Intermittent access ethanol consumption dysregulates CRF function in the hypothalamus and is attenuated by the CRF-R1 antagonist, CP-376395
Abstract
Corticotrophin-releasing factor (CRF) is a mediator of stress responses and a key modulator of ethanol-mediated behaviors. We report here that the CRF receptor 1 (CRF-R1) antagonist, CP-376395 reduces 20% ethanol consumption in animals trained to consume ethanol on an intermittent, but not a continuous, schedule. Furthermore, using [35S]GTPγS binding assays, we demonstrate that CRF-mediated G-protein signaling in the hypothalamus of the intermittent drinkers is decreased when compared to controls suggesting that the effects of CP-376395 are mediated by extrahypothalamic mechanisms. The present study provides further support for the use of CRF-R1 antagonists for the treatment of alcohol use disorders and suggests that ethanol consumption dysregulates CRF function in the hypothalamus.
There remains a need for development of more effective pharmacotherapies for the treatment of alcohol use disorders (AUDs). While the current list of Food and Drug Administration-approved compounds provides some relief, all suffer from limited effect sizes and poor patient compliance. One promising target for future development is the corticotrophin-releasing factor (CRF) system. Beginning with a study that examined the selective CRF-Receptor 1 (CRF-R1) antagonist, LWH-63 (Sabino et al. 2006), several studies have established the utility of CRF-R1 antagonists in attenuating the increased ethanol consumption that results from dependence induction by ethanol vapor (for review, see Koob 2010). While these early studies emphasized the importance of dependence for CRF effects, a recent study showed that binge-like, but not moderate, ethanol consumption in non-dependent mice is decreased following treatment with the CRF-R1 antagonist, CP-154,526 (Sparta et al. 2008). Another recent report demonstrated that the CRF-R1 antagonist, antalarmin, significantly decreased consumption of ethanol in non-dependent Wistar rats consuming 20% ethanol on both an intermittent and a continuous access (CA) schedule (Cippitelli et al. 2012). Taken together, these studies suggest that the CRF system may be recruited before the development of alcohol dependence and creates the possibility that early interventions with CRF-R1 antagonists in binge drinkers may yield positive therapeutic results.
The aim of the present study is to explore the utility of the highly selective, potent, soluble, CRF-R1 antagonist, CP-376395, in decreasing ethanol intake in Long-Evans rats trained to consume ethanol using the intermittent access ethanol (IAE) drinking procedure. In the initial study describing CP-376395, the compound was shown to exhibit decreased side effects on feeding behavior in rats and increased efficacy in fear potentiated startle and locus coeruleus excitation models when compared to other candidate CRF-R1 antagonists developed by Pfizer (Chen et al. 2008). This is the first study to our knowledge exploring the effects of this compound on ethanol self-administration.
All behavioral procedures were preapproved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines for the Humane Care and Use of Laboratory Animals. Briefly, adult male Long-Evans rats (Harlan, Indianapolis, IN, USA: 200–225 g at first drinking session) were trained to consume 20% ethanol using one of two schedules: CA (24 hours a day 7 days a week; n = 10) or IAE (three 24 hours sessions per week on Monday, Wednesday and Friday, as previously described (Simms et al. 2008), n = 19 [the data from two separate cohorts of animals (n = 9–10 per group) were combined]. Importantly, both groups of animals were trained to consume ethanol without the use of initiation procedures, such as sucrose fading, and food and water were available ad libitum throughout the experiment. Following at least 9 weeks of ethanol consumption (67 and 27 drinking sessions for the CA and IAE animals, respectively), we examined the effects of systemic (intraperitoneal; i.p.) administration of the CRF-R1 antagonist, CP-376395 (Tocris, Ellisville, MO, USA), on drinking behavior. CP-376395 (5 or 10 mg/kg) or vehicle (2% dimethyl sulfoxide in distilled water) were tested across a 3-week test period in a Latin square design, thus, each animal served as its own control. The injections were administered 30 minutes before the start of the drinking session, which was initiated approximately 1 hour after the lights were turned off in the 12 hours reversed light-dark cycle room (lights off 10 a.m.). Bottles were weighed 30 minutes, 6 hours and 24 hours following the start of the session. One animal from each group was excluded from the study owing to failure to complete the full dose response curve.
As in our previous studies, animals trained on the IAE schedule consumed significantly greater amounts of ethanol on average during each individual 24 hours drinking session (4.93 ± 0.55 gram per kilogram (g/kg) for the IAE group versus 2.86 g/kg ± 0.45 for the CA group) and during the first 30 minutes of each session (0.93 ± 0.06 g/kg for the IAE group versus 0.57 g/kg ± 0.05 for the CA group). A repeated-measures two-way analysis of variance (ANOVA) examining the effect of CP-376395 treatment on ethanol consumption at the 30 minutes time point revealed a significant effect of dose [F(3, 107) = 2.83, P < 0.05] and a significant group × dose interaction [F(3, 107) = 3.05, P < 0.05] but no significant effect of group [F(1, 107) = 2.58, n.s.]. Student-Newman-Keuls post hoc analysis revealed a significant difference in baseline consumption between the two groups and a significant reduction in ethanol intake following pretreatment with the 10 mg/kg dose in the IAE, but not the CA, group when compared to both the pretreatment baseline and the vehicle treatment (Fig. 1a). Linear regression analysis comparing baseline ethanol consumption (g/kg/30 minutes) with the efficacy of the 10 mg/kg dose of CP-376395 (% change from baseline) revealed a significant correlation in the IAE group (P < 0.05, r2 = 0.26, Fig. 1b) but not in the CA 0.26 group (n.s., r2 = 0.22, Fig. 1c). The correlation in the IAE group suggests that CP-376395 is more effective at decreasing drinking in the higher consuming animals. A repeated-measures two-way ANOVA of the ethanol intake 6 hours into the session revealed a significant effect of group [F(1, 107) = 10.86, P < 0.01] and a significant group × dose interaction [F(3, 107) = 3.59, P < 0.05] but no significant effect of dose [F(3, 107) = 1.92, n.s.]. Post hoc analysis revealed significant differences in vehicle and baseline consumption between the two groups and a significant reduction in ethanol intake following pretreatment with the 10 mg/kg dose in the IAE, but not the CA, group when compared to both the pretreatment baseline and the vehicle treatment (Supporting Information Fig. 1a). Two-way ANOVA analysis of the consumption 24 hours after CP-376395 treatment revealed a significant effect of group [F(1, 107) = 4.67, P < 0.05] but failed to reveal a significant effect of dose [F(3, 107) = 0.82, n.s.] or an interaction [F(3, 107) = 2.32, n.s., P = 0.08; data not shown].
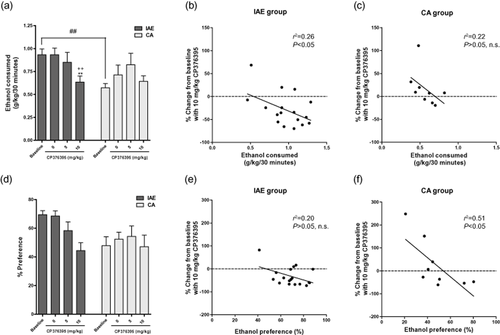
CP-376395 significantly reduced ethanol consumption (g/kg) in animals trained on the intermittent access (IAE) schedule 30 minutes after the onset of the drinking session but had no effect in the continuous access (CA) group (a). The efficacy of the 10 mg/kg dose was significantly correlated with the amount of ethanol consumed (g/kg/30 minutes) in the IAE group (P < 0.05, b), but not in the CA group (n.s., c). Statistical analysis of ethanol preference revealed no significant interaction between group and dose following CP-376395 pretreatment (c). There was a nonsignificant trend (P = 0.07) for the efficacy of the 10 mg/kg dose to be correlated with ethanol preference in the IAE group (e) and a significant correlation between ethanol preference and CP-376395 efficacy in the CA group (P < 0.05, f). The values are expressed as mean ± SEM. ** P < 0.01, compared to IAE vehicle treatment, ++ P < 0.01, compared to IAE baseline, ## P < 0.01, comparing IAE baseline to CA baseline. Two-way ANOVA with repeated measures, Newman–Keuls post hoc test
A repeated-measures two-way ANOVA examining the effect of CP-376395 treatment on ethanol preference at the 30 minutes time point revealed a significant effect of group [F(1, 107) = 4.95, P < 0.05] and an effect of dose [F(3, 107) = 2.79, P < 0.05] but no significant group × dose interaction [F(3, 107) = 1.95, n.s.: Fig. 1d]. Linear regression analysis comparing baseline ethanol preference (% preference at 30 minutes) with the efficacy of the 10 mg/kg dose of CP-376395 (% change from baseline) failed to reveal a significant correlation in the IAE group (n.s., r2 = 0.20, Fig. 1e), however, there was a strong trend (P = 0.07) for a correlation between high ethanol preference and increased efficacy of CP-376395. Linear regression analysis of the ethanol preference in the CA group did reveal a significant correlation between ethanol preference and the efficacy of CP-376395 (P < 0.05, r2 = 0.51, Fig. 1f). The efficacy of CP-376395 in decreasing ethanol preference in the CA group was highest in animals that exhibited the highest baseline preference for ethanol. This inverse correlation is similar in direction to the trend seen in the IAE group and indicates that higher levels of baseline ethanol preference tend to be correlated with increased efficacy of the drug, independent of the drinking schedule. A repeated-measures two-way ANOVA of the ethanol preference 6 hours into the session revealed a significant effect of group [F(1, 107) = 15.25, P < 0.001] and a significant group × dose interaction [F(3, 107) = 5.09, P < 0.01] but no significant effect of dose [F(3, 107) = 1.96, n.s.]. Post hoc analysis revealed significant differences in baseline preference and preference following vehicle and 5 mg/kg treatment between the two groups and a significant reduction in ethanol preference following pretreatment with the 10 mg/kg dose in the IAE, but not the CA, group when compared to both the pretreatment baseline and the vehicle treatment (Supporting Information Fig. 1b). Two-way ANOVA analysis of the preference 24 hours after CP-376395 treatment revealed a significant effect of group [F(1, 107) = 13.30, P = 0.001] and a significant interaction [F(3, 107) = 3.72, P < 0.05] but failed to reveal a significant effect of dose [F(3, 107) = 0.70, n.s.; data not shown]. Two-way ANOVA analysis of the water intake revealed a significant interaction at the 6 hours time point [F(3, 107) = 2.79, P < 0.05] but not at the 30 minutes or 24 hours time points. Post hoc analysis of the 6 hours data revealed significant differences in baseline, vehicle and 5 mg/kg water consumption between the groups and a significant increase in water consumption following pretreatment with the 10 mg/kg dose in the IAE, but not the CA, group when compared to both the pretreatment baseline and the vehicle treatment (Supporting Information Fig. 1c).
To further explore the differences between the IAE and CA groups, brains were collected from animals in each of the two groups and an age-matched (20 weeks old), ethanol-naïve, water control group. CRF binding in the hypothalamus was then analyzed using the [35S]GTPγS binding assay using methods previously established in our laboratory for examining opioid binding (Nielsen et al. 2012) and described in detail in the Supporting Information. Importantly, brains were collected from the two ethanol drinking groups 21 hours after their last ethanol exposure. This time point was chosen to ensure that all the ethanol was eliminated from the body and the animal was in a state of acute withdrawal. The potency of CRF-mediated [35S]GTPγS binding was highest in the water group (EC50 = 137 ± 21 nM; P < 0.05; Fig. 2a, compared to the CA group, Student's t-test), moderate in the CA group (EC50 = 548 ± 21 nM; Fig. 2b) and very low in the IAE group (EC50 > 10 μM; Fig. 2c). A one-way ANOVA of the efficacy of CRF-mediated [35S]GTPγS binding, as measured by the Emax, revealed a significant effect of group [IAE versus CA versus water; F(2, 34) = 36.09, P < 0.0001]. Post hoc analysis further revealed that the Emax for the IAE group was significantly less than both the CA group and the age-matched water controls and the Emax was significantly greater in the water group than in the CA group (Fig. 2d). Linear regression analysis comparing baseline ethanol consumption (g/kg/24 hours) with the CRF binding efficacy at the highest concentration (% of basal binding at CRF 10 μM) failed to reveal a significant correlation for the CA group (n.s., r2 = 0.28, Fig. 2e) but did show a significant correlation for the IAE group (P < 0.05, r2 = 0.34, Fig. 2f). This correlation suggests that CRF activation in the hypothalamus of the IAE group is lower in the higher consuming animals.
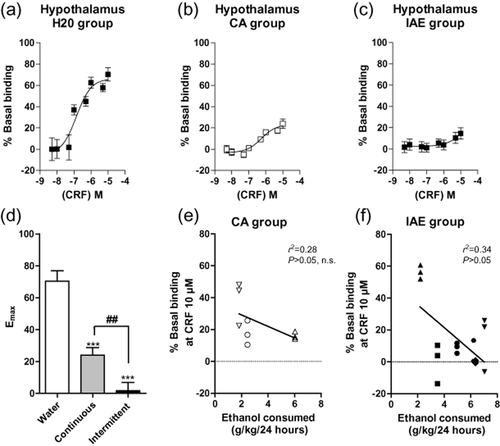
CRF-mediated [35S]GTPγS binding in the hypothalamus is highest in rats consuming water only (a), moderate in continuous access (CA) animals (b) and very low in intermittent access (IAE) animals (c). The efficacy (Emax value) of CRF-mediated [35S]GTPγS binding in the hypothalamus is attenuated in the IAE group and lower in the CA ethanol group compared to the water group (d). Linear regression analysis comparing baseline ethanol consumption (g/kg/24 hours) with the CRF binding efficacy at the highest concentration (% of basal binding at CRF 10 μM) failed to reveal a significant correlation for the CA group (e) but did show a significant correlation for the IAE group (P < 0.05, f). The values are expressed as mean ± SEM percentage increase in basal [35S]GTPγS binding. *** P < 0.001, ** P < 0.01, comparison between water and CA or IAE groups. ## P < 0.001, comparison between CA and IAE groups. One-way ANOVA with repeated measures, Newman–Keuls post hoc test. Each symbol in panels e and f signify triplicate binding for an individual animal
The present results provide more evidence that excessive ethanol consumption before the onset of dependence causes significant changes in CRF system function. In agreement with previous studies of binge-like ethanol consumption in mice (Sparta et al. 2008; Lowery-Gionta et al. 2012), we found that the CRF-R1 antagonist, CP-376395, was effective in rats that exhibited elevated ethanol intake (IAE) but not in those with moderate consumption levels (CA). Additionally, we found that the efficacy of the compound in reducing ethanol consumption was significantly correlated with the amount of ethanol consumed such that CP-376395 caused a greater reduction in the higher consuming animals trained on the IAE schedule. The IAE schedule yields significantly greater ethanol intake in each 24 hours drinking session when compared to the CA group. However, we have previously shown that the cumulative lifetime ethanol consumption for the two groups is significantly greater in animals consuming ethanol continuously (Nielsen et al. 2012). This suggests that differences in the drinking pattern are vital to the recruitment of the CRF system in Long-Evans rats. However, in contrast, a recently published study in Wistar rats found that antalarmin decreased drinking in animals consuming 20% ethanol on both an IAE and a CA schedule (Cippitelli et al. 2012). This discrepancy is most likely the result of strain differences as important basal differences in stress hormone levels (including CRF) have been described between Long-Evans and Wistar rats (Tannahill et al. 1988). There are also a couple of methodological differences between the studies that may contribute to the disparate findings including the length of exposure history and age differences upon first ethanol exposure. Interestingly, Cippitelli et al. showed that when Wistar rats were given a lower concentration of ethanol (10%), IAE, but not CA, drinking was attenuated by antalarmin. This result suggests that Wistar rats may have a different threshold for CRF activation following ethanol self-administration than Long-Evans rats. Also of note, the relative decreases caused by the CRF-R1 antagonists in the two studies are quite similar (∼25% reduction) and both studies report a significant increase in water consumption following treatment with the respective CRF-R1 antagonists.
The decreased G-protein coupled signaling in both the CA and IAE groups observed in the present study indicates that ethanol consumption dysregulates CRF function in the hypothalamus. However, this effect is more pronounced in the IAE group, which further suggests that the pattern in which ethanol is consumed has important physiological consequences. Combining the two major findings of this report, the low CRF binding in the hypothalamus of rats trained on the IAE schedule and the increased efficacy of systemically administered CP-376395 in these animals, leads to the conclusion that the effect of CP-376395 occur in extrahypothalamic brain structures. It has long been postulated that the effects of CRF on ethanol-mediated behaviors are controlled by extrahypothalamic brain circuitry (for review, see Koob 2010). Indeed, several studies have shown that chronic ethanol liquid diet exposure leads to alterations in hypothalamic-pituitary-adrenal axis function (Lee & Rivier 1993; Rasmussen et al. 2000). More recently, it has been shown that the attenuation of binge-like drinking in mice by a CRF-R1 antagonist is evident in adrenalectomized animals (Lowery et al. 2010) providing further support for an extrahypothalamic CRF effect on drinking. The blunted CRF response in the hypothalamus following chronic ethanol exposure may lead to compensatory changes in extrahypothalamic brain regions involved in the response to stress, such as the bed nucleus of the stria terminalis, the posterior shell of the nucleus accumbens and the central amygdala; areas shown to play a critical role in development of drug dependence (Koob 2010). Future studies aimed at examining changes in CRF receptor function in these regions following IAE exposure would perhaps lead the way to behavioral studies using local microinfusions of CRF-R1 antagonists into these brain sites to determine the region (or regions) that mediate these effects. Taken together, our data provide more evidence for the utility of CRF-R1 antagonists for the treatment of AUDs and further suggest that extrahypothalamic CRF receptors play a role in ethanol-mediated behaviors.
Acknowledgements
We would like to thank Dr. Subhashini Srinivasan for assistance editing the manuscript and Dr. T. Michael Gill for statistical help. This work was supported by funding from the State of California for Medical Research through UCSF to S.E.B and Department of Defense Grant W81XWH-10-1-0247 to S.E.B.
Authors Contribution
JAS and SEB were responsible for the development and design of the study and manuscript. JAS was responsible for collecting behavioral data. RL and CKN performed in vitro binding assays. JAS drafted the manuscript. CKN and SEB provided critical review of the article. All authors reviewed contents of the study and have approved the final version for publication.




