. PCR products were resolved on a 1.8% agarose gel containing 0.05% ethidium bromide for visualization under ultraviolet light. Images were captured using Eagle Eye 2 software. Band intensities were quantified using NIH ImageJ software. For the qRT-PCR, cDNA samples were analyzed in triplicate on a 384-well plate using the Taqman gene expression 2X master mix, and the following rat-specific gene expression primer/probe kits: GDNF, Rn00569510_m1 and GAPDH, Rn99999916_s1. Relative GDNF and GAPDH mRNA concentrations were determined based on a standard curve constructed using triplicate Ct readings of serial dilutions of whole striatal cDNA, which was prepared in parallel with the samples to be tested.
Western blot analysis
VTAs were immediately snap-frozen in liquid nitrogen until the time of analysis. Frozen tissues were mechanically homogenized in ice-cold radio-immunoprecipitation assay buffer containing 50 mM Tris-HCl, pH 7.4, 5 mM ethylenediaminetetraacetic acid, 120 mM NaCl, 1% NP-40, 0.1% deoxycholate, 0.5% sodium dodecyl sulfate and the protease inhibitor mini tablet. After homogenization, samples were briefly sonicated and placed on ice for 30 minutes. Protein concentrations were determined using the BCA protein assay kit. Samples (25 μg) were resolved on NuPAGE 4–12% Bis-Tris gels, and transferred onto nitrocellulose membranes for analysis. Primary antibody concentrations used were 1:250 (GDNF) and 1:5000 (GAPDH). HRP-conjugated secondary antibodies were used at a concentration of 1:1000. Immunoreactivity was detected via an ECL reaction, and developed on Kodak film. The film was digitally scanned for relative densitometric quantification using NIH ImageJ software.
Adenoviral-mediated small hairpin (shRNA) downregulation of GDNF
The construction, preparation and quantification of GDNF knockdown using a recombinant adenovirus expressing shRNA targeting GDNF (AdV-shGDNF), as well as the construction and preparation of a recombinant adenovirus expressing a scrambled RNA sequence, which was used as a control (AdV-SCR), are described in detail in Wang et al. (2010). Intra-VTA infusion of the viruses (1.3 × 108 TU/ml) is detailed in Barak et al. (2011a). The intermittent access to 20% ethanol two-bottle choice paradigm started 10 days after the infusion of the viruses. This time point was chosen based on our previous results demonstrating significant shRNA-mediated knockdown of GDNF in the mesolimbic system beginning between days 7 and 14 post-virus infusion (Wang et al. 2010; Barak et al. 2011a).
Statistical Analysis
For gene expression analysis, statistical significance was determined by Student's t-test or one-way analysis of variance (ANOVA), with post hoc Student-Newman–Keuls test. Ethanol consumption was analyzed in a mixed model two-way ANOVA with a between-subjects factor of virus infection and a repeated measurement factor of day post-infection, followed by comparisons using the method of contrasts.
Results
Acute, systemic administration of ethanol increases GDNF levels in the VTA, but not the NAc
First, we tested whether a single exposure of rats to ethanol alters the expression of GDNF in either the VTA and/or the NAc. Rats were given a single intraperitoneal (i.p.) administration of a non-hypnotic dose of ethanol (1.8 g/kg, in a 20% v/v ethanol solution) and the VTA and NAc were collected 0.5, 2, 4, 10 or 24 hours later. We observed a significant upregulation of GDNF mRNA in the VTA 10 hours after the single administration of ethanol as compared with the saline-treated control group (Fig. 1a). No alteration in the expression of GDNF in the NAc was found at any of the time points tested (Fig. 1b), suggesting that ethanol-mediated effects on GDNF expression in the mesolimbic VTA-NAc circuit are restricted to the VTA. We next set out to determine whether the increase in GDNF mRNA levels in the VTA corresponds to a functional increase in the protein levels of the growth factor. To do so, rats were administered a single 1.8 g/kg dose of ethanol, and 10 hours later, the GDNF protein levels in the VTA were assessed. As shown in Fig. 1c, we found a significant increase in the level of the growth factor in the VTA of rats that received an i.p. administration of ethanol, as compared with the saline-injected controls. Together, these findings indicate that ethanol exposure causes a functional upregulation of the GDNF gene in the VTA.
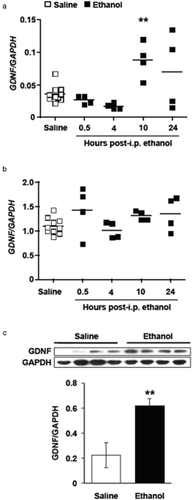
Systemic administration of ethanol increases glial cell line-derived neurotrophic factor (GDNF) expression in the ventral tegmental area (VTA). Rats were injected intraperitoneally (i.p.) with 1.8 g/kg ethanol (20% v/v solution; black squares) or saline (white squares). The VTA and nucleus accumbens (NAc) were collected 0.5, 4, 10 and 24 hours after the injection. GDNF and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) expression in the VTA (a) and the NAc (b) were measured by quantitative real-time polymerase chain reaction. Each square represents the GDNF/GAPDH ratio of an individual subject, with the group mean depicted by a bar. n = 10 animals for the saline group and four for each ethanol time point. ** P < 0.01, as compared with the saline group. (c) GDNF protein levels in the VTA were assessed by Western blot 10 hours after a single administration of 1.8 g/kg ethanol. Data are expressed as the mean GDNF/GAPDH ± SEM, n = 4 per group. **P < 0.01, as compared with the water control group
GDNF expression in the VTA is altered by voluntary excessive ethanol consumption
We next tested whether the level of GDNF expression is altered following voluntary ethanol drinking. We utilized the intermittent access to 20% ethanol two-bottle choice procedure to obtain high levels of voluntary ethanol drinking in rats. This procedure, first developed by Wise (1973), has been recently extensively used as it reliably results in a rapid escalation of excessive ethanol consumption (Simms et al. 2008; Carnicella et al. 2009c). In this paradigm, rats had intermittent access to a 20% v/v ethanol solution, in addition to water, for 1 week (total of four ethanol-drinking sessions). Control rats consumed only water. The average ethanol consumption was 4.03 ± 0.61 g/kg during the last 24-hour ethanol-drinking session (Table 1), and the VTA and NAc were collected immediately after the end of the session. We found that GDNF levels in the VTA were significantly upregulated in rats consuming ethanol for 1 week, as compared with water-drinking controls (Fig. 2a), whereas GDNF levels in the NAc were unaffected by ethanol intake (Fig. 2b). We next asked whether long-term, excessive consumption of ethanol would continue to alter GDNF expression. Rats were trained to voluntarily consume excessive amounts of ethanol in the intermittent-access two-bottle choice paradigm for a period of 7 weeks, achieving a stable baseline range averaging 5.48 ± 0.88 g/kg of ethanol consumed over 24 hours (Table 1), and the VTA and NAc were collected immediately after the last drinking session. We observed that the levels of GDNF in both the VTA and NAc of subjects that consumed ethanol for 7 weeks did not differ from those in the water-only controls (Fig. 2a, b). These results indicate that voluntary ethanol consumption leads to fluctuations in the level of GDNF expression in the VTA.
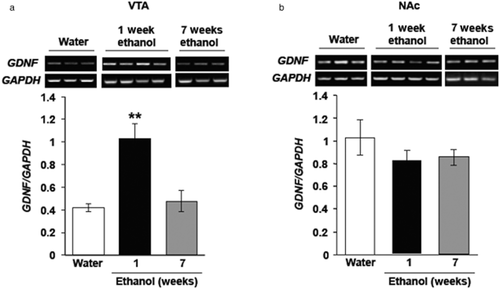
Glial cell line-derived neurotrophic factor (GDNF) is increased in the ventral tegmental area (VTA), but not nucleus accumbens (NAc), following a short training period of ethanol intake but not after a long period of intermittent excessive ethanol consumption. Rats consumed ethanol in the intermittent access to 20% ethanol two-bottle choice paradigm for 1 week (black bars) or 7 weeks (grey bars). Controls consumed water only for 7 weeks (white bars). The VTA and NAc were collected immediately following the end of the last ethanol-drinking session. GDNF (upper panels in gel images) and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) (lower panels in gel images) expression in the VTA (a) and the NAc (b) were determined by quantitative real-time polymerase chain reaction. Bar graphs represent the mean GDNF/GAPDH ratio ± SEM, n = 7–8 per group. ** P < 0.01, as compared with water control
| Drinking history | Ethanol intake during last drinking session (Mean ± SEM) |
|---|---|
| 1 week ethanol | 4.03 ± 0.61 g/kg/24 hours |
| 7 week ethanol | 5.48 ± 0.88 g/kg/24 hours |
| 7 week ethanol + ‘binge’ (excessive drinkers) | 1.28 ± 0.24 g/kg/30 minutes |
| 7 week ethanol + ‘binge’ (low drinkers) | 0.20 ± 0.10 g/kg/30 minutes |
Next, we determined whether GDNF expression is altered during deprivation from ethanol. In order to test this possibility, rats consumed 20% ethanol or water only, for 7 weeks as described above, and the level of GDNF mRNA in the VTA and NAc were measured 24 hours after the end of the last ethanol-drinking session. Interestingly, we found that GDNF mRNA in the VTA of ethanol-deprived rats was markedly reduced below the baseline level of the growth factor's expression that was measured in the rats consuming only water (Fig. 3a). However, GDNF expression in the NAc of the ethanol-deprived rats was similar to those found in the rats that consumed only water (Fig. 3b). These findings suggest that, after a long history of high levels of ethanol intake, GDNF expression in the VTA is abnormally low during deprivation, a period of time during which ethanol is neither systemically present nor available for consumption.
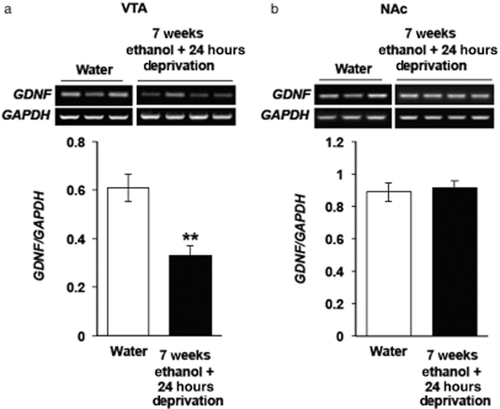
Glial cell line-derived neurotrophic factor (GDNF) is reduced in the ventral tegmental area (VTA) after a 24-hour period of deprivation following 7 weeks of excessive ethanol intake. Rats consumed ethanol in the intermittent access to 20% ethanol two-bottle choice paradigm 7 weeks (black bars). Controls consumed water only for 7 weeks (white bars). The VTA and nucleus accumbens (NAc) were dissected 24 hours after the end of the last ethanol-drinking session. GDNF (upper panels of gel images) and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) (lower panels of gel images) in expression levels in the VTA (a) and the NAc (b) were determined by quantitative real-time polymerase chain reaction. Bar graph represents the mean GDNF/GAPDH ratio, ±SEM. ** P < 0.01, n = 6–8 (a) and 4–5 (b) per group
GDNF expression in the VTA is significantly increased after a period of excessive, ‘binge-like’ ethanol intake
Since we observed a significant decrease in GDNF expression during deprivation, and no change in the mRNA levels of the growth factor at the end of the 24-hour ethanol-drinking session, we next set out to determine whether and how the expression of GDNF is altered during the drinking session. Previously, we found that after 7 weeks of ethanol consumption in the intermittent access to 20% ethanol two-bottle choice paradigm, rats showing a typical pattern of drinking escalation consumed an excessive amount of ethanol (approximately 1–1.5 g/kg) during the first 30 minutes of the drinking session, resulting in a blood ethanol concentration (BEC) of 80.9 ± 7.2 mg% (17.5 ± 1.5 mM) (Carnicella et al. 2009c), which meets the NIAAA definition of binge drinking in humans (BEC of 80 mg% or more) (NIAAA 2004). Although the intermittent access to 20% ethanol two-bottle choice procedure generally produces a high number of excessively drinking rats (approximately 60–70%), not all rats will develop a typical pattern of excessive drinking. As shown in Fig. 4a, ethanol-exposed rats can be segregated into two distinct groups: those whose ethanol intake progressively increases over time (excessive drinkers), and those whose consumption stagnates and does not increase (low drinkers). Thus, we compared the levels of GDNF expression after the period of ‘binge-like’ ethanol intake in these two groups. As shown in Table 1, excessive drinkers consumed 1.28 ± 0.24 g/kg of ethanol, corresponding to a BEC of approximately 70 mg% [according to Carnicella et al. (2009c)], while rats with a low-drinking profile consumed 0.20 ± 0.1 g/kg of ethanol, which corresponds to a BEC of approximately 10 mg% [according to Carnicella et al. (2008)], during the first 30 minutes of the ethanol-drinking session. We observed a significant increase in GDNF mRNA in the VTA in both the excessive- and low-drinking groups 30 minutes after the beginning of an ethanol-drinking session, as compared with the water-only control group (Fig. 4b). Interestingly, GDNF expression was elevated to a greater degree in the low drinkers, as compared with rats whose ethanol intake showed a typical escalation to excessive levels (Fig. 4b). These results suggest that an acute binge drinking session rapidly induces the expression of GDNF in the VTA of both excessive- and low-ethanol-drinking rats.
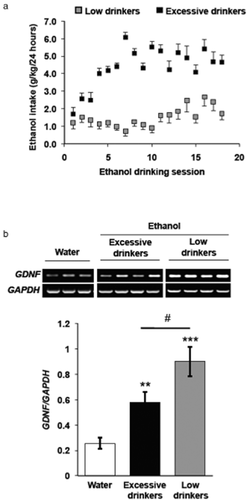
A binge drinking session elevates glial cell line-derived neurotrophic factor (GDNF) levels in the ventral tegmental area (VTA). Rats consumed ethanol in the intermittent access to 20% ethanol two-bottle choice paradigm 7 weeks. Controls consumed water only for 7 weeks. (a) Rats' drinking patterns segregated into groups of excessive- (black squares) and low-ethanol-drinking (grey squares) subjects. (b) The VTAs of excessive (black bar) and low (grey bar) drinkers, as well as water-only controls (white bar), were collected 30 minutes after the start of the last ethanol-drinking session. GDNF (upper panels of gel image) and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) (lower panels of gel image) expression levels were measured by quantitative real-time polymerase chain reaction. Bar graph represents the average GDNF/GAPDH ratio ± SEM, n = 5–10 per group. ** P < 0.01, ***P < 0.001, as compared with the water control group. # P < 0.05, as compared with the excessive-drinking group
Knockdown of endogenous GDNF expression in the VTA elevates ethanol intake by previously ethanol-naïve rats
Next, we tested the possible behavioral consequences of the ethanol-mediated fluctuation in GDNF expression in the VTA in response to ethanol consumption. We previously showed GDNF negatively regulates alcohol consumption (Carnicella et al. 2008, 2009c; Carnicella & Ron 2009; Barak et al. 2011a,b). We, therefore, hypothesized that upregulation of GDNF in the VTA is response to ethanol may be part of a homeostatic mechanism that controls the level of ethanol intake. To test this possibility, we used viral-mediated gene delivery in combination with RNA interference (Sliva & Schnierle 2010) to specifically knockdown GDNF expression in the VTA (AdV-shGDNF) and assessed whether ethanol drinking is altered. Using this virus, we previously determined that expression of the growth factor in the mesolimbic system is significantly decreased beginning 7 days after viral infusion, and that this downregulation persists up to at least day 18 post-virus infusion, whereas the expression of GDNF was unaltered upon infection with a control adenovirus expressing a scrambled shRNA sequence (AdV-SCR) (Wang et al. 2010; Barak et al. 2011a). Here, we infused AdV-shGDNF, or AdV-SCR, into the VTAs of ethanol-naïve rats, allowed 10 days for both surgical recovery and downregulation of GDNF, and then began to assess the level of ethanol intake in the intermittent access 20% ethanol two-bottle choice paradigm.
As shown in Fig. 5a, we observed significantly higher levels of ethanol consumption in subjects that received AdV-shGDNF, as compared with those who had received the control AdV-SCR during the first four drinking sessions (e.g. 1 week of intake, days 11–18 post-virus infusion). ANOVA revealed no main effect of virus [F(1,16) = 1.08, P = 0.31], but a significant main effect of day post-virus infusion [F(10, 160) = 3.41, P < 0.001] and a significant interaction [F(10, 106) = 2.55, P < 0.01]. Further analysis using the method of contrasts showed a significant difference in ethanol intake between the AdV-shGDNF- and AdV-SCR-treated animals during days 11–18 post-virus infusion [F(1,16) = 8.92, P < 0.01]. Importantly, water consumption was similar in both the AdV-shGDNF and AdV-SCR groups (Fig. 5b). These findings suggest that endogenous GDNF in the VTA suppresses the escalation of ethanol consumption during the first week of ethanol drinking.
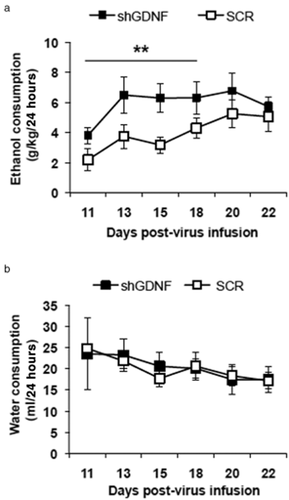
Knockdown of ventral tegmental area (VTA) glial cell line-derived neurotrophic factor (GDNF) facilitates ethanol drinking escalation by ethanol-naïve rats. Recombinant adenoviruses containing small hairpin RNA (shRNA) targeting GDNF (shGDNF; black squares) or a scrambled control sequence (SCR; white squares) were bilaterally infused into the VTA of rats. Ethanol (a) and water (b) consumption in the intermittent access to 20% ethanol two-bottle choice paradigm was measured beginning 10 days after the virus infusion. Graph represents amount of ethanol (a) or water (b) consumed ± SEM, n = 9 per group. **P < 0.01, as compared with the SCR control group
Knockdown of endogenous GDNF after a history of excessive drinking does not affect ethanol consumption
Since GDNF knockdown in the VTA during the early stages of drinking results in rapid escalation of ethanol consumption, we tested whether downregulation of GDNF after a history of excessive ethanol consumption would alter ethanol intake. To do so, an independent group of rats first consumed ethanol in the intermittent access to 20% ethanol two-bottle choice paradigm described above for 7 weeks, reaching a baseline level of 5.65 ± 0.44 g/kg of ethanol consumed over 24 hours. AdV-shGDNF or AdV-SCR was then infused into the VTA. Rats were re-exposed to ethanol in the same two-bottle choice procedure 10 days following viral infusion, and ethanol intake was measured. We found that knockdown of GDNF in the VTAs of rats that had previously been trained to consume excessive amounts of ethanol did not affect either the amount of ethanol (Fig. 6a) or water (Fig. 6b) consumed. These findings indicate that knockdown of GDNF does not alter ethanol consumption of rats with a history of repeated excessive ethanol intake.
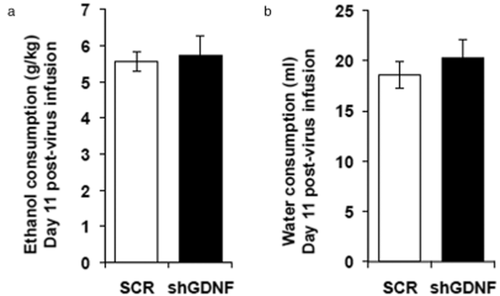
Knockdown of glial cell line-derived neurotrophic factor (GDNF) does not alter ethanol consumption by rats that previously consumed excessive amounts of ethanol for 7 weeks. Rats consumed ethanol in the intermittent access to 20% ethanol two-bottle choice paradigm 7 weeks. Controls consumed water only for 7 weeks. Recombinant adenovirus expressing small hairpin RNA (shRNA) targeting GDNF (shGDNF; black bars) or a scrambled control sequence (SCR; white bars) was then infused into the ventral tegmental area (VTA). Ethanol (a) and water (b) consumption in the intermittent access to 20% ethanol two-bottle choice paradigm was assessed starting 10 days after the virus infusion. Bar graphs represent the mean ethanol (a) or water (b) intake ± SEM on day 11 post-virus infusion, n = 7 per group.
Discussion
Several important findings stem from this study. First, we show that GDNF is a novel ethanol-responsive gene, and that its expression levels fluctuate as a function of drinking history in a brain region-specific manner. In addition, we show that the endogenous growth factor in the VTA plays an important role in gating the level of excessive ethanol intake. Based on our findings, we propose the following model: GDNF expression in the VTA increases during early ethanol-drinking experiences and functions to homeostatically suppress the development and progression of excessive ethanol consumption. Protracted, excessive intermittent ethanol consumption results in abnormally low levels of endogenous GDNF expression during deprivation, which contributes to the persistence of high levels of ethanol drinking. A short period of ‘binge-like’ ethanol intake following deprivation transiently elevates GDNF expression levels, but is insufficient to control ethanol consumption by rats with a history of excessive ethanol intake. This indicates that long-term repeated cycles of ethanol drinking and withdrawal induces a breakdown of the growth factor's protective homeostatic response.
GDNF and its signaling pathway have previously been shown to be susceptible to alterations in the level of expression and/or activation caused by stimulants and opiates (Carnicella & Ron 2009; Ghitza et al. 2010). For instance, levels of phosphorylated, and thus activated, Ret were lowered after chronic exposure to cocaine or morphine (Messer et al. 2000). Notably, endogenous GDNF mRNA levels were decreased in the dorsal striatum, but not in the NAc, 24 hours after the end of a 12-day-long cocaine self-administration procedure (Green-Sadan et al. 2003). In addition, GDNF expression in the VTA of rats was decreased on the first day of withdrawal from a 10-day-long heroin self-administration procedure (Airavaara et al. 2011). In the present study, we found that ethanol affects GDNF expression in the VTA, but not the NAc. This finding is in agreement with previous studies showing that the level of GDNF expression in the VTA is inducible and is enhanced in response to systemic administration of drugs, such as phencyclidine (Semba et al. 2004), ibogaine (He et al. 2005) and cabergoline (Carnicella et al. 2009a), as well as in response to an infusion of the growth factor itself (Barak et al. 2011a). However, since the basal level of GDNF expression in the NAc is much higher than that in the VTA, it is possible that increases in the growth factor's expression in response to ethanol can be difficult to detect. Thus, while we did not observe a difference in the level of GDNF mRNA in the NAc, we cannot rule out the possibility that ethanol consumption also alters the growth factor's expression in this brain region.
We found that GDNF mRNA and protein levels were increased in the VTA after a single systemic administration of ethanol. Moreover, in a physiologically relevant form of ethanol exposure, 1 week of voluntary ethanol intake in the intermittent-access two-bottle choice drinking model, the expression of the growth factor was likewise increased. Interestingly, rats infected with AdV-shGDNF in the VTA prior to any ethanol exposure showed an escalation of subsequent ethanol intake during the first week of ethanol consumption, as compared with control rats infected with AdV-SCR. Together, these results suggest that initial episodes of excessive ethanol intake upregulate GDNF levels, which in turn acts to hinder the progressive escalation of ethanol consumption. Notably, 3 weeks post-virus infusion, ethanol intake levels were equal between the AdV-shGDNF and AdV-SCR groups. This is likely due to the characteristic progression and escalation of ethanol intake by the AdV-SCR group in this drinking model. This temporary behavioral effect also correlates well with the transient nature of adenoviral-mediated gene delivery and expression (Ghosh, Gopinath & Ramesh 2006).
Binding of GDNF to the Ret receptor leads to the activation of the extracellular signal-regulated kinases 1/2, which subsequently activate the transcription factor cAMP response element binding protein in the nucleus (Hayashi et al. 2000). As a result, GDNF signaling plays a role in the alteration of gene expression (Hayashi et al. 2000; Jongen et al. 2005). Several genes whose products are involved in synaptic plasticity, including the calcineurin subunits ppp3R1 and pppC3B, and calcium-calmodulin-dependent protein kinase II-β (Consales et al. 2007), as well as the calcium binding proteins frequenin (Wang et al. 2001) and calbindin (Wang et al. 2008) have been shown to be modulated by GDNF signaling. Thus, it is plausible that the increase in the level of GDNF during early drinking experiences alters the expression of these and other genes to mitigate the development of excessive-drinking behaviors.
We observed that GDNF expression levels are reduced below basal levels during a period of deprivation from ethanol. Interestingly, infusion of the recombinant GDNF protein into the VTA of rats significantly lowered operant responding for ethanol and blocked excessive intake and relapse (Carnicella et al. 2008, 2009c; Barak et al. 2011a,b). Therefore, it is plausible that the recombinant protein replaces the deficit in the endogenous growth factor, negating the need to consume excessive ethanol to restore GDNF levels, and ultimately resulting in a decrease in ethanol consumption. Interestingly, a recent study reported reduced levels of GDNF in the blood serum of human alcoholics undergoing protracted withdrawal (Heberlein et al. 2010), suggesting that the deprivation-associated decrease in GDNF expression that we observed in rats may be a common, long-lasting molecular neuroadaptation to chronic cycles of ethanol drinking and withdrawal.
We show here that a short period of binge drinking of ethanol induces GDNF expression in the VTA. This suggests that although the long-term, excessive consumption of ethanol has resulted in lower basal levels of GDNF in the absence of ethanol, GDNF is still upregulated when ethanol is present. Moreover, we observed a recovery of GDNF expression to levels similar to those of the control, water-only group at the end of a 24-hour drinking session. Whether this normalization is a consequence of the actual act of continued drinking or a result of a change in the overall regulation of the GDNF gene because of the long history of excessive drinking is unknown. Our findings also indicate that several weeks of excessive ethanol consumption alters the time-course of the ethanol-induced expression of the growth factor. For example, while a short history of ethanol drinking causes a significant upregulation of GDNF that is apparent at the end of a 24-hour drinking session, a long history of excessive intake results in an increase in the level of the growth factor that is observed 30 minutes after the start, but not at the end of the drinking session. Thus, the effect of a protracted history of repeated excessive ethanol drinking and withdrawal on the sensitivity, as well as the overall transcriptional control of the GDNF gene in response to ethanol merits further inquiry.
As mentioned above, the mesolimbic dopaminergic system, and in particular the dopaminergic projection from the VTA to the NAc, is targeted by drugs of abuse, including ethanol (Nestler 2001). Interestingly, several studies have shown that dopamine levels in the NAc are reduced in ethanol-dependent rats experiencing withdrawal (Rossetti et al. 1992; Diana et al. 1993; Weiss et al. 1996; Barak et al. 2011b). We previously reported that GDNF in the VTA enhances the dopaminergic tone of the mesolimbic system (Wang et al. 2010), we propose that the abnormally low level of GDNF we observed during deprivation can contribute to withdrawal-associated dopamine deficiency, which can be associated with an abnormal affective state and susceptibility to relapse (Weiss et al. 1996; Koob & Le Moal 2001). Importantly, we recently demonstrated that infusion of GDNF into the VTA of rats reversed the ethanol withdrawal-induced dopamine deficiency in the mesolimbic system (Barak et al. 2011b), an effect that may be due to the growth factor's ability to increase the spontaneous firing rate of VTA dopaminergic neurons (Wang et al. 2010). These findings suggest that normalization of the deprivation-induced deficit in GDNF, which can function to rescue dopamine levels, is one possible mechanism by which ethanol-induced GDNF regulates ethanol intake after a long history of cycles of excessive drinking and withdrawal.
Notably, we found that low-ethanol-drinking rats displayed an even greater increase in GDNF expression 30 minutes after the start of a drinking session, as compared with rats whose intake reached excessive levels. It is plausible that some individual animals, before any exposure to ethanol, have a higher basal level of GDNF, which may be an underlying cause of the low-drinking phenotype. Because it is impossible to determine a priori which individuals' ethanol drinking will escalate and which will not based on the basal level of GDNF in the brains of living subjects, testing this hypothesis may prove to be an insurmountable challenge. Interestingly, we observed a very low level of inter-individual variation in VTA GDNF expression in the water-only control groups, which contain rats that could develop into either low or excessive drinkers. This suggests that large differences in the levels of the growth factor from subject to subject may not manifest until after exposure to ethanol. Therefore, if the ethanol-mediated upregulation of GDNF is a consequence of ethanol intake, then such a sensitive and robust transcriptional response could contribute to a mechanism that protects some individuals from becoming excessive drinkers. Intriguingly, we found that the level of GDNF in rats that had all received an identical systemic dose of ethanol varied between individuals, suggesting that the response of this gene to an ethanol challenge differs between subjects. Thus, the inter-individual differences in the level of GDNF expression after exposure to ethanol, or at the end of a 24-hour ethanol-drinking session, and whether they correlate with excessive ethanol consumption warrant additional examination.
Interestingly, infection of VTA neurons with adenovirus expressing shRNA targeting GDNF did not alter ethanol intake in rats with a history of excessive ethanol consumption. It is possible that a ceiling of ethanol consumption has been reached, and thus further increases in ethanol intake in response to GDNF knockdown cannot be observed. It is also plausible that long-lasting neuroadaptations resulting from a history of excessive ethanol consumption counteract the effect of downregulation of endogenous GDNF on ethanol intake. Alternatively, dissociation between ethanol-induced GDNF and consumption may have occurred as a result of the protracted drinking history. For example, chronic exposure to ethanol may alter the secretion and/or the post-translational modifications (e.g. the proteolytic cleavage of the pro-GDNF precursor) that lead to the availability of mature, extracellular GDNF (Airaksinen & Saarma 2002). The development of tolerance to the effects of GDNF on ethanol intake, however, is less likely, as we have previously demonstrated that infusion of exogenous GDNF into the VTA of rats that have chronically consumed excessive amounts of ethanol readily causes a robust decrease in the level of voluntary drinking (Carnicella et al. 2009c; Barak et al. 2011a) and operant self-administration of ethanol (Carnicella et al. 2008; Barak et al. 2011b). This suggests that the GDNF signaling pathway and the consequent behavioral response on ethanol drinking remains intact even after a long history of excessive intake.
We previously reported that endogenous brain-derived neurotrophic factor (BDNF) is acutely upregulated in response to moderate ethanol consumption to control the level of subsequent ethanol intake (McGough et al. 2004; Jeanblanc et al. 2009; Logrip, Janak & Ron 2009). We propose that GDNF is another such homeostatic mediator of ethanol consumption. There are however, major differences in the modes of action of BDNF and GDNF. For example, while BDNF is elevated in the dorsolateral striatum of moderately drinking rats, GDNF is increased in the rat VTA in response to excessive ethanol consumption. Moreover, the ethanol-mediated increase in BDNF expression disappears after a history of ethanol consumption (Logrip et al. 2009), whereas GDNF maintains the ability to be upregulated by ethanol intake even after 7 weeks of excessive ethanol consumption.
In conclusion, our results infer that ethanol leads to a long-lasting dysregulation of endogenous GDNF expression, which contributes to the manifestation and persistence of excessive-drinking behaviors, and may add to the development of alcoholism and alcohol use disorders.
Acknowledgements
This work was supported by National Institutes of Health (NIH)-National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant RO1 AA014366 (D.R.), the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (D.R.), the National Research Service Award (NRSA)-NIAAA Fellowship F31 AA017801 (S.A.), and the Merck Scholar Award (S.A.).
Authors Contribution
SA, SB and DR were responsible for the research concept and design, and interpretation of the data. SA and SB performed the experiments. SA, SB and DR wrote the manuscript. All authors critically reviewed the manuscript and approved the final version for publication.




