Association of smoking with μ-opioid receptor availability before and during naltrexone blockade in alcohol-dependent subjects
Abstract
Persons with a history of alcohol dependence are more likely to use tobacco and to meet criteria for nicotine dependence compared with social drinkers or non-drinkers. The high levels of comorbidity of nicotine and alcohol use and dependence are thought to be related to interactions between nicotinic, opioid and dopamine receptors in mesolimbic regions. The current study examined whether individual differences in regional μ-opioid receptor (MOR) availability were associated with tobacco use, nicotine dependence and level of nicotine craving in 25 alcohol-dependent (AD) subjects. AD subjects completed an inpatient protocol, which included medically supervised alcohol withdrawal, monitored alcohol abstinence, transdermal nicotine maintenance (21 mg/day) and Positron Emission Tomography (PET) imaging using the MOR agonist [11C]-carfentanil (CFN) before (basal scan) and during treatment with 50 mg/day naltrexone (naltrexone scan). Subjects who had higher scores on the Fagerström Nicotine Dependence Test had significantly lower basal scan binding potential (BPND) across mesolimbic regions, including the amygdala, cingulate, globus pallidus, thalamus and insula. Likewise, the number of cigarettes per day was negatively associated with basal scan BPND in mesolimbic regions. Higher nicotine craving was significantly associated with lower BPND in amygdala, globus pallidus, putamen, thalamus and ventral striatum. Although blunted during naltrexone treatment, the negative association was maintained for nicotine dependence and cigarettes per day, but not for nicotine craving. These findings suggest that intensity of cigarette smoking and severity of nicotine dependence symptoms are systematically related to reduced BPND across multiple brain regions in AD subjects.
There is a strong association between heavy alcohol drinking and tobacco use. The rates of smoking, daily tobacco use and nicotine dependence increase with increasing levels of alcohol consumption and the presence of an alcohol use disorder (Falk, Yi & Hiller-Sturmhofel 2006). Approximately 80% of alcohol-dependent (AD) persons report regular tobacco use, and persons with alcohol use disorders endorse more nicotine dependence symptoms and find it more difficult to quit smoking (DiFranza & Guerrera 1990; Marks et al. 1997).
Clinical and preclinical research has established that the endogenous opioid system plays a key role in alcohol and nicotine reward and dependence (Oswald & Wand 2004; Hadjiconstantinou & Neff 2011). In rodents, systemic administration of nicotine increases the release of endogenous opioids in several brain areas, including the nucleus accumbens and striatum (Dhatt et al. 1995; Walters et al. 2005). Nicotine-induced behavioral sensitization, conditioned rewarding effects, antinociception and dependence are attenuated in mice with a targeted disruption in either the MOR gene or the preproenkephalin gene (Berrendero et al. 2010). Opiate antagonists (naloxone and naltrexone) block the effects of nicotine on various behaviors, including food-maintained operant responding (Corrigall, Herling & Coen 1988), and antinociception (Aceto et al. 1993) and also precipitate behavioral signs of withdrawal in nicotine-dependent rats (Malin et al. 1993). In mice that were tolerant to the antinociception effects of nicotine following chronic nicotine treatment, density of MOR was decreased in the caudate–putamen and nucleus accumbens (Galeote et al. 2006). However, others have reported that chronic nicotine increased MOR density in striatum in female rats and in the ventral tegmental area in male and female mice (Wewers et al. 1999; Walters et al. 2005).
Interactions between nicotinic, opioid and dopamine mechanisms are thought to contribute to the high levels of comorbidity of nicotine and alcohol dependence. Opioid receptors and nicotinic receptors are colocalized with dopamine (DA) receptors in several mesolimbic structures including the nucleus accumbens and amygdala (for review see McGehee 2006). Nicotine activates nicotinic acetylcholine receptors, which in turn increases the release of DA and endogenous opioids. Similar to preclinical findings (Malin et al. 1993), acute intravenous administration of the opioid antagonist naloxone increased withdrawal symptoms in nicotine-dependent smokers (Krishnan-Sarin, Rosen & O'Malley 1999). Studies in human subjects have shown that cigarette smoking increased plasma levels of endogenous opioid peptides (Pomerleau et al. 1983), and increased DA release in the ventral striatum (Brody et al. 2004). In controlled laboratory studies, administration of naltrexone reduced the relative reinforcing value of nicotinized cigarettes in a cigarette smoking choice paradigm (Rukstalis et al. 2005) and blocked the pleasurable subjective effects of cigarette smoking in heavy smokers (King & Meyer 2000).
There has been very limited research using positron emission tomography (PET) imaging to examine nicotine effects on the opioid system. Using the MOR-selective radiotracer [11C]carfentanil (CFN), heavy smokers had lower MOR availability in the thalamus, ventral basal ganglia and amygdala after smoking a denicotinized cigarette when compared with baseline scans in nonsmoking controls (Scott et al. 2007). To date, no studies have examined the chronic effects of smoking in persons with comorbid alcohol dependence. Using [11C]CFN PET studies, we demonstrated that MOR availability was higher across mesolimbic regions in recently abstinent AD subjects when compared with age-matched healthy control subjects (Weerts et al. 2011). In addition, we demonstrated that there was near maximum blockade of MOR throughout the brain in AD subjects maintained on the FDA recommended therapeutic dose of 50 mg/day naltrexone (Weerts et al. 2008). The current report is a secondary analysis of MOR availability as a function of nicotine use and dependence in brain volumes involved in alcohol and nicotine reinforcement and dependence in the 25 AD subjects who completed these previous studies. Based on the previous findings in heavy smokers and nonsmokers (Scott et al. 2007), we hypothesized that greater smoking intensity, severity of nicotine dependence and nicotine craving would be associated with lower MOR availability. We also speculated that naltrexone treatment would attenuate these relationships.
Methods
Subjects
Screening procedures for study enrollment including assessment instruments and demographics information have been reported previously (Weerts et al. 2011). Subjects provided informed consent in the sober state, using an Institutional Review Board–approved informed consent document. All subjects met DSM-IV criteria for alcohol dependence as determined with the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA-II) and were actively drinking prior to admission to the Clinical Research Unit (CRU) as measured using the Time Line Follow Back (TLFB) (Sobell & Sobell 1992). Subjects were excluded if they met current or lifetime DSM-IV diagnostic criteria for another Axis I disorder, including other drug abuse/dependence (except nicotine); had a positive urine drug toxicology test for cocaine, meth-amphetamine, tetrahydrocannabinol (THC), opiates, oxycodone or benzodiazepines at screening or hospital admission; had any ongoing health problems or reported maternal problem drinking during pregnancy. In order to avoid potential confounds of withdrawal-related medications during study procedures, subjects were excluded who reported alcohol-related seizures or the need for medication (e.g. benzodiazepines) during previous detoxifications. The secondary analysis included 15 Caucasian and 10 black AD subjects. Subjects ranged in age from 25 to 61 (mean 43.6 years) and were mostly male (n = 18 male and 7 female). Twenty of the 25 subjects were current smokers.
General procedures
The 19-day inpatient protocol included medically supervised alcohol detoxification and PET imaging as described previously (Weerts et al. 2008). During the inpatient stay, the Clinical Institute Withdrawal Assessment-Alcohol Revised (CIWA-Ar) was administered three times daily (at 8:00, 14:00 and 20:00 hours) during the first 5 days. No subject required withdrawal medication based on CIWA scores, vital signs and physician assessment. For the current analysis, we examined PET imaging data from [11C]CFN scans conducted before (basal scan) and during naltrexone treatment (naltrexone scan). The basal scan was conducted on day 5 of alcohol abstinence, after alcohol withdrawal symptoms as measured by CIWA-AR, had subsided (mean score on day 5 = 0.44 + 1.1 SD). The naltrexone scan was completed on day 18 of the inpatient stay, while subjects were maintained on naltrexone. Oral naltrexone dosing (50 mg) was initiated on day 15, and subjects received four doses of 50 mg naltrexone prior to the naltrexone scan. The duration of naltrexone dosing was determined based our prior laboratory study (McCaul et al. 2000) showing stable levels of naltrexone and its active metabolite (6-β-naltrexol) in plasma were reached over three consecutive days of administration of 50 mg naltrexone (see also Weerts et al. 2008).
Cigarette smoking was prohibited throughout the inpatient stay. Smokers who were nicotine dependent, as determined by the Fagerström Nicotine Dependence Test (FNDT) score of 3 or more (n = 15), were medicated at inpatient admission, each morning and 3 hours prior to the PET scans with transdermal nicotine replacement therapy (NRT; 21 mg nicotine). Smokers with FNDT scores of 0–2 (n = 5) were not medicated with NRT. This standardized approach of treatment with the same nicotine dose patch across subjects was used to reduce potential confounds introduced by different levels of nicotine and to minimize the impact of nicotine withdrawal. Each day of the inpatient stay (at 8:00, 14:00 and 20:00 hours), subjects completed four nicotine craving questions using visual analog scales (VAS): How much do you WANT a smoke or nicotine? How much do you NEED a smoke or nicotine? How much do you DESIRE a smoke or nicotine? How much do you CRAVE a smoke or nicotine? Subjects were instructed to mark items on a scale ranging from 1 (not at all) to 9 (extremely) to reflect how they felt since the last measurement.
Before the PET scan, urine toxicology screens and breath alcohol tests were conducted to verify abstinence from alcohol and drugs. On the day of the scan, all subjects received a calorie-controlled breakfast 3 hours before the scan.
PET imaging procedures
A thermoplastic mask was individually fitted to each subject's face for immobilization and positioning during MRI and PET imaging. Subjects underwent magnetic resonance imaging (MRI) to allow anatomical localization and alignment of PET imaging planes within subjects (Meltzer et al. 1990). PET scans were acquired in 3D mode on a GE Advance PET scanner (GE Medical Systems, Milwaukee, WI). A transmission scan of 10-minute duration was obtained using rotating germanium-68 rods before intravenous bolus injection of the radiotracer, [11C] carfentanil (CFN) (19.4 ± 2.1 mCi SA: 17 298 ± 13 907 mCi/μmol). A set of 25 images with variable time periods (6 × 30 seconds, 5 × 60 seconds, 5 × 120 seconds, 9 × 480 seconds) was acquired during a 90-minute period for each study. PET images were reconstructed using the back projection algorithm with a ramp filter using the software provided by the manufacturer, correcting for attenuation, scatter and dead time. Radioactivity was corrected for physical decay to the injection time. Each PET frame consisted of a 128 × 128 × 35 matrix with voxel size of 2 × 2 × 4.25 mm.
PET image analyses
MOR availability was determined as CFN regional non-displaceable binding potential (BPND), as defined by Innis et al. (2007). BPND values were obtained via reference tissue graphical analysis (RTGA) using occipital lobe as the reference region and setting the k2 value (k2R) of the reference region at 0.104/minute. Detailed information on PET image analyses has been provided previously (Weerts et al. 2011).
Volumes of interest (VOI)
Eight brain VOIs were selected from mesolimbic opioid-rich regions based on their association with alcohol and nicotine reinforcement, dependence and craving: ventral striatum, amygdala, caudate nucleus, cingulate, globus pallidus, insula, putamen and thalamus. VOIs were manually defined on SPGR MRI for putamen, caudate nucleus, insula, and thalamus using a locally developed VOI defining tool. Ventral striatum was separated as previously described (Oswald et al. 2005). Additional VOIs were customized using a VOI template (available at http://www.loni.ucla.edu). VOIs were spatially transferred to individual subjects' MRI using the MRI-to-MRI spatial normalization parameters from the SPM spatial normalization module available at http://www.fil.ion.ucl.ac.uk/spm and edited to suit outlines of the structures given by the MRI. Those VOIs were transferred to PET space according to the MRI-to-PET co-registration parameters obtained with the SPM2 co-registration module and applied to individual PET frames to obtain time–radioactivity curves (TACs) of VOIs.
Statistical analysis
Since the effects of NRT on BPND have not been studied, we conducted an analysis to compare BPND as a function of NRT status (patch versus no patch) in smokers only. As indicated in the results, there was no significant difference between groups, so NRT status was not included in the final model. Mixed effect models with random intercept were constructed separately for the basal and naltrexone scans to test the correlation between BPND from the eight brain VOIs and smoking measures. The primary independent variables for each model were (1) severity of nicotine dependence symptoms (FNDT score), (2) smoking intensity (cigarettes per day) and (3) level of nicotine craving reported prior to each of the PET scans (VAS score). Gender and recent drinking (total number of standard drinks for the 90-day TLFB period that preceded study assessment) were included as covariates in the model. Gender differences in BPND have been previously reported (Zubieta, Dannals & Frost 1999). Previous studies have demonstrated a strong association of alcohol drinking intensity with both smoking intensity (i.e. number of cigarettes per day) and severity of nicotine dependence symptoms (Falk et al. 2006). A compound symmetry variance/covariance structure was used to account for the high correlation between individual brain VOIs. Significance was accepted at P < 0.05. All analyses were carried out using SAS 9.3.
Results
Table 1 shows means and ranges for alcohol consumption and nicotine dependence measures in AD subjects. In smokers only, there was no significant effect of NRT status on the basal scan BPND using our mixed model with patch status (patched versus unpatched) and VOI with random intercept (P = 0.5499), or when adding gender to the model (P = 0.5841). Thus, patch status was not included in subsequent models, and all smokers were pooled in further analyses.
| Mean (Range) | |
|---|---|
| All subjects (n = 25) | |
| Total drinks in 90 days before assessment | 915.2 (200–2749) |
| Average number of drinks per drinking day | 12.4 (4.2–37.2) |
| Smokers only (n = 20) | |
| Number of cigarettes per day | 17.2 (2–40) |
| Fagerström nicotine dependence test score | 4.4 (0–9) |
| Nicotine craving score before basal scan | 14.4 (0–36) |
| Nicotine craving score before naltrexone scana | 9.6 (0–36) |
- a Significant difference (P = 0.0033) when compared with the basal scan craving as determined via Wilcoxon matched pairs test.
Basal scan
When adjusting for gender and recent drinking as covariates in our main model, there was a strong negative association between severity of nicotine dependence symptoms (as measured by FNDT score) and basal scan BPND across all eight VOI (Table 2, Column 1). Figure 1 shows partial residual plots for FNDT score and BPND in four regions (amygdala, insula, cingulate and thalamus) that have been previously associated with smoking (Ray et al. 2011). Basal scan BPND decreased as a function of increasing FNDT score. There was also a negative association for smoking intensity and basal scan MOR BPND in six brain VOIs. Specifically, basal scan BPND in amygdala, cingulate, globus pallidus, insula, putamen and thalamus decreased significantly as a function of increasing numbers of cigarettes per day (Table 2, column 2). When we examined the relationship between nicotine craving score on the day of the scan and basal scan BPND, nicotine craving was also inversely related to BPND in the amygdala, the globus pallidus, putamen, thalamus and ventral striatum (Table 2, column 3). The strongest relationship was in the amygdala. As shown in Fig. 2 (left panel), basal scan BPND in the amygdala decreased as a function of increased nicotine craving. The magnitude of VAS nicotine craving score on the day of the basal scan was positively correlated with the severity of nicotine dependence symptoms measured via the FNDT (P = 0.0058).
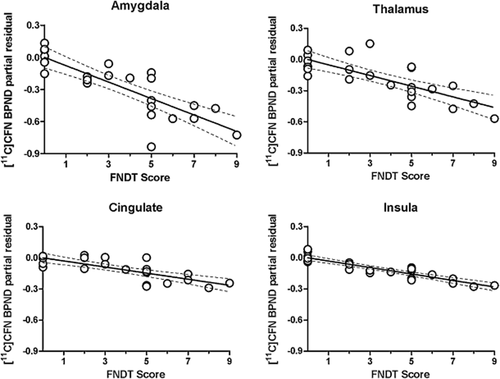
Partial residual plots showing the relationship between [11C]CFN BPND and FNDT Scores in amygdala, thalamus, cingulate and insula controlling for gender and recent drinking (total drinks in the 90 days prior to assessment as determined via the TLFB). Data points are individual subjects. The regression line indicates the partial fit. Dotted lines represent ±95% confidence intervals. Statistics [β and P] are displayed in Table 2
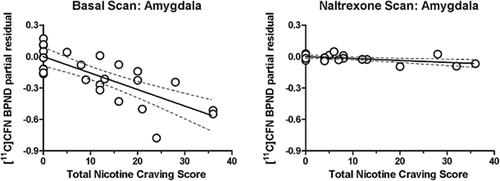
Partial residual plots showing the relationship between [11C]CFN BPND and total nicotine craving score in amygdala for the basal scan (left) and the naltrexone scan (right), adjusted for gender and recent drinking. Nicotine craving was determined via visual analog scales (VAS). Other details as in Fig. 1. Statistics [β and P] are displayed in Tables 2 and 3
| VOI | 1. FNDT Scorea | 2. Cigarettes per day | 3. Nicotine craving score | |||
|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | |
| Amygdala | −0.077 (0.015) | < 0.0001 | −0.014 (0.003) | < 0.0001 | −0.016 (0.004) | 0.0002 |
| Caudate nucleus | −0.037 (0.018) | 0.037 | −0.004 (0.004) | 0.325 | −0.007 (0.004) | 0.129 |
| Cingulate | −0.029 (0.012) | 0.016 | −0.005 (0.003) | 0.058 | −0.004 (0.003) | 0.179 |
| Globus pallidus | −0.055 (0.015) | 0.0003 | −0.009 (0.003) | 0.005 | −0.009 (0.004) | 0.026 |
| Insula | −0.031 (0.011) | 0.007 | −0.006 (0.002) | 0.021 | −0.005 (0.003) | 0.1 |
| Putamen | −0.038 (0.013) | 0.004 | −0.005 (0.003) | 0.062 | −0.008 (0.003) | 0.017 |
| Thalamus | −0.051 (0.014) | 0.0004 | −0.008 (0.003) | 0.008 | −0.008 (0.004) | 0.032 |
| Ventral Striatum | −0.051 (0.022) | 0.020 | −0.008 (0.005) | 0.087 | −0.012 (0.005) | 0.027 |
- a The FNDT score was missing in one subject; n = 24 for this analysis.
| VOI | 1. FNDT Score | 2. Cigarettes per day | 3. Nicotine craving score | |||
|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | |
| Amygdala | −0.014 (0.006) | 0.013 | −0.003 (0.001) | 0.016 | −0.002 (0.002) | 0.246 |
| Caudate nucleus | −0.008 (0.006) | 0.217 | −0.002 (0.002) | 0.269 | −0.001 (0.002) | 0.508 |
| Cingulate | −0.012 (0.006) | 0.037 | −0.003 (0.001) | 0.023 | −0.001 (0.002) | 0.453 |
| Globus pallidus | −0.014 (0.006) | 0.028 | −0.003 (0.002) | 0.031 | −0.003 (0.002) | 0.147 |
| Insula | −0.01 (0.005) | 0.057 | −0.003 (0.001) | 0.031 | −0.002 (0.002) | 0.272 |
| Putamen | −0.01 (0.006) | 0.144 | −0.003 (0.002) | 0.111 | −0.003 (0.002) | 0.131 |
| Thalamus | −0.011 (0.007) | 0.107 | −0.002 (0.002) | 0.142 | −0.001 (0.002) | 0.501 |
| Ventral Striatum | −0.012 (0.007) | 0.072 | −0.003 (0.002) | 0.046 | −0.002 (0.002) | 0.288 |
- a Two subjects did not complete the naltrexone scan due to problems with synthesis of [11C]CFN on the scheduled day of the scan.
Naltrexone scan
The 50 mg/day naltrexone dose attenuated or eliminated the relationships between the smoking variables and BPND observed in the basal scan. Specifically, the negative association between FNDT score and BPND was maintained in the amygdala, cingulate and globus pallidus (Table 3, column 1); but a relationship was no longer observed in the other VOIs. Likewise, there was negative association between number of cigarettes per day and BPND in the amygdala, cingulate, globus pallidus, insula and ventral striatum for the naltrexone scan (Table 3, column 2); but the strength of the association was reduced.
Nicotine craving scores were significantly reduced while subjects were maintained on naltrexone (Table 1, P = 0.0033), and there was no association between craving scores and naltrexone scan BPND across regions (Table 3, column 3). Figure 2 shows this change in the association of BPND and craving from baseline to naltrexone administration.
To visually present regional differences associated with severity of nicotine dependence, we selected subjects with FNDT scores indicative of severe nicotine dependence (FNDT score ≥ 6; n = 5) and subjects with no nicotine dependence (FNDT score = 0; n = 7). Separate mean BPND volume images were constructed for each group for the basal scan (Fig. 3, top panel) and the naltrexone scan (Fig. 3, bottom panel). As shown in Fig. 3 (top panel), subjects with FNDT scores of 6 or more showed lower basal BPND at the level of the amygdala, insula, thalamus and cingulate, when compared with subjects with FNDT scores of 0. As shown in Fig. 3 (bottom panel), naltrexone blocked CFN binding across these VOIs, but to a greater extent in subjects with FNDT scores of 6 or more (column C).
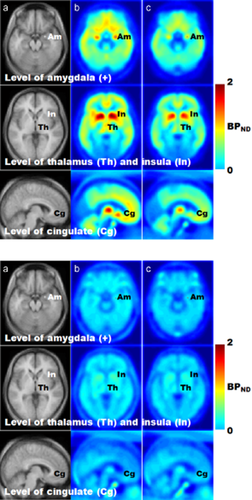
MRI images (a) and averaged [11C]CFN BPND brain images for the basal scan (top panel) and the naltrexone scan (bottom panel) in subjects with no nicotine dependence (n = 7) (b) and severe nicotine dependence (n = 5) (c) as defined by their FNDT scores. For each panel: the upper row shows trans-axial images at the level of amygdala (Am). Middle row shows trans-axial images at the level of thalamus (Th) and insula (In). Lower row shows sagittal images, 6 mm left from the midline at the level of cingulate (Cg). Images shown are color coded according to the [11C]CFN BPND ranging from lowest (0, blue color) to highest (2, red color)
Discussion
In AD subjects, severity of nicotine dependence symptoms, number of cigarettes smoked and craving for nicotine was negatively associated with MOR availability in many mesolimbic regions at the basal scan. Specifically, basal scan BPND in amygdala, caudate, cingulate, globus pallidus, insula, putamen, thalamus and ventral striatum decreased as a function of increased severity of nicotine dependence symptoms. Similarly, basal BPND in the amygdala, cingulate, globus pallidus, insula, putamen and thalamus decreased as a function of the numbers of cigarettes per day. Lower basal scan BPND in the amygdala, globus pallidus, putamen and thalamus as well as the ventral striatum was associated with higher nicotine craving. Importantly, nicotine craving was significantly decreased during naltrexone treatment compared with baseline levels. In addition, during naltrexone treatment, the relationships between smoking variables and BPND were attenuated or eliminated depending on the VOI.
It is most likely that these negative associations between smoking intensity and severity of nicotine dependence symptoms with MOR availability reflect the effects of chronic nicotine administration. Although the effects of chronic nicotine per se on the MOR have been inconsistent across animal studies (for review see Berrendero et al. 2010), nicotine exposure resulting in the development of nicotine tolerance is strongly associated with lower MOR availability (Galeote et al. 2006). Likewise, nicotine withdrawal symptoms were significantly attenuated in mice with targeted disruptions of the MOR when compared with wild-type mice (Berrendero et al. 2010). Scott et al. (2007) observed that heavy smokers had lower [11C]CFN BPND after smoking a denicotinized cigarette when compared with the baseline scan in nonsmoking controls. This could reflect a nicotine-induced down-regulation of MOR associated with chronic, heavy nicotine use, as in the current study. However, the lower MOR availability following the denicotinized cigarette in the Scott et al. study may also reflect acute changes in endogenous opioids associated with exposure to smoking cues and nicotine deprivation. As reported previously, we found no relationship between severity of alcohol withdrawal symptoms (as determined via the CIWA) and MOR availability in AD subjects (Weerts et al. 2011). Lastly, there are no preclinical or clinical studies to guide prediction of directional effects of nicotine craving severity on BPND.
Compared with nicotine craving reported at the basal scan, nicotine craving was decreased on the day of the naltrexone scan, and there was an attenuated association between BPND and nicotine craving at the time of the naltrexone scan. The weakening of association between nicotine craving and BPND in the context of naltrexone treatment is likely due in part to the near maximal occupancy of the MOR by naltrexone (mean + SD% inhibition = 94.9 + 4.9%), as reported previously (Weerts et al. 2008). However, it also is possible that the weakening of association between nicotine craving and BPND during the naltrexone scan is due to naltrexone's direct effects on nicotine craving. In laboratory studies, naltrexone administration reduced craving and urges to smoke after smoking a cigarette (King & Meyer 2000) or exposure to smoking cues (Hutchison et al. 1999); although other studies have reported that naltrexone failed to alter nicotine craving (Wong et al. 1999; Rohsenow et al. 2003; Toll et al. 2010), or produced increased negative effects on mood (Brauer et al. 1999). Similar to these laboratory studies, the efficacy of naltrexone for smoking cessation in clinical trials has also been mixed (for review see Schnoll & Lerman 2006; Berrendero et al. 2010). Thus, the effects of naltrexone on craving and BPND may be interdependent in that naltrexone may have reduced both concurrently. Finally, it is possible that nicotine craving simply decreased over time while the participants received NRT on the inpatient unit. The relative contribution of these different factors on the attenuated relationship between nicotine craving and BPND during naltrexone treatment cannot be determined from the current data. For an improved understanding of these relationships, future studies are needed to evaluate whether the association between nicotine craving and BPND is altered when abstinent smokers are maintained on naltrexone versus placebo.
A recent analysis of data from the COMBINE (Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence) study (Anton et al. 2006) suggests that smoking status may modulate naltrexone effects on drinking in AD subjects (Fucito et al. 2012). Although in general smokers had poorer treatment outcomes than nonsmokers, smokers who received naltrexone had significantly greater drinking reductions than smokers who received placebo. In contrast, there was no difference in drinking outcomes for nonsmokers as a function of naltrexone treatment assignment. This effect was independent of differences in behavioral interventions, alcoholism typography and baseline demographics. The authors proposed nicotine-induced changes in the endogenous opioid system as one of the primary mechanisms contributing to these differences in naltrexone treatment response. Our observed inverse relationships between BPND and smoking intensity, severity of nicotine dependence symptoms and nicotine craving provide direct, in vivo evidence in humans of the changes in the opioid system related to chronic smoking, which may in turn affect naltrexone efficacy for alcohol dependence.
Do our findings inform the well-documented comorbidity between alcohol and nicotine dependence in humans? There is pharmacological interaction between alcohol and nicotine resulting in greater behavioral and physiological effects when both drugs are consumed in combination compared with the effects of each drug administered alone (Perkins et al. 1995; Kouri et al. 2004; Sayette et al. 2005; Barrett et al. 2006). When AD subjects are compared with healthy control subjects, PET imaging studies in our laboratory (Weerts et al. 2011) and others (Heinz et al. 2005) have demonstrated that MOR availability is increased in mesolimbic regions in AD subjects following brief alcohol abstinence. In one study, the MOR availability observed in AD subjects was positively correlated with severity of alcohol craving measured using the Obsessive Compulsive Drinking Scale (Heinz et al. 2005). In the current analysis of AD subjects only, BPND was lower as a function of both increasing numbers of cigarettes smoked per day and severity of nicotine dependence symptoms. Thus, smoking appears to modulate the upregulation in MOR associated with alcohol withdrawal and increased alcohol craving. In this model, AD persons may smoke in part to minimize the onset and severity of alcohol craving.
Although the data from the current study are compelling, there are limitations that may influence generalization of these findings. First, all of the subjects were AD. Because of the smaller number of healthy control subjects who smoked (Weerts et al. 2011), we were not able to conduct similar analyses in these subjects. Second, the current findings may not extend to persons with greater severity of alcohol dependence, as we selected subjects without prior histories of serious alcohol withdrawal symptoms. Third, genetic variations in the mu opioid receptor alter MOR binding characteristics and appear to play a role in the effects of nicotine. Previous studies in human subjects have found that the single nucleotide polymorphism (SNP) Asn40Asp (A118G) of the MOR gene OPRM1 was associated with nicotine reinforcement (Ray et al. 2006), smoking initiation and dependence (Zhang, Kendler & Chen 2006) and successful short-term smoking abstinence with transdermal NRT (Lerman et al. 2004; Munafo et al. 2007). In a recent [11C]CFN PET study, Ray et al. (2011) found that smokers carrying one or two copies of the minor G allele had lower [11C]CFN BPND in bilateral amygdala, left thalamus and left anterior cingulate cortex compared with smokers homozygous for the major A allele. Our previous analysis of A118G genotype effects on global [11C]CFN BPND also showed that AD and healthy control subjects who were carriers of the G allele had lower global BPND when controlling for both alcohol dependence diagnosis and smoking status (Weerts et al. 2012). We do not believe that variation in the OPRM1 gene contributed to our findings, as the proportion of subjects carrying the minor allele of the A118G SNP did not differ in smokers and nonsmokers.
Despite the above limitations, there are a number of important strengths in the current study design. First, AD subjects completed all procedures while remaining on the inpatient unit for the duration of the study. This provided the opportunity to collect PET images and subjective assessments under conditions in which alcohol and smoking abstinence were verified, and nicotine exposure was controlled using nursing-monitored NRT. Second, the analyses included covariates to control for possible BPND differences related to gender or recent alcohol consumption.
The current findings add to the growing literature demonstrating the importance of the opioid system in both alcohol and nicotine dependence. Several of the key regions associated with smoking intensity and severity of nicotine dependence symptoms in the current study (e.g. amygdala, cingulate and insula) have been previously associated with acute effects of smoking on [11C]CFN BPND (Scott et al. 2007; Ray et al. 2011) and higher neuronal activity in fMRI studies in smokers exposed to acute nicotine administration or nicotine-associated cues (Janes et al. 2010; Addicott et al. 2012). The amygdala in particular has been proposed to play a key role in nicotine addiction and dependence (Mihov & Hurlemann 2012). Indeed, the association of BPND with smoking intensity and severity as well as nicotine craving was strongest in the amygdala in the current study.
In conclusion, these data support that cigarette smoking intensity and severity of nicotine dependence symptoms are associated with MOR availability in mesolimbic regions of the human brain. Nicotine craving, which was positively correlated with severity of nicotine dependence symptoms, also was negatively associated with [11C]CFN BPND. These data are compelling given the high comorbidity of alcohol and nicotine use and dependence and evidence from previous studies that AD subjects in early abstinence had higher [11C]CFN BPND in mesolimbic regions than healthy control subjects. Importantly, these data suggest that nicotine and alcohol dependence may be associated with opposite effects on the μ-opioid system and that such interactions may contribute to the comorbidity of nicotine and alcohol use and dependence.
Acknowledgements
This research was supported by the National Institute of Alcohol Abuse and Alcoholism (AA11872, AA11855 and AA12303). The authors would like to acknowledge the technical support of Dr. Hayden T. Ravert, Mr. Robert Smoot and Mr. Daniel Holt for their radiochemistry expertise, Ms. Karen Edmonds and David Clough for their PET acquisition and reconstruction expertise.
Disclosure/Conflict of Interest
Dr. Wand is the recipient of a gift fund from the Kenneth Lattman Foundation. He is an investigator in a post-marketing study for Eli Lilly & Company, entitled The Global Hypopituitary Control and Complications Study (HypoCCS). He is an investigator in a post marketing study for Ipsen entitled, Somatuline Depot (lanreotide) Injection for Acromegaly (SODA). Dr. Wong is a consultant for Amgen. Between 2009 and present, Dr. Wong has received funding from the following companies: Acadia, Amgen, Avid, Biotie, Bristol Myers Squibb, GE, Intracellular, J&J, Lilly, Luhdeck, Merk, Orexigen, Otuska, Roche, Sanofi-Aventis and Sepracor. Dr. McCaul was principal investigator on a contract, ‘A Phase 2 Study of LY2196044 Compared with Naltrexone and Placebo in the Treatment of Alcohol Dependence’, funded by Lilly Research Laboratories. Drs. Weerts and Wand were co-investigators on this project. Dr. Kuwabara and Mr. Xu have no financial disclosures.
Authors Contribution
All authors contributed to the experimental design, data collection, analysis and manuscript preparation. MEM McCaul, GSW and JJF secured grant funding for the data represented in the current manuscript. All authors critically reviewed content and approved the final version for publication.




