The dopamine beta-hydroxylase inhibitor nepicastat increases dopamine release and potentiates psychostimulant-induced dopamine release in the prefrontal cortex
Abstract
The dopamine-beta-hydroxylase inhibitor nepicastat has been shown to reproduce disulfiram ability to suppress the reinstatement of cocaine seeking after extinction in rats. To clarify its mechanism of action, we examined the effect of nepicastat, given alone or in association with cocaine or amphetamine, on catecholamine release in the medial prefrontal cortex and the nucleus accumbens, two key regions involved in the reinforcing and motivational effects of cocaine and in the reinstatement of cocaine seeking.
Nepicastat effect on catecholamines was evaluated by microdialysis in freely moving rats.
Nepicastat reduced noradrenaline release both in the medial prefrontal cortex and in the nucleus accumbens, and increased dopamine release in the medial prefrontal cortex but not in the nucleus accumbens. Moreover, nepicastat markedly potentiated cocaine- and amphetamine-induced extracellular dopamine accumulation in the medial prefrontal cortex but not in the nucleus accumbens. Extracellular dopamine accumulation produced by nepicastat alone or by its combination with cocaine or amphetamine was suppressed by the α2-adrenoceptor agonist clonidine.
It is suggested that nepicastat, by suppressing noradrenaline synthesis and release, eliminated the α2-adrenoceptor mediated inhibitory mechanism that constrains dopamine release and cocaine- and amphetamine-induced dopamine release from noradrenaline or dopamine terminals in the medial prefrontal cortex.
Introduction
Cocaine addiction represents an important public health problem worldwide. Due to the high social cost of cocaine addiction, the development of even modestly effective pharmacotherapy will have great economic and socio-sanitary benefits. However, in spite of considerable effort on drug development, no proven pharmacotherapy for this condition is presently available.
However, clinical and epidemiological studies have identified disulfiram, the classic aversive medication for alcoholism, as a potentially promising treatment for cocaine dependence (George et al. 2000; Petrakis et al. 2000; Carroll et al. 2004; Sofuoglu & Kosten 2006). Indeed, human laboratory studies indicate that disulfiram does not affect cocaine ‘high’ but decreases craving for cocaine and increases dysphoria, anxiety and paranoia from cocaine (Hameedi et al. 1995; McCance-Katz, Kosten & Jatlow 1998; Mutschler, Diehl & Kiefer 2009).
The mechanism of disulfiram efficacy in the treatment of cocaine dependence has been attributed to its ability to inhibit dopamine beta-hydroxylase (DBH), the enzyme that converts dopamine to noradrenaline within noradrenergic neurons (Goldstein et al. 1964; Musacchio et al. 1966). However, since as result of DBH inhibition brain noradrenaline is reduced but dopamine concentration is increased (Musacchio et al. 1966; Goldstein & Nakajima 1967; Karamanakos et al. 2001; Bourdélat-Parks et al. 2005), there is a debate on whether disulfiram's efficacy in the treatment of cocaine dependence is mediated by the increase of central dopamine or to the loss of noradrenaline. Thus, Haile et al. (2003) have shown that disulfiram facilitates the development and expression of locomotor sensitization to cocaine in rats, and suggested that this effect is mediated by disulfiram-induced increase of brain dopamine. These authors maintain that cocaine sensitization is a neurochemical correlate of anxiety and dysphoria from cocaine, and that it contributes to disulfiram's efficacy in clinical trials.
On the other hand, Schroeder et al. (2010) have shown that disulfiram suppresses cocaine-primed reinstatement of drug-seeking behavior following extinction, but is ineffective in reducing maintenance of cocaine self-administration behavior. These authors maintain that the suppressant effect of disulfiram on reinstatement of cocaine seeking is due to the depletion of brain noradrenaline, and that the latter has a critical role in the response to environmental stimuli that cause relapse to cocaine seeking.
Recently, we found that disulfiram, consistent with its ability to inhibit DBH, profoundly reduced noradrenaline release in the medial prefrontal cortex (mPFC), occipital cortex, nucleus accumbens and caudate nucleus. However, we also found that disulfiram produced a concomitant increase of dopamine release in the mPFC and occipital cortex, where noradrenergic prevails over dopaminergic innervation, but did not modify extracellular dopamine in the nucleus accumbens and caudate nucleus, where dopaminergic innervation is predominant (Devoto et al. 2012). In addition, consistent with its ability to inhibit DBH, disulfiram reduced cocaine-induced noradrenaline release both in the mPFC and nucleus accumbens. However, disulfiram potentiated cocaine-induced dopamine release in the mPFC but had no effect in the nucleus accumbens. The regional selectivity of disulfiram effect on dopamine release, together with the fact that such release was suppressed by the α2-adrenoceptor agonist clonidine but not by the D2-agonist quinpirole, led us to postulate that, as a consequence of DBH inhibition, disulfiram caused a selective release of dopamine instead of noradrenaline from noradrenergic terminals.
However, disulfiram, being a copper chelator, is a relatively non-selective inhibitor of different enzymes, including DBH, aldehyde dehydrogenase, two cocaine metabolizing enzymes, cholinesterase and carboxyl esterase, and tyrosine hydroxylase (Zemaitis & Greene 1976; Nousiainen & Törrönen 1984; Savolainen et al. 1984; Stanley et al. 1997; Schroeder et al. 2010). These findings raise the question of the mechanism responsible for the regional selective increase of dopamine release produced by disulfiram and the possible role of dopamine in disulfiram ability to prevent the reinstatement of cocaine seeking and/or to facilitate locomotor sensitization to cocaine in rats. Thus, in order to determine if disulfiram effects on catecholamines were mediated by DBH inhibition, we tested if they were reproduced by nepicastat, a selective competitive inhibitor of DBH (Stanley et al. 1997).
Nepicastat has been found to reproduce the ability of disulfiram to suppress the reinstatement of cocaine seeking triggered by cocaine priming (Schroeder et al. 2010) and, like disulfiram, to be unable to reduce the ongoing cocaine-reinforced operant responding. Nepicastat effects have been attributed to the depletion of brain noradrenaline due to DBH inhibition (Schroeder et al. 2010), consistent with the view that noradrenaline has a critical role in the response to environmental stimuli that reinstate cocaine seeking, but has no effect on maintenance of responding to cocaine (Weinshenker & Schroeder 2007).
Since nepicastat, originally developed for treating congestive heart failure and hypertension (Stanley et al. 1997), is presently under advanced clinical evaluation for the treatment of cocaine addiction (http://www.clinicaltrials.gov/ct2/show/NCT01704196), to uncover the neurochemical correlates of its potential therapeutic action would bear important clinical other than scientific relevance.
Materials and Methods
Experiments were approved by the local ethical committee and performed according to the guidelines for care and use of experimental animals of the European Union (EEC Council 86/609; D.L. 27/01/1992, n. 116). A total of 103 male Sprague-Dawley rats (Harlan Italy, S. Pietro al Natisone, Italy) were used. Animals weighed 175–200 g on arrival, and were housed in groups of five per cage for at least 7 days before experiment, at standard conditions of temperature and humidity and artificial light from 8 a.m. to 8 p.m.; food and water were available ad libitum.
Experiments were conducted as previously described (Devoto et al. 2003), with slight modifications. Briefly, rats were anesthetized with Equithesin (0.97 g pentobarbital, 2.1 g MgSO4, 4.25 g chloral hydrate, 42.8 ml propylene glycol, 11.5 ml 90% ethanol, distilled water up to 100 ml, 5 ml/kg, i.p.) and placed in a Kopf stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). Animals were implanted with in-house constructed vertical microdialysis probes (membrane AN 69-HF, Hospal-Dasco, Bologna, Italy; cut-off 40 000 Dalton) in the mPFC (AP +3.0, L ± 0.6, V −6.5 from bregma, 3 mm active membrane length) or nucleus accumbens shell (AP 1.7, L ± 0.5, V −8.5 from bregma, 2 mm active membrane length) according to the coordinates of the atlas by Paxinos & Watson (1997). Rats were given antibiotic therapy (enrofloxacin, Bayer HealthCare, Shawnee Mission, KS, USA) and allowed to recover in their home cages before testing. The day after probe implantation, an artificial cerebrospinal fluid (147 mM NaCl, 4 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, pH 6–6.5) was pumped through the dialysis probes at a constant rate of 2.2 μl/minute via a CMA/100 microinjection pump (Carnegie Medicine, Stockholm, Sweden). Samples were collected every 20 minutes and immediately analyzed for noradrenaline and dopamine content by high-performance liquid chromatography (HPLC) with electrochemical detection. The HPLC systems were equipped with 3.0 × 150 mm C18 (3.5 μ) Symmetry columns (Waters, Milan, Italy), kept at 35°C by Series 1100 thermostats (Agilent Technologies, Waldbronn, Germany), and ESA Coulochem II detectors (Chelmford, MA, USA). The mobile phase consisted of 80 mM Na2HPO4, 0.27 mM Ethylenediaminetetraacetic acid, 0.6 mM sodium octyl sulfate, 8% methanol, 3% acetonitrile, pH 2.8 with H3PO4, delivered at 0.35 ml/minute; the Coulochem analytical cell first electrode was set at +200 mV, the second at −300 mV. Only the second electrode signal was recorded and analyzed. In these conditions, the detection limit (signal to noise ratio 3:1) was 0.3 pg of noradrenaline and dopamine on column.
Mean basal extracellular catecholamine values were calculated from three consecutive samples with a variance not exceeding 15%, and were expressed as pg/40 μl dialysate. Drugs were administered after basal value collection and changes were calculated as percent of basal value. On completion of testing, rats were killed by an Equithesin overdose, the brains removed and sectioned by a cryostat (Leica CM3050 S) in 40 μm thick coronal slices to verify locations of dialysis probes. Animals with errant location of the device were excluded from analysis.
To evaluate noradrenaline and dopamine tissue content, rats were killed by decapitation 2 hours after treatment, the brains were rapidly removed and placed on a brain-cutting block kept on ice. The mPFC was dissected out from slices, according to the method described by Heffner, Hartman & Seiden (1980), immediately frozen on dry ice and stored at −80°C until processed for catecholamine content. Briefly, tissues were homogenized by sonication in 0.1 N HClO4 (1:20, weight/volume), centrifuged at 10 000 × g, the supernatant filtered on microcentrifuge filters (0.22 μm nylon filter, Costar Spin-X, Corning, NY, USA) and directly injected into the HPLC under analytical conditions as described for microdialysis experiments. Data are expressed as pg/mg tissue.
Nepicastat was a gift from Biotie Therapies (Basel, Switzerland). RS 79948, clonidine and quinpirole were purchased from Tocris (Bristol, UK), cocaine hydrochloride from Macfarlan Smith Ltd. (Edinburgh, UK), d-amphetamine sulfate from Sigma Chemical Co. (St. Louis, MO, USA). Nepicastat was used at the doses of 25, 50 and 100 mg/kg/ml i.p., dissolved in DMSO; RS 79948 (3 mg/kg), clonidine (0.15 mg/kg), quinpirole (0.5 mg/kg), amphetamine (2.5 mg/kg) and cocaine (10 mg/kg) were dissolved in sterile water. All drugs were administered i.p. in a volume of 1 ml/kg weight.
Statistical significance was calculated by means of Statistical Package for the Social Sciences 16.0 software (SPSS Inc., Chicago, IL, USA), applying one-way repeated measure analysis of variance (ANOVA) or mixed-design ANOVA, and with appropriate post hoc test as detailed in Results.
Results
Nepicastat effect on catecholamine tissue content in the mPFC
As shown in Table 1, nepicastat (molecular weight 349.83) and disulfiram (molecular weight 296.54), at equimolar dose (143 and 169 μmoles/kg, respectively), decreased tissue noradrenaline levels by about 40% (one-way ANOVA: F(2,29) = 23.28, P < 0.0001) and increased tissue dopamine content by 100% (one-way ANOVA: F(2,29) = 29.61, P < 0.0001) in the mPFC. Post hoc analysis (Tukey multiple comparison test) revealed a significant difference (P < 0.001) between vehicle versus disulfiram and vehicle versus nepicastat, but not disulfiram versus nepicastat treatment groups, for both noradrenaline and dopamine results.
| Treatment | Noradrenaline | Dopamine |
|---|---|---|
| Vehicle (15) | 319.0 ± 13.2 | 66.2 ± 3.6 |
| Nepicastat (6) | 178.0 ± 7.3* | 133.4 ± 12.5* |
| Disulfiram (9) | 203.1 ± 21.2* | 133.8 ± 12.5* |
- Nepicastat and disulfiram were dissolved in DMSO (vehicle) and the dose of 50 mg/kg was administered i.p., 2 hours before the animals were killed. Data are expressed as pg/mg tissue, and are the mean ± standard error of the mean of the number of animals indicated in brackets. *P < 0.001 versus vehicle (Tukey multiple comparison test).
For microdialysis experiments, a total of 118 rats were used; six animals were excluded from result calculation due to malposition of dialysis probe. Basal values (mean ± standard error of the mean) expressed as pg/40 μl dialysate, were: mPFC: noradrenaline = 2.91 ± 0.13 (n = 87), dopamine = 1.63 ± 0.11 (n = 88); nucleus accumbens shell: noradrenaline = 2.44 ± 0.21 (n = 23), dopamine = 7.26 ± 1.27 (n = 24). Due to chromatographic technical problems, noradrenaline data of one trial in the nucleus accumbens and in the mPFC was not included in calculations.
Nepicastat effect on catecholamine release in the mPFC
Consistent with its ability to inhibit DBH, nepicastat reduced extracellular noradrenaline in the mPFC (Fig. 1). Two hours after treatment, nepicastat at the dose of 25 and 50 mg/kg reduced extracellular noradrenaline by about 40 and 50% of baseline, respectively; a higher dose of 100 mg/kg produced no further depletion. We examined extracellular noradrenaline levels by mixed-design ANOVA with treatment (four levels: vehicle, nepicastat 25 mg, nepicastat 50 mg, nepicastat 100 mg) as between-subject factor and time (nine levels: 80, 100, 120, 140, 160, 180, 200, 220 and 240 minutes) as within-subject factor.

Effect of nepicastat (25, 50 and 100 mg/kg, i.p.) or its vehicle on extracellular noradrenaline and dopamine levels in the medial prefrontal cortex. Results are expressed as percent of mean basal level and are the mean ± standard error of the mean of at least five rats. Arrows indicate the time of administration. Black symbols indicate P < 0.05 with respect to each correspondent vehicle point (Bonferroni's post hoc comparison). Nep, nepicastat
ANOVA showed that both treatment (F(3,25) = 4.916, P < 0.01) and time (F(8,200) = 28.918, P < 0.001) per se and their interaction (F(24,200) = 3.148, P < 0.001) have significant effects on noradrenaline extracellular levels. Specifically, Bonferroni's post hoc comparisons of the means evidenced that nepicastat, consistent with its ability to inhibit DBH, reduced extracellular noradrenaline in mPFC compared to its vehicles (see Fig. 1 for statistical details).
The same statistical design was used to analyze the effect of nepicastat on extracellular dopamine levels. ANOVA showed that both treatment (F(3,24) = 6.371, P < 0.01) and time (F(8,192) = 8.377, P < 0.001) per se and their interaction (F(24,176) = 3.996, P < 0.001) have significant effects. Bonferroni's post hoc test showed that nepicastat increased dopamine levels compared to its vehicles (see Fig. 1 for statistical details). Indeed, at the doses of 25 and 50 mg/kg nepicastat increased dopamine by about 65 and 180% the basal level, respectively, while 100 mg/kg were not more effective than 50 mg/kg. Therefore, nepicastat was used at the dose of 50 mg/kg throughout the study for subsequent trials. DMSO (vehicle) administration did not affect noradrenaline and dopamine levels.
Stimulation of α2- and D2-receptors on nepicastat-induced effects on catecholamines in the mPFC
As shown in Fig. 2, the α2-adrenoceptor agonist clonidine (0.15 mg/kg i.p.), which per se reduced extracellular dopamine by about 60%, totally prevented nepicastat-induced extracellular dopamine accumulation in the mPFC. In contrast, the D2 receptor agonist quinpirole (0.5 mg/kg i.p.), as already demonstrated (Devoto et al. 2003), failed to modify extracellular dopamine and to prevent nepicastat-induced dopamine release in the mPFC. The effect of clonidine and quinpirole pre-treatment on extracellular dopamine levels was examined by mixed-design ANOVA with pre-treatment (three levels: vehicle, clonidine, quinpirole) and treatment (two levels: vehicle, nepicastat) as between-subject factors and time (six levels: 140, 160, 180, 200, 220 and 240 minutes after treatment) as within-subject factor. ANOVA showed that both pre-treatment (F(2,35) = 20.379, P < 0.001) and treatment (F(1,35) = 30.080, P < 0.001) per se had significant effects on dopamine extracellular levels. Similarly treatment × time interaction (F(5,175) = 2.761, P < 0.05) and pre-treatment × treatment interaction (F(2,35) = 20.379, P < 0.01) had significant effects. Conversely, no significant effects were found for time per se (F(5,175) = 0.718, n.s.), time × pre-treatment (F(10,175) = 0.657, n.s.) and time × pre-treatment × treatment (F(10,175) = 1.558, n.s.). Specifically, Bonferroni's post hoc comparisons of the means evidenced that nepicastat treated group was significantly different from clonidine + nepicastat group (P < 0.05) and not significantly different from quinpirole + nepicastat group.
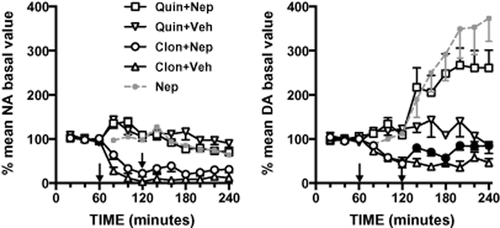
Effect of clonidine (Clon, 0.15 mg/kg i.p.) or quinpirole (Quin, 0.5 mg/kg, i.p.) pre-treatment on nepicastat (Nep, 50 mg/kg, i.p.) effect on extracellular catecholamines in the medial prefrontal cortex. Results are expressed as percent of mean basal level and are the mean ± standard error of the mean of at least five rats. The first arrow indicates the time of clonidine or quinpirole administration, the second arrow indicates time of nepicastat administration. Nepicastat data from Fig. 1 are reported for comparison. Black symbols indicate significant (P < 0.05) Bonferroni's post hoc comparison for the interaction pre-treatment × treatment. DA, dopamine; NA, noradrenaline, Veh, vehicle
On the other hand, nepicastat did not further reduce extracellular noradrenaline level below the clonidine-induced decrease, and its effect was not modified by quinpirole pre-treatment. Extracellular noradrenaline variations were evaluated with the same experimental design used for dopamine. ANOVA showed that pre-treatment (F(2,34) = 46.966, P < 0.001), treatment (F(1,34) = 7.876, P < 0.01) and time per se (F(5,170) = 6.477, P < 0.001) had significant effects on noradrenaline extracellular levels. Similarly pre-treatment × treatment interaction (F(2,34) = 6.003, P < 0.01) had significant effect. Conversely no significant effects were found for time × pre-treatment (F(10,170) = 0.955, n.s.), time × treatment (F(5,170) = 0.878, n.s.) and time × pre-treatment × treatment (F(10,170) = 321.818, n.s.) interactions. Bonferroni's post hoc comparisons of the means evidenced that no significant differences were found between clonidine- versus clonidine + nepicastat group and quinpirole- versus quinpirole + nepicastat group.
Nepicastat effect on cocaine- and amphetamine-induced catecholamine release in the mPFC
As shown in Fig. 3, cocaine (10 mg/kg, i.p.) increased extracellular noradrenaline and dopamine by 370 and 270%, respectively. Nepicastat attenuated cocaine-induced increase of extracellular noradrenaline, although the effect did not reach statistic significance. Conversely, nepicastat markedly potentiated cocaine-induced elevation of extracellular dopamine in the mPFC.
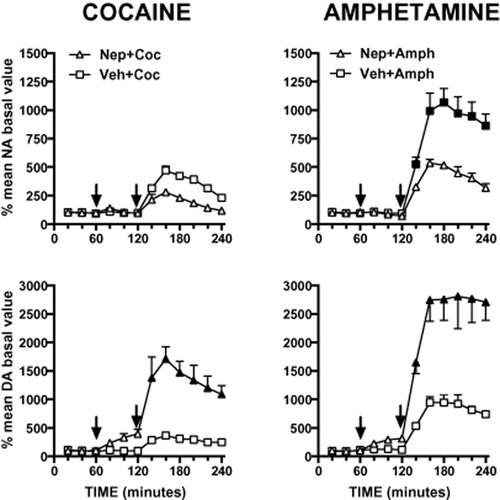
Consequence of nepicastat (Nep, 50 mg/kg i.p.) pre-treatment on cocaine- (Coc, 10 mg/kg, i.p.) or amphetamine- (Amph, 2.5 mg/kg i.p.) induced increase of extracellular noradrenaline (NA) and dopamine (DA) levels in the medial prefrontal cortex. Results are expressed as percent of mean basal level and are the mean ± standard error of the mean of at least four rats. The first arrow indicates the time of nepicastat or its vehicle administration; the second arrow indicates time of cocaine or amphetamine administration. Black symbols indicate P < 0.05 for Bonferroni's post hoc comparisons with respect to each correspondent Veh + Coc or Veh + Amph time point
The administration of amphetamine (2.5 mg/kg, i.p.) increased extracellular noradrenaline and dopamine by 900 and 850%, respectively. Pre-treatment with nepicastat significantly reduced amphetamine-induced noradrenaline release, but markedly potentiated dopamine release produced by amphetamine (Fig. 3). We examined the effect of nepicastat on extracellular dopamine and noradrenaline increases induced by cocaine and amphetamine by mixed-design ANOVA with treatment (two levels: veh, nepicastat) as between-subject factor and time (six levels: 140, 160, 180, 200, 220 and 240 minutes after treatment) as within-subject factor. Analysis of cocaine-induced noradrenaline increase showed a significant main effect of time (F(5,30) = 6.4929, P < 0.001), while no significant effects were found for treatment per se (F(1,6) = 0.551, n.s.) and time × treatment interaction (F(5,30) = 1.449, n.s.). ANOVA analysis on cocaine-induced dopamine accumulation showed significant effect of time (F(5,30) = 8.711, P < 0.001), treatment (F(1,6) = 24.027, P < 0.01) and their interaction (F(5,30) = 3.220, P < 0.05). ANOVA on amphetamine-induced noradrenaline increase showed significant effect of time (F(5,35) = 11.352, P < 0.01), treatment (F(1,7) = 16.652, P < 0.01) and their interaction (F(5,35) = 3.050, P < 0.05). ANOVA on amphetamine-induced dopamine increase showed significant effect of time (F(5,30) = 9.630, P < 0.01), treatment (F(1,6) = 23.986, P < 0.01) and their interaction (F(5,30) = 2.742, P < 0.05). Bonferroni's post hoc comparison results are indicated in Fig. 3 legend.
Consistent with the hypothesis that nepicastat potentiated amphetamine effect by inactivating α2-adrenoceptors, the potentiating effect of nepicastat on amphetamine response was reproduced by the administration of the α2-adrenoceptor blocker RS 79948 (3 mg/kg, i.p.) (Fig. 4a) and reversed by the α2-adrenoceptor agonist clonidine (0.15 mg/kg, i.p.) (Fig. 4b). On the other hand, clonidine, administered after amphetamine alone, did not affect amphetamine-induced dopamine increase (Fig. 4b). We compared the role of RS 79948 to the role of nepicastat on amphetamine-induced extracellular dopamine increase by mixed-design ANOVA with treatment (three levels: vehicle, RS, nepicastat) as between-subject factor and time (six levels: 140, 160, 180, 200, 220 and 240 minutes after treatment) as within-subject factor.
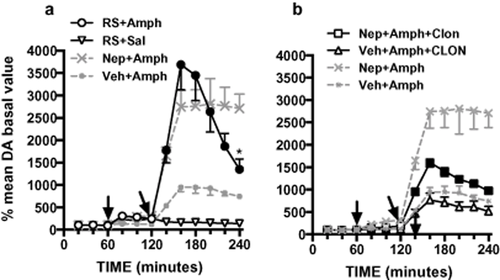
Effect of RS 79948 on amphetamine-induced extracellular dopamine accumulation in the mPFC (a), and antagonism of nepicastat-induced amphetamine potentiation by clonidine (b). Nepicastat + amphetamine and vehicle + amphetamine data from Fig. 3 are reported for comparison. Results are expressed as percent of mean basal level and are the mean ± standard error of the mean of at least five rats. The first arrow indicates the time of nepicastat (Nep, 50 mg/kg i.p.), its vehicle or RS 79948 (RS, 3 mg/kg i.p.) administration; the second arrow indicates time of amphetamine administration (Amph, 2.5 mg/kg, i.p.); the third arrow (in b) indicates clonidine administration (Clon, 0.15 mg/kg, i.p.). Black symbols in (a) indicate significant difference between RS + Amph and Veh + Amph groups; asterisk indicate significant difference with corresponding point in Nep + Amph group (P < 0.05, Bonferroni's post hoc). Black symbols in (b) indicate significant difference between Nep + Amph + Clon, Nep + Amph and Nep + Veh + Amph groups (P < 0.05, Bonferroni's post hoc)
ANOVA showed significant effect of time (F(5,50) = 11.818, P < 0.001), treatment (F(2,10) = 11.075, P < 0.01) and their interaction (F(10,50) = 5.5528, P < 0.001) on extracellular dopamine levels. Bonferroni's post hoc confirmed a similar action of nepicastat and RS 70048, the two groups resulting significantly different only in the last time point, due to different duration of effect.
We investigated the effect of clonidine on the potentiation of amphetamine effect induced by nepicastat on extracellular dopamine levels by mixed-design ANOVA with pre-treatment (two levels: vehicle, clonidine) and treatment (two levels: vehicle, nepicastat) as between-subject factor and time (six levels: 140, 160, 180, 200, 220 and 240 minutes after treatment) as within-subject factor. ANOVA showed that time (F(5,80) = 22.349, P < 0.001), pre-treatment (F(1,16) = 23.165, P < 0.001) and treatment (F(1,16) = 52.98, P < 0.001) per se had significant effects on extracellular dopamine levels. Moreover time × pre-treatment (F(5,80) = 6.002, P < 0.001), time × treatment (F(5,80) = 4.056, P < 0.01), time × pre-treatment × treatment (F(5,80) = 3.046, P < 0.05) and pre-treatment × treatment interactions (F(1,16) = 12.575, P < 0.01) had significant effects on dopamine extracellular levels.
Nepicastat effect on catecholamine release in the nucleus accumbens shell
As shown in Fig. 5, nepicastat (50 mg/kg) decreased extracellular noradrenaline by 42% of the basal values in the nucleus accumbens (Repeated measures ANOVA: F(11,5) = 16.6, P < 0.0001), but unlike in the mPFC, it failed to increase extracellular dopamine level in this area (Repeated measures ANOVA: F(11,5) = 0.915, n.s.) (Fig. 5). Vehicle administration did not affect extracellular catecholamine levels (data not shown).

Effect of nepicastat (50 mg/kg, i.p.) on extracellular noradrenaline and dopamine levels in the nucleus accumbens. Results are expressed as percent of mean basal level and are the mean ± standard error of the mean of six rats. Arrows indicate the time of administration. Black symbols indicate P < 0.05 with respect to basal values (Bonferroni's post hoc)
As shown in Fig. 6, nepicastat attenuated cocaine- and amphetamine-induced noradrenaline release in the nucleus accumbens. However, unlike in the mPFC, it failed to significantly modify cocaine- and amphetamine-induced dopamine release in this region. We examined the effect of nepicastat on extracellular dopamine and noradrenaline levels induced by cocaine and amphetamine by mixed-design ANOVA with treatment (two levels: vehicle, nepicastat) as between-subject factor and time (six levels: 140, 160, 180, 200, 220 and 240 minutes after treatment) as within-subject factor. ANOVA on cocaine-induced noradrenaline increase showed only a significant main effect of time (F(5,35) = 0.304, P < 0.01). A statistical trend for treatment per se was found (F(1,7) = 4.559, P = 0.070, n.s.), while no significant effect for time × treatment interaction was found (F(5,35) = 0.304, n.s.). ANOVA on cocaine-induced dopamine increase showed significant effect of time (F(5,35) = 6.709, P < 0.001) but not for treatment (F(1,7) = 0.001, n.s.) and their interaction (F(5,35) = 0.854, n.s.). ANOVA on amphetamine-induced noradrenaline increase showed significant effect of time (F(5,30) = 32.386, P < 0.001), treatment (F(1,6) = 10.626, P < 0.05) and their interaction (F(5,30) = 5.345, P < 0.01). ANOVA on amphetamine-induced dopamine increase showed only a significant main effect of time (F(5,25) = 12.945, P < 0.001). No significant effects were found for treatment per se (F(1,5) = 0.544, n.s.) and time × treatment interaction (F(5,25) = 0.672, n.s.).
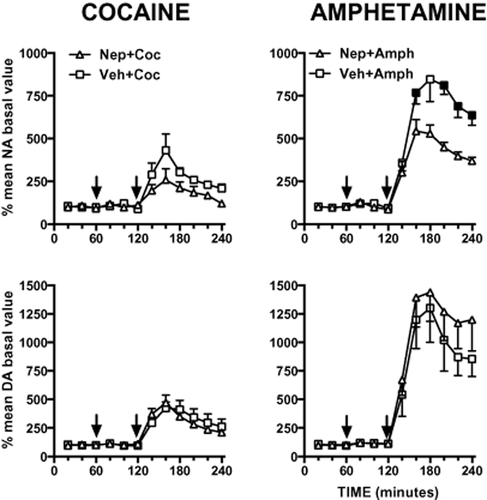
Consequence of nepicastat (Nep, 50 mg/kg i.p.) administration on cocaine (Coc, 10 mg/kg, i.p.) or amphetamine (Amph, 2.5 mg/kg i.p.) effects on extracellular noradrenaline (NA) and dopamine (DA) in the nucleus accumbens. Results are expressed as percent of mean basal level and are the mean ± standard error of the mean of at least four rats for each treatment. The first arrow indicates the time of nepicastat or its vehicle administration; the second arrow indicates time of cocaine or amphetamine administration. Black symbols indicate P < 0.05 for Bonferroni's post hoc comparisons with respect to each correspondent Veh + Amph time point
Discussion
In agreement with previous results with disulfiram (Devoto et al. 2012), nepicastat reduced tissue and extracellular noradrenaline both in the mPFC and in the nucleus accumbens and, like disulfiram, increased tissue and extracellular dopamine in the mPFC but not in the nucleus accumbens.
Nepicastat- and disulfiram-induced reduction of noradrenaline is consistent with their ability to inhibit DBH. However, the fact that the two DBH inhibitors were approximately equally potent in modifying brain catecholamines, in spite of nepicastat being far more potent than disulfiram in inhibiting DBH in vitro (Schroeder et al. 2010), may be explained with a poor penetration of nepicastat across the blood brain barrier.
Effect of nepicastat on catecholamine release in the mPFC
Nepicastat-induced increase of extracellular dopamine should likely represent a nerve impulse mediated exocytotic release, since our previous studies demonstrated that disulfiram-induced extracellular dopamine accumulation was reversed by the local perfusion with tetrodotoxin (Devoto et al. 2012). Moreover, nepicastat-induced dopamine release in the mPFC was suppressed, as previously observed with disulfiram (Devoto et al. 2012), by the administration of the α2-adrenoceptor agonist clonidine, but not of the D2-agonist quinpirole, suggesting that α2-adrenoceptors but not D2-receptors are involved in the action of DBH inhibitors.
On the basis of these considerations, it might be suggested that both disulfiram and nepicastat, by suppressing noradrenaline synthesis and release, cause a lack of noradrenaline at α2-autoreceptors, resulting in noradrenergic disinhibition and, eventually, increased release of dopamine instead of noradrenaline from noradrenergic terminals in the mPFC.
This hypothesis is supported by the finding that disulfiram increased extracellular dopamine in the occipital cortex by the same magnitude as in the mPFC, consistent with similar noradrenergic innervation in the two cortices but inconsistent with the scanty dopaminergic innervation of the occipital cortex.
However, the possibility that mesoprefrontal dopaminergic neurons might be a source of dopamine released by DBH inhibitors should be also considered.
Electrophysiological and biochemical evidence indicates that mesoprefrontal dopamine neurons lack functional impulse-regulating somatodendritic- (Chiodo et al. 1984; Grace et al. 2007; Lammel et al. 2008) and synthesis-modulating nerve terminal autoreceptors (Chiodo et al. 1984; Galloway, Wolf & Roth 1986), have the lowest dopamine transporter expression compared with other subtype of dopaminergic neurons (Sesack et al. 1998; Lammel et al. 2008) and fire at higher frequency in a sustained fashion compared with nigrostriatal dopamine neurons (White & Wang 1984; Tam et al. 1990; Lammel et al. 2008). These peculiarities place mesoprefrontal dopamine neurons well suited to mediate a sustained dopamine release, as that produced by DBH inhibitors. These considerations suggest that DBH inhibitors may reduce noradrenaline concentration not only at α2-autoreceptors on noradrenergic neurons but also at α2-heteroreceptors on mesoprefrontal dopamine neurons and thereby increase dopamine release from nerve terminals of both types of neurons. Consistent with this possibility, α2-adrenoceptor immunoreactivity has been detected in most dopamine neurons in the ventral tegmental area (Lee et al. 1998) and α2-adrenoceptor antagonists, locally or systemically administered, have been shown to selectively increase dopamine release in the prefrontal cortex, similarly to DBH inhibitors (Matsumoto et al. 1998; Hertel, Fagerqist & Svensson 1999; Devoto et al. 2004a,b; Masana, Bortolozzi & Artigas 2011).
It is pertinent to note that nepicastat-induced increase of extracellular dopamine was associated with reduced extracellular noradrenaline, which argues against the notion that dopamine increase produced by α2-adrenoceptor antagonists, results from impaired reuptake of dopamine from extracellular spaces due to competition with noradrenaline for the same transporter (Carboni et al. 1990; Pozzi et al. 1994).
However, while the suppressant effect of clonidine on the effect of DBH inhibitors is compatible with the hypothesis that extracellular dopamine might originate from either noradrenergic or dopaminergic nerve terminals, quinpirole ineffectiveness to suppress dopamine release is apparently inconsistent with the hypothesis that dopamine may originate from dopaminergic terminals, since several in vivo (Galloway et al. 1986; Altar et al. 1987; Ozaki et al. 1989; Gobert et al. 1996) and in vitro studies (Talmaciu, Hoffmann & Cubeddu 1986; Plantjé et al. 1987; Hoffmann et al. 1988) have demonstrated the inhibitory effect of D2-like agonists on dopamine release in the mPFC. A possible explanation for the negative effect of quinpirole in our experiments might be that D2-autoreceptors were saturated by the high dopamine concentration present in the mPFC after DBH inhibition.
Potentiation of cocaine- and amphetamine-induced dopamine release
Irrespective of the source of dopamine released by DBH inhibitors, our results suggest that noradrenaline acting on α2-adrenoceptors exerts a tonic inhibitory control on dopamine release in the mPFC.
This hypothesis may explain why nepicastat and disulfiram potentiated cocaine- and amphetamine-induced dopamine release in the mPFC. In fact, cocaine does not directly release catecholamines from nerve terminals but inhibits their retrieval from extracellular spaces by blocking dopamine, noradrenaline and serotonin transporters (Torres, Gainetdinov & Caron 2003). Therefore, the magnitude of cocaine-induced extracellular dopamine accumulation should depend on the amount of nerve-impulse dependent dopamine release. Accordingly, nepicastat by reducing noradrenaline concentration at α2-adrenoceptors would eliminate the major constraint on cocaine-induced dopamine release. The same mechanism may explain nepicastat potentiation of amphetamine-induced dopamine release in the mPFC. Indeed, consistent with the hypothesis that amphetamine effect, as that of cocaine, is constrained by noradrenaline acting on α2-adrenoceptors, the effect of the combination of nepicastat-amphetamine was suppressed by clonidine. The finding that the α2-adrenoceptor antagonist RS 79948 reproduced nepicastat ability to potentiate amphetamine-induced dopamine release supports this hypothesis.
These results also suggest that amphetamine-induced dopamine release in the mPFC is impulse-dependent rather than being mediated by reverse transport (see Sulzer et al. 2005).
It is possible that mesoprefrontal dopamine neurons, due to their low uptake capacity (Sesack et al. 1998; Lammel et al. 2008) unlike nigrostriatal ones (Sulzer et al. 2005; Wallace 2012), take up an amount of dopamine insufficient to cause an impulse-independent reverse transport of dopamine.
On the other hand, since extracellular dopamine in the mPFC is mainly cleared from extracellular space by noradrenaline transporter (Carboni et al. 1990; Pozzi et al. 1994), it is likely that the high level of extracellular dopamine produced by amphetamine, cocaine and their combination with nepicastat is due to the blockade of noradrenaline rather than dopamine transporter.
Failure of nepicastat to modify cocaine and amphetamine in the nucleus accumbens
The finding that nepicastat, like disulfiram, failed to modify dopamine output and to potentiate cocaine- and amphetamine-induced dopamine release in the nucleus accumbens, indicates that in this region dopamine release is not tonically controlled by noradrenaline, as in the mPFC. Moreover, the failure of nepicastat and disulfiram to modify both basal extracellular dopamine concentrations and cocaine- and amphetamine-induced dopamine output in the nucleus accumbens is in contrast to previous observations in mice, indicating that chronic deficiency of prefrontal noradrenaline (with lesions or genetic elimination of DBH) was associated both with a reduced basal extracellular dopamine in the nucleus accumbens and striatum, and with the elimination of amphetamine- and cocaine-induced dopamine release in these regions (Ventura et al. 2003; Weinshenker et al. 2008). The discrepancy suggests that chronic, not acute, depletion of cortical noradrenaline is needed to produce reduction of dopaminergic transmission and insensitivity to psychostimulant-induced dopamine release in the nucleus accumbens. Future studies should clarify this issue.
Conclusion
A major outcome of this study is that nepicastat, like disulfiram, selectively increased dopamine in the mPFC and potentiated cocaine- and amphetamine-induced dopamine release. Moreover, this study has provided evidence that DBH inhibitors, by reducing noradrenaline release inactivate α2-adrenoceptor mediated inhibitory control of dopamine release in the mPFC.
The hypothesis that DBH inhibitors act by inactivating α2-adrenoceptors, suggests that they have a common mechanism of action with α2-adrenergic blockers in controlling dopamine release in the mPFC. Therefore, it will be of great interest to determine if DBH inhibitors share with α2-adrenoceptor antagonists the ability to potentiate, other than the effect of psychostimulants, also that of L-DOPA (Eltayb, Wadenberg & Svensson 2005), antipsychotics (Hertel et al. 1999; Wadenberg, Wilker & Svensson 2007; Masana et al. 2011) and antidepressants (Masana et al. 2012), on dopamine release in the mPFC. It is pertinent to note that pharmacological blockade of α2-adrenoceptors has been shown to induce reinstatement of cocaine-seeking behavior (Lee et al. 2004), while nepicastat and disulfiram, which putatively inactivate α2-adrenoceptors, have been shown to block reinstatement of cocaine-seeking behavior (Schroeder et al. 2010). To clarify the homologies between the action of DBH inhibitors and that of α2-adrenoceptor blockers, might help to differentiate the relative role of noradrenaline and dopamine in the action of the two classes of compounds.
Acknowledgements
We thank Biotie Therapies for the generous gift of nepicastat. This research was supported by the ‘Guy Everett Laboratory’ Foundation. The Authors declare no competing financial interests.
Authors Contribution
PD and GLG were responsible for the study concept and design. PD and GLG drafted the manuscript; PD supervised experiments and analyzed the data; VB performed statistics; PS and GF performed microdialysis and HPLC analysis. All authors reviewed content and approved final version for publication.




