Donepezil, an acetylcholinesterase inhibitor, attenuates nicotine self-administration and reinstatement of nicotine seeking in rats
Abstract
Nicotine craving and cognitive impairments represent core symptoms of nicotine withdrawal and predict relapse in abstinent smokers. Current smoking cessation pharmacotherapies have limited efficacy in preventing relapse and maintaining abstinence during withdrawal. Donepezil is an acetylcholinesterase inhibitor that has been shown previously to improve cognition in healthy non–treatment-seeking smokers. However, there are no studies examining the effects of donepezil on nicotine self-administration and/or the reinstatement of nicotine-seeking behavior in rodents. The present experiments were designed to determine the effects of acute donepezil administration on nicotine taking and the reinstatement of nicotine-seeking behavior, an animal model of relapse in abstinent human smokers. Moreover, the effects of acute donepezil administration on sucrose self-administration and sucrose seeking were also investigated in order to determine whether donepezil's effects generalized to other reinforced behaviors. Acute donepezil administration (1.0 or 3.0 mg/kg, i.p.) attenuated nicotine, but not sucrose self-administration maintained on a fixed-ratio 5 schedule of reinforcement. Donepezil administration also dose-dependently attenuated the reinstatement of both nicotine- and sucrose-seeking behaviors. Commonly reported adverse effects of donepezil treatment in humans are nausea and vomiting. However, at doses required to attenuate nicotine self-administration in rodents, no effects of donepezil on nausea/malaise as measured by pica were observed. Collectively, these results indicate that increased extracellular acetylcholine levels are sufficient to attenuate nicotine taking and seeking in rats and that these effects are not due to adverse malaise symptoms such as nausea.
Introduction
Approximately, 59.8 million Americans (23.9% of the population) smoke cigarettes on a regular basis (Substance Abuse and Mental Health Services Administration 2009). Cigarette smoking remains the leading cause of preventable death in the United States, accounting for approximately 443 000 premature deaths annually, or about 1 out of every 5 deaths (CDC 2012). Smoking cessation, at any age, results in significant increases in life expectancy (Taylor et al. 2002). Even though 70% of smokers express desire to quit, only 3% of smokers quit successfully on their own (Benowitz 2010). Indeed, most smokers relapse within the first 8 days following a quit attempt (Hughes, Keely & Naud 2004). Medications approved by the Food & Drug Administration (FDA) for smoking cessation effectively maintain long-term abstinence (>12 months) in only 1 out of every 4 smokers attempting to quit (Schnoll & Lerman 2006). Therefore, there is a critical need to develop more efficacious smoking cessation pharmacotherapies for nicotine dependence.
Nicotine is the principal psychoactive chemical in tobacco smoke that mediates tobacco's reinforcing effects (Baker, Massey & Smith 2004). Nicotine binds to and stimulates nicotinic acetylcholine receptors, ligand-gated ion channels activated by the endogenous neurotransmitter acetylcholine. Chronic nicotine exposure is associated with altered levels of acetylcholine. For example, extracellular acetylcholine levels are elevated and expression of choline acetyltransferase, the enzyme responsible for synthesizing acetylcholine, is increased in the brain following repeated nicotine administration in rats (Arnold et al. 2003; Hernandez & Terry 2005). In contrast, choline acetyltransferase activity is decreased in rats during nicotine withdrawal (Slotkin et al. 2008). Smoking cessation and nicotine withdrawal are also associated with drug craving and cognitive impairments (Hughes & Hatsukami 1986; Kenny & Markou 2001). A growing literature indicates that cognitive deficits represent a core symptom of nicotine withdrawal that predict relapse during abstinence (Patterson et al. 2010; Powell et al. 2010). Thus, it has recently been proposed that cognitive-enhancing medications may prevent drug craving and relapse, in part, by reversing or normalizing nicotine withdrawal-induced cognitive impairments (Sofuoglu 2010; Brady, Gray & Tolliver 2011). Consistent with these findings, nicotine re-exposure (Davis et al. 2005; Myers et al. 2008), nicotine replacement therapies (Atzori et al. 2008) and the α4β2 nicotinic acetylcholine receptor partial agonist varenicline (Raybuck et al. 2008) reverse abstinence-induced cognitive deficits and blunt relapse in both humans and rodents. Taken together, these findings suggest that other cognitive-enhancing drugs that modulate endogenous acetylcholine levels and cholinergic transmission in the brain may prevent smoking relapse.
Acetylcholinesterase inhibitors increase extracellular levels of acetylcholine in the brain and augment cholinergic transmission through inhibition of acetylcholinesterase, a catabolic enzyme responsible for metabolizing acetylcholine in the synapse. Acetylcholinesterase inhibitors are FDA-approved for treating cognitive impairments associated with mild to moderate Alzheimer's disease (Terry & Buccafusco 2003; Pepeu & Giovannini 2009). Recent evidence demonstrates that administration of galantamine, an acetylcholinesterase inhibitor that also functions as a positive allosteric modulator of nicotinic acetylcholine receptors (Harvey 1995; Maelicke & Albuquerque 2000; Samochocki et al. 2003), improves cognitive performance following nicotine withdrawal in mice (Wilkinson & Gould 2011) and attenuates nicotine taking and seeking in rats (Hopkins et al. 2012). These results suggest that galantamine and other acetylcholinesterase inhibitors may serve as potential pharmacotherapies for smoking cessation. However, it is not clear whether drugs that act exclusively as acetylcholinesterase inhibitors attenuate nicotine taking and seeking.
The present study examined the potential effects of the acetylcholinesterase inhibitor donepezil on nicotine taking and the reinstatement of nicotine seeking, an animal model of relapse in abstinent human smokers (Shaham et al. 1997; Mathieu-Kia et al. 2002). Donepezil has a different pharmacokinetic profile than galantamine and functions solely as a potent, reversible acetylcholinesterase inhibitor (Jann, Shirley & Small 2002; Goh et al. 2011). In addition to examining the effects of systemic donepezil administration on nicotine reinforcement and nicotine-seeking behavior, these experiments also assessed the role of donepezil in modulating sucrose self-administration and reinstatement in order to examine the specificity of this drug treatment in appetitive/reinforced behaviors. The reported adverse effects of donepezil are similar to other drugs that increase cholinergic transmission and include malaise symptoms, such as nausea and vomiting (Dunn, Pearce & Shakir 2000; Pratt et al. 2002; Farlow et al. 2010). Therefore, the effects of acute donepezil administration on ad libitum food intake and pica, an animal model that is used to assess rodent consumption of non-nutritive materials (e.g. kaolin clay) in response to nauseating agents (Mitchell et al. 1976; Kanoski et al. 2012), were tested in separate cohorts of rats. We hypothesized that acute donepezil administration would attenuate nicotine, but not sucrose, taking and seeking.
Materials and Methods
Animals and housing
Male Sprague Dawley rats (Rattus norvegicus) weighing 225–250 g were obtained from Taconic Laboratories (Germantown, NY, USA). Initially, animals were single-housed with access to standard lab chow and water ad libitum. Rats used for nicotine self-administration and reinstatement studies were mildly food restricted (approximately 20 g chow daily) to 85–90% of their free-feeding body weight following recovery from surgery. Mild food restriction was used to facilitate acquisition and maintenance of nicotine self-administration similar to previously published reports (Corrigall & Coen 1989; Fowler et al. 2011; Yan et al. 2012b). Food intake experiments were conducted in separate cohorts of animals that were maintained on ad libitum access to chow, except as noted below. All animals were housed in a colony maintained on a 12-hour/12-hour reverse light/dark cycle, with lights off at 7:00 a.m. All experimental procedures were conducted during the dark phase of the light/dark cycle. All experimental protocols were in accordance with the guidelines set forth by the National Institutes of Health and were approved by the University of Pennsylvania School of Medicine Institutional Animal Care and Use Committee.
Materials
All self-administration experiments were conducted in ventilated, sound-attenuating operant chambers purchased from Med-Associates Inc. (East Fairfield, VT, USA). Each operant chamber was equipped with both active and inactive response levers, a sucrose pellet dispenser, cue lights, tone generator, as well as an automated injection pump for administering drug or vehicle solutions intravenously.
Surgery
Rats were handled daily and allowed 1 week to acclimate to their home cages upon arrival. Prior to surgery, the rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine (Sigma Aldrich/RBI, St. Louis, MO, USA). An indwelling silicone catheter (CamCaths, Cambridge, UK) was inserted into the right, external jugular vein and sutured securely in place. The catheter was connected to a mesh backmount, which was implanted subcutaneously above the shoulder blades. To prevent infection and maintain patency, catheters were flushed daily with 0.3 ml of a solution of the antibiotic Timentin (0.93 mg/ml; Fisher, Pittsburgh, PA, USA) dissolved in heparinized 0.9% saline (Butler Schein, Dublin, OH, USA). When not in use, catheters were sealed with plastic obturators.
Nicotine self-administration
Rats were allowed 7 days to recover from surgery before behavioral testing commenced. Initially, rats were placed in operant chambers and allowed to lever press for intravenous nicotine (0.03 mg/kg nicotine/59 μl saline, infused over 5 seconds) on a fixed-ratio 1 (FR1) schedule of reinforcement. Each nicotine infusion was paired with a light/tone cue. Stable responding on the FR schedules of reinforcement was defined as less than 20% variation in response rates over three consecutive self-administration days. After stable responding was achieved, the schedule of reinforcement was increased to fixed-ratio 3 (FR3) for 3–5 days and then finally increased to a fixed-ratio 5 (FR5) schedule. For all FR schedules, a 20 seconds time-out period followed each nicotine infusion, during which time active lever responses were recorded but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during each 2-hour daily self-administration session and were used as a measure of nonspecific behavioral activation.
Sucrose self-administration
Rats were trained initially to lever press for 45 mg sucrose pellets (Research Diets, Inc., New Brunswick, NJ, USA) on a FR1 schedule of reinforcement during 1-hour, daily operant sessions. Once animals achieved stable responding for sucrose (defined as <20% variation in responding over three consecutive sessions) on the FR1 schedule of reinforcement, the response requirement was increased to a FR5 schedule of reinforcement. Animals were limited to 30 sucrose pellets within a 1-hour operant session and were restricted to 20 g of lab chow daily (Harlan Teklad, Wilmington, DE, USA) in their home cages for the duration of the experiment. Subjects were mildly food restricted in order to maintain consistency with the nicotine self-administration experiments (i.e. to ensure similar motivational states). Each successful completion of the response requirement resulted in delivery of a sucrose pellet as well as contingent presentation of light/tone cues.
Experiment 1: effects of donepezil on nicotine and sucrose self-administration behaviors
The effects of acute donepezil were examined in rats that acquired stable nicotine or sucrose self-administration on a FR5 schedule of reinforcement. A between-session, within-subjects design was used to test the effects of donepezil on nicotine- and sucrose-taking behaviors. Each test day was separated by at least 2 days of nicotine or sucrose self-administration to ensure that nicotine or sucrose taking had stabilized between test sessions. In all experiments, subjects were pretreated with donepezil (0, 0.3, 1.0 or 3.0 mg/kg, i.p.) 20 minutes prior to the beginning of the operant session. All doses of donepezil were counterbalanced and tested in each rat. Doses of donepezil and time course of administration were selected based on previously published reports (Geerts et al. 2005; Reid & Sabbagh 2008; Giorgetti et al. 2010; Kendall et al. 2011).
Experiment 2: effects of donepezil on the reinstatement of nicotine- and sucrose-seeking behaviors
The effects of acute donepezil on nicotine and sucrose seeking were studied in separate cohorts of animals that were not previously tested with donepezil during the self-administration phase of the experiment. Following approximately 21 days of daily nicotine self-administration sessions, drug-taking behavior was extinguished by replacing the nicotine solution with 0.9% saline. Light/tone cues previously paired with nicotine infusions during the self-administration phase were turned off. Daily 2-hour extinction sessions continued until responding on the active lever was <20% of the response rate maintained by nicotine self-administration under the FR5 schedule of reinforcement. Typically, it took approximately 3–7 days for rats to meet this criterion. Once nicotine self-administration was extinguished, the ability of a priming injection of nicotine (0.2 mg/kg, s.c.) plus light/tone cues to reinstate nicotine seeking was assessed. During each reinstatement test session, subjects were allowed to respond for light/tone cues that were previously paired with nicotine infusions. When an animal completed each response requirement, an intravenous infusion of 0.9% saline was delivered along with contingent presentations of the light/tone cues. Moreover, non-contingent presentation of the light/tone cues was delivered for 5 seconds at the beginning of each reinstatement test session and repeated once every 2 minutes for the first 10 minutes of the reinstatement session. On subsequent reinstatement test days, donepezil (0, 0.3, 1.0 and 3.0 mg/kg, i.p.) was administered 20 minutes prior to a priming injection of nicotine. Animals were placed immediately into the operant chambers following administration of a priming injection of nicotine and the 2-hour reinstatement session began. Using a between-session design, each reinstatement test session was followed by extinction days until responding was <20% of the maximum number of responses maintained by nicotine self-administration.
The effect of acute donepezil on the reinstatement of sucrose-seeking behavior was examined in a separate cohort of animals. After 14 days of sucrose-maintained responding on the FR5 schedule of reinforcement, sucrose self-administration was extinguished by inactivating the sucrose pellet dispenser. Light/tone cues that were previously paired with delivery of sucrose pellets during the self-administration phase were turned off. Once lever responding decreased to <20% of the maximum number of responses completed during sucrose self-administration, animals proceeded to reinstatement testing. During the reinstatement test session, subjects were allowed to respond for light/tone cues that were previously paired with sucrose pellet delivery during the self-administration phase. Acute injections of donepezil (0, 0.3, 1.0 and 3.0 mg/kg, i.p.) were administered 20 minutes prior to the beginning of the reinstatement session. The experimenter remotely administered one sucrose pellet every 2 minutes for the first 10 minutes of the reinstatement session. Moreover, non-contingent presentation of the light/tone cues was delivered for 5 seconds at the beginning of each reinstatement test session and repeated once every 2 minutes for the first 10 minutes of the reinstatement session. A between-session paradigm was used such that each daily 1-hour reinstatement session was followed by extinction sessions on subsequent days until responding was again <20% of the response rate maintained by sucrose. A within-subjects design was used for these studies and doses of donepezil were counterbalanced across reinstatement sessions.
Experiment 3: effects of donepezil on ad libitum food intake and pica
The effects of acute donepezil on ad libitum food intake and pica were studied in two separate cohorts of rats. All animals were single housed on a 12-hour/12-hour reverse light/dark cycle with the lights off at 7:00 am. Consistent with the aforementioned studies of donepezil on nicotine self-administration, all experimental procedures were performed during the dark phase of the light/dark cycle. Initially, rats were habituated to ad libitum kaolin pellets (Research Diets; K50001) for 4 days while maintained on standard rodent chow. Prior to experimental testing, rats were mildly food restricted from 4:00 pm to 7:00 am, with the majority of food restriction occurring during the animals' light cycle to ensure an acute, energy-depleted state similar to the chronic deprivation state of rats used in the nicotine self-administration studies (Hopkins et al. 2012). Subjects were pretreated with donepezil (0, 0.3, 1.0 and 3.0 mg/kg, i.p.) 20 minutes prior to testing. Pre-weighed standard rodent chow (Purina 5001) and kaolin pellets were placed in each cage at the onset of the dark cycle. Cumulative chow and kaolin intake (± 0.1g) were recorded 2 and 24 hours following onset of the dark cycle. Changes in bodyweight were also recorded over the 24-hour testing session. A within-subject, Latin-square design was used to test the potential effects of donepezil on ad libitum food consumption and kaolin intake. Testing days were separated by a minimum of 48 hours to ensure that animals maintained stable feeding behavior and body weight gain between treatments.
Statistical analyses
Total active and inactive lever responses during all donepezil experiments were analyzed with one-way mixed-factors analyses of variance (ANOVAs). Total chow and kaolin consumption as well as changes in bodyweight were also analyzed with one-way ANOVAs. Time-course data were analyzed with mixed-factors ANOVAs, with repeated measures over time. Pairwise comparisons were made with Tukey's honestly significant difference (HSD) (P < 0.05).
Drugs
(–)Nicotine hydrogen tartrate salt (Sigma Aldrich, St. Louis, MO, USA) was dissolved in sterile 0.9% saline (pH was adjusted to 7.4 ± 0.5 with sodium hydroxide). Donepezil hydrochloride (Tocris, Ellisville, MS, USA) was dissolved in sterile 0.9% saline. Nicotine doses are reported as freebase concentrations.
Results
Acute donepezil administration dose-dependently attenuated nicotine, but not sucrose, self-administration in rats
The average number of nicotine infusions [mean ± standard error of the mean (SEM)] self-administered during the last operant session prior to testing the effects of donepezil on nicotine taking was 15.4 ± 0.65, which corresponds to an average daily consumption of 462 ± 53 μg/kg nicotine. The amount of nicotine consumed is within the range of nicotine intake reported in other short access nicotine self-administration paradigms, albeit on the lower end of the range (Matta et al. 2007). While it is not clear whether the level of nicotine intake observed in the present study produces a state of physical dependence in these animals, previous studies that employed a similar short access paradigm have demonstrated physical dependence as measured by mecamylamine-precipitated somatic withdrawal signs and changes in brain reward thresholds (Matta et al. 2007).
Total lever responses (mean ± SEM) for animals self-administering nicotine are shown in Fig. 1a (n = 14). The active lever data from Fig. 1a were analyzed with a one-way ANOVA, which revealed a significant main effect of treatment [F(3,52) = 6.589, P < 0.05]. Subsequent pairwise analyses showed that total active lever responses were significantly different between subjects pretreated with saline and those pretreated with 1.0 or 3.0 mg/kg donepezil (Tukey's HSD, P < 0.05). Total inactive lever responses shown in Fig. 1a were analyzed with a one-way ANOVA. No significant differences were found on inactive lever responding between treatments [F(3,52) = 0.562, P < 0.64]. Figure 1b displays total active lever responses (mean ± SEM) completed during each 10-minute component of the 2-hour operant session for subjects treated with donepezil. A mixed-factors ANOVA with repeated measures over time was used to analyze these data. The results of this analysis showed significant main effects of treatment [F(3,50) = 9.353, P < 0.0001] and time [F(11,550) = 3.954, P < 0.0001], as well as a treatment × time interaction [F(33,550) = 1.653, P < 0.02]. Subsequent pairwise analyses showed that the active lever response rate was significantly different between animals treated with saline and 1.0 mg/kg donepezil for the 50 and 90 minutes time periods and 3.0 mg/kg donepezil during the 10, 20, 30 and 50 minutes time periods (Tukey's HSD, P < 0.05).
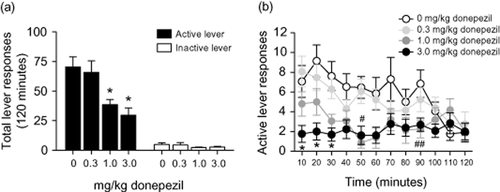
Systemic administration of the acetylcholinesterase inhibitor donepezil dose-dependently attenuated nicotine self-administration. (a) Data in panel (a) depict the total number of responses [mean ± standard error of the mean (SEM) ] on the active and inactive levers following systemic administration of 0, 0.3 1.0 or 3.0 mg/kg donepezil in rats self-administering nicotine on a fixed-ratio 5 schedule of reinforcement (n = 14 per treatment). The asterisk represents a significant difference from vehicle and 1.0 or 3.0 mg/kg donepezil [Tukey's honestly significant difference (HSD), P < 0.05]. No significant differences in responding on the inactive lever (mean ± SEM) were found between treatments. (b) The timecourses of active lever responses (mean ± SEM) for the data presented in panel (a). The active lever responses for the 3.0 mg/kg donepezil treatment were significantly different from saline control animals during the 12-, 20- and 30-minute time components as indicated by the asterisks (Tukey's HSD, P < 0.05). Active lever responding was also significantly different between subjects pretreated with saline and those pretreated with 1.0 and 3.0 mg/kg donepezil at the 50-minute time component as indicated by the pound sign (Tukey's HSD, P < 0.05). The active lever responses for the 1.0 mg/kg donepezil treatment were also significantly different from saline control animals during the 90-minute time component as indicated by the double pound sign (Tukey's HSD, P < 0.05)
The behaviorally relevant doses of donepezil (i.e., 1.0 and 3.0 mg/kg) that attenuated nicotine self-administration had no effect on sucrose taking (see Fig. 2). Total active lever responses (mean ± SEM) were analyzed with a one-way ANOVA in animals pretreated with donepezil (n = 10). No significant differences in active lever responding were found between treatments [F(3,36) = 2.204, P < 0.11].
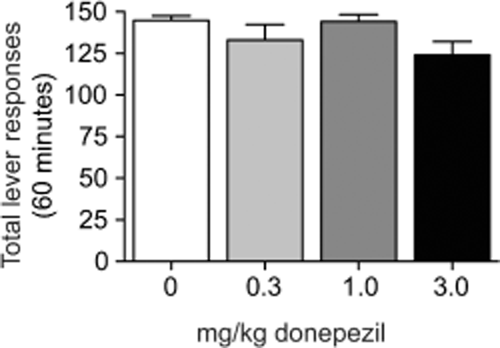
Acute, systemic administration of donepezil did not alter sucrose self-administration. Total number of lever responses [mean ± standard error of the mean (SEM) ] in subjects (n = 10 per treatment) self-administering sucrose pellets on a fixed-ratio 5 schedule of reinforcement. No significant differences in responding were noted between subjects pretreated with vehicle, 0.3, 1.0 or 3.0 mg/kg donepezil
Acute donepezil administration dose-dependently attenuated the reinstatement of nicotine- and sucrose- seeking behavior in rats
Total lever responses (mean ± SEM) following systemic administration of donepezil prior to the nicotine reinstatement test session are plotted in Fig. 3a (n = 10). Total active lever responses were analyzed using a one-way ANOVA, which revealed a significant main effect of treatment [F(3,36) = 13.41, P < 0.0001]. Pairwise analyses revealed a significant difference in responding on the active lever between the saline and 1.0 and 3.0 mg/kg donepezil treatments (Tukey's HSD, P < 0.05). Significant differences in active lever responding were also revealed between animals treated with 0.3 or 1.0 mg/kg donepezil and those subjects treated with 3.0 mg/kg donepezil (Tukey's HSD, P < 0.05). No significant differences on inactive lever responding were found between treatments [F(3,36) = 2.235, P < 0.10]. The active lever response rate for each 10-minute component of the reinstatement test session is shown in Fig. 3b. These data were analyzed using a mixed-factors ANOVA, with repeated measures over time. This analysis revealed significant main effects of treatment [F(3,36) = 12.99, P < 0.0001] and time [F(11,396) = 22.86, P < 0.0001], as well as a treatment × time interaction [F(33,396) = 4.578, P < 0.0001]. Subsequent pairwise analyses showed that the active lever response rate was significantly different between animals treated with saline and 1.0 or 3.0 mg/kg donepezil during the 10- and 20-minute time periods (Tukey's HSD, P < 0.05). A significant difference in active lever responding was also revealed between animals treated with saline and 3.0 mg/kg donepezil during the 30-minute time component (Tukey's HSD, P < 0.05).
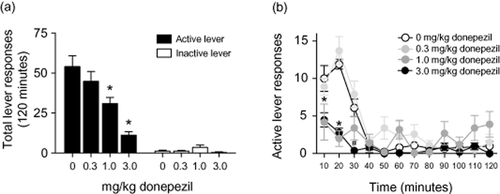
Systemic donepezil administration dose-dependently attenuated the reinstatement of nicotine-seeking behavior in rats. (a) Total number of lever responses [mean ± standard error of the mean (SEM) ] on the active and inactive levers following systemic administration of 0, 0.3, 1.0 or 3.0 mg/kg donepezil (n = 10 per treatment). Donepezil dose-dependently attenuated drug seeking induced by a priming injection of nicotine and cues previously associated with nicotine self-administration. The asterisk represents a significant decrease in responding on the active lever in animals treated with 1.0 or 3.0 mg/kg donepezil when compared with animals treated with saline [Tukey's honestly significant difference (HSD), P < 0.05]. No significant differences in responding on the inactive lever (mean ± SEM) were noted between treatments. (b) The timecourses of active lever responses (mean ± SEM) for the data summarized in panel a. The active lever responses for the 1.0 and 3.0 mg/kg donepezil treatments were significantly different from the saline control animals during the 10- and 20-minute time bins (Tukey's HSD, P < 0.05). A significant difference in active lever responding also was noted between saline controls and subjects treated with 3.0 mg/kg donepezil during the 30-minute time component as indicated by the pound sign (Tukey's HSD, P < 0.05)
The effects of systemic donepezil administration (n = 16) on sucrose reinstatement are shown in Fig. 4. Total active lever responses (mean ± SEM) were analyzed with a one-way ANOVA, which revealed a significant main effect of treatment [F(3,60) = 7.332, P < 0.001]. Subsequent pairwise analyses showed that total active lever responses were significantly different between subjects pretreated with saline or 0.3 mg/kg donepezil and those pretreated with 3.0 mg/kg donepezil (Tukey's HSD, P < 0.05). No significant differences in active lever responding were noted between animals pretreated with vehicle and those receiving 1.0 mg/kg donepezil (Tukey's HSD, P > 0.05).

Acute, systemic administration of donepezil dose-dependently attenuated the reinstatement of sucrose seeking in rats. Total number of lever responses (mean ± standard error of the mean) completed during the 1-hour sucrose reinstatement session for animals receiving systemic saline, 0.3, 1.0 or 3.0 mg/kg donepezil (n = 16 per treatment). A significant difference in responding was found between animals pretreated with saline or 0.3 mg/kg donepezil and those subjects pretreated with 3.0 mg/kg donepezil 20-minute prior to the sucrose reinstatement session (Tukey's honestly significant difference, P < 0.05)
Acute donepezil administration transiently decreased food intake without producing pica in rats
In order to determine whether the effects of donepezil on nicotine self-administration were due to drug-induced nausea/malaise, total chow and kaolin intake were measured in two separate cohorts of animals. Total chow consumed (mean ± SEM) following systemic administration of donepezil is plotted in Fig. 5a. A one-way ANOVA revealed a significant main effect of treatment on chow intake 2 hours following injection [F(3,42) = 3.467, P < 0.03]. Subsequent pairwise analyses revealed a significant difference in 2 hours chow intake between saline and 3.0 mg/kg donepezil treatments (Tukey's HSD, P < 0.05). No significant differences were found on chow intake between treatments 24 hours following injection [F(3,42) = 0.4710, P < 0.70]. Figure 5b depicts total kaolin intake (mean ± SEM) following systemic administration of donepezil. No significant differences were found between treatments on kaolin intake at 2 hours [F(3,42) = 0.757, P < 0.52] or 24 hours [F(3,42) = 1.024, P < 0.39] following injection. Total body weight (mean ± SEM) 24 hours following injection of donepezil is plotted in Fig. 5c. Systemic donepezil administration did not affect 24 hours body weight gain [F(3,42) = 0.671, P < 0.57].
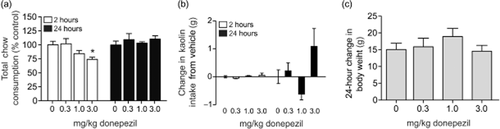
Systemic donepezil administration transiently altered feeding behavior without producing pica in rats. Total chow (a) and kaolin (b) consumed [mean ± standard error of the mean (SEM) ] 2- and 24-hour after systemic administration of 0, 0.3, 1.0 and 3.0 mg/kg donepezil are plotted (n = 10–12 subjects per treatment). A significant difference in total chow consumed was found between animals receiving 3.0 mg/kg donepezil when compared with saline controls 2-hour following injection (Tukey's honestly significant difference, P < 0.05). In contrast, no significant differences in total chow consumed 24-hour following injection were noted. Kaolin consumption 2- and 24-hour following injection was not significantly different between treatments. Total kaolin consumed (mean ± SEM) 2-hour following administration of 0, 0.3, 1.0, and 3.0 mg/kg donepezil was 0.1 ± 0.07, 0.04 ± 0.02, 0.08 ± 0.03, and 0.16 ± 0.08 g, respectively. Total kaolin consumed (mean ± SEM) 24-hour following administration of 0, 0.3, 1.0, and 3.0 mg/kg donepezil was 0.35 ± 0.27, 0.56 ± 0.29, 0.84 ± 0.23, and 1.44 ± 0.63 g, respectively. (c) No significant changes in body weight gain were noted 24-hour following systemic administration of saline, 0.3, 1.0 or 3.0 mg/kg donepezil
Discussion
This is the first study to demonstrate that systemic administration of the acetylcholinesterase inhibitor donepezil attenuates nicotine taking and seeking in rats. Here, we show that acute administration of donepezil dose-dependently attenuated nicotine, but not sucrose, self-administration in animals maintained on a FR schedule of reinforcement. Moreover, acute donepezil administration dose-dependently attenuated the reinstatement of nicotine- and sucrose-seeking behaviors. However, acute donepezil administration transiently decreased ad libitum feeding behavior at the highest dose tested. Decreased nicotine taking and seeking were not due to drug-induced nausea/malaise (as measured by kaolin intake), as donepezil administration did not produce pica in these animals. These results indicate that increased cholinergic transmission in the brain is sufficient to attenuate nicotine reinforcement and the reinstatement of nicotine seeking. However, further studies are required to determine whether the present findings reflect a leftward shift in the nicotine dose–response curve, which would indicate a decrease in the reinforcing efficacy of nicotine.
Nicotine reinforcement and acetylcholinesterase inhibitors
A growing body of evidence indicates that enhanced cholinergic signaling is critically involved in nicotine addiction (Changeux 2010; De Biasi & Dani 2011; Tuesta, Fowler & Kenny 2011). Galantamine and donepezil increase cholinergic transmission in the brain by inhibiting acetylcholinesterase, thereby increasing endogenous acetylcholine levels (Harvey 1995; Giacobini et al. 1996; Kosasa et al. 1999; Snape et al. 1999). While donepezil functions solely as a pharmacological inhibitor of acetylcholinesterase (Jann et al. 2002; Goh et al. 2011), galantamine also acts as a positive allosteric modulator of nicotinic acetylcholine receptors (Harvey 1995; Maelicke & Albuquerque 2000; Samochocki et al. 2003). Extracellular acetylcholine levels in the brain are significantly increased 20 minutes following acute administration of galantamine or donepezil and remain elevated for at least 60 minutes and 3 hours, respectively (Giorgetti et al. 2010). A recent report demonstrated that acute galantamine administration attenuated nicotine, but not sucrose, self-administration in rats maintained on FR and progressive ratio schedules of reinforcement, suggesting that galantamine pretreatment decreased both the reinforcing efficacy of nicotine as well as the animals' motivation to self-administer nicotine (Hopkins et al. 2012). Consistent with these results, the present study demonstrated that the net effect of donepezil pretreatment in animals self-administering nicotine is an overall decrease in responding. These findings suggest that pharmacological inhibition of acetylcholinesterase alone (i.e. without the positive allosteric modulatory properties of galantamine) is sufficient to attenuate nicotine self-administration in rats. Future studies are required to determine exactly how donepezil shifts the dose-response curve for nicotine self-administration. Decreased responding for nicotine in animals pretreated with 1.0 and 3.0 mg/kg donepezil may reflect an increase in the reinforcing efficacy of nicotine and/or increased drug satiation analogous to higher unit doses of nicotine. Consistent with this hypothesis, a recent study demonstrated that acetylcholinesterase inhibitor administration generalizes to the discriminative stimulus properties of nicotine, which suggests that these compounds share similar interoceptive stimulus properties (Giarola, Auber & Chiamulera 2011). Therefore, it is likely that donepezil decreases nicotine taking, in part, by increasing endogenous acetylcholine levels in the brain and producing subjective effects similar to nicotine. Further support for this hypothesis comes from recent studies demonstrating that the α4β2 nicotinic acetylcholine receptor partial agonist varenicline also generalizes to the discriminative stimulus properties of nicotine (Rollema et al. 2007; Smith et al. 2007) and attenuates nicotine taking and seeking in rats (O'Connor et al. 2010; George et al. 2011).
It is unlikely that donepezil-induced attenuation of nicotine taking is due to drug-induced motor suppressant effects or increased adverse effects (i.e. sickness, etc.). Each operant chamber was equipped with an inactive lever, responses upon which are commonly used as a measure of nonspecific alterations in lever responding. Systemic donepezil administration had no effect on inactive lever responding. However, one could argue that responses on the inactive lever are uniformly too low to meaningfully assess potential rate suppressant effects of drug treatment. Therefore, we also assessed the ability of systemic donepezil infusions to alter sucrose self-administration. Donepezil pretreatment did not significantly affect sucrose taking, which suggests that the effects of donepezil are not due to general motor disruption or sedation and do not generalize to other reinforced behaviors. The selectivity of donepezil's effects on nicotine reinforcement are also supported by the ad libitum feeding data which revealed that acute donepezil administration does not reduce normal feeding behavior up to doses of 1.0 mg/kg. The ability of the highest dose (3.0 mg/kg) of donepezil to decrease 2 hour chow intake is consistent with a previous report demonstrating that high doses of an acetylcholinesterase inhibitor attenuate food taking in rats (Grasing, He & Yang 2008). Thus, an intermediate dose of donepezil selectively attenuated nicotine taking without disrupting responding for nondrug reinforcers.
An emerging literature indicates that donepezil administration may also attenuate psychostimulant and opiate addictions. For example, donepezil pretreatment significantly decreased the reinstatement of methamphetamine-seeking behavior in rats, albeit at lower doses than those that attenuated nicotine seeking in the present study (Hiranita et al. 2006). Moreover, the same doses of donepezil that attenuated nicotine taking and seeking (present study) have also been shown to block the rewarding effects of cocaine and morphine (Hikida et al. 2003). Taken together, these findings suggest that donepezil may be an efficacious pharmacotherapy for treating nicotine, psychostimulant and/or opiate dependence.
Reinstatement of nicotine-seeking behavior and acetylcholinesterase inhibitors
Similar to relapse in humans, reinstatement of drug-seeking behavior in rodents can be precipitated by three major stimuli: a stressful life event, an environmental stimulus previously associated or paired with the drug-taking event, or re-exposure to the previously self-administered drug itself (Shalev, Grimm & Shaham 2002; Schmidt et al. 2005). For example, following extinction of nicotine self-administration, a subcutaneous injection of nicotine or re-exposure to light/tone cues previously paired with nicotine infusions reinstates operant responding in the absence of drug reinforcement in rodents (Shaham et al. 1997; Bespalov et al. 2005; Fowler & Kenny 2011; Hopkins et al. 2012; Yan et al. 2012a). A combination of a priming injection of nicotine and cues elicits more robust nicotine seeking than a drug prime or cues alone (O'Connor et al. 2010; Feltenstein, Ghee & See 2012). The present study demonstrated that acute donepezil administration attenuated the reinstatement of nicotine-seeking behavior precipitated by a combination of a priming injection of nicotine and re-exposure to cues previously paired with nicotine infusions. These results are consistent with a previous study that demonstrated that acute administration of galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator of nicotinic receptors, blunts nicotine seeking in rats (Hopkins et al. 2012). However, the present findings suggest that increased endogenous acetylcholine levels alone are sufficient to block nicotine craving and relapse. Given the possible substitution-like mechanism of acetylcholinesterase inhibitors, it is conceivable that donepezil administration alone (i.e. without a priming injection of nicotine) could reinstate nicotine seeking. However, donepezil administration did not promote nicotine-seeking in a preliminary study (data not shown). It is also possible that donepezil has differential effects on cue- versus drug-primed reinstatement of nicotine seeking. Future studies are required to identify the precise role of donepezil in regulating these two aspects of nicotine seeking.
Studies demonstrating that acetylcholinesterase inhibitors attenuate nicotine seeking (present study and Hopkins et al. 2012) add to a growing literature indicating that increased cholinergic transmission plays a critical role in the reinstatement of nicotine seeking. For example, administration of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline (O'Connor et al. 2010) attenuates nicotine-seeking behavior in rats. Future studies are required to determine the exact contributions of nicotinic acetylcholine receptor subtypes to nicotine craving and relapse.
Acute donepezil administration also dose-dependently attenuated the reinstatement of sucrose-seeking behavior, which suggests that high doses of donepezil reduce cravings for highly palatable food. The same dose of donepezil that attenuated sucrose seeking also reduced total chow consumption 2 hours post injection. The effects of donepezil on ad libitum feeding behavior were transient and total chow consumption normalized across treatments 24 hours post injection. Taken together, these results indicate that, in addition to attenuating nicotine reinforcement and the reinstatement of nicotine seeking, high doses of donepezil also blunt highly palatable food seeking in rats. Smoking cessation is associated with a significant increase in bodyweight (∼4–5 kg) with the majority of the weight gain occurring within the first three months following a quit attempt (Audrain-McGovern & Benowitz 2011; Aubin et al. 2012). Therefore, high doses of donepezil may not only inhibit smoking relapse, but also, curb withdrawal-induced hyperphagia that contributes to weight gain in abstinent human smokers. A limitation of the current experiments is that they do not examine the effect of donepezil on sucrose consumption in animals undergoing nicotine-induced withdrawal. The neurobiological mechanisms underlying nicotine withdrawal-induced changes in food reinforcement are not clear (Donny et al. 2011). Furthermore, it is possible that donepezil-induced attenuation of nicotine and sucrose seeking is due to reduced salience of cues previously paired with reinforcer delivery during self-administration. Regardless, these novel findings identify: (1) an intermediate dose of donepezil (i.e. 1.0 mg/kg) that selectively attenuates nicotine, but not sucrose, taking and seeking, and (2) a high dose of donepezil (i.e. 3.0 mg/kg) that blocks both nicotine- and sucrose-seeking behaviors, as well as transiently reduces food intake in rats.
Adverse effects of donepezil administration
Nausea and vomiting are two adverse events commonly reported in 5–16% of Alzheimer's patients treated with donepezil (Dunn et al. 2000; Pratt et al. 2002; Farlow et al. 2010). Studies of emesis in rodents are limited due to the lack of a vomiting reflex in rats and mice (for review see Andrews & Horn 2006). Despite the lack of a vomiting reflex, rodents display pica, or the consumption of non-nutritive substances such as kaolin clay, following administration of emetic agents (Mitchell et al. 1976; Liu et al. 2005; De Jonghe et al. 2009; Kanoski et al. 2012). For example, administration of the chemotherapeutic agent cisplatin, which induces nausea and vomiting in humans (Evans et al. 1981), significantly increases kaolin intake in rats (Andrews & Horn 2006; Hopkins et al. 2012). In contrast, the present findings indicate that behaviorally relevant doses of donepezil that attenuate nicotine taking and seeking do not induce pica in rats. These results suggest that donepezil-induced attenuation of nicotine self-administration and the reinstatement of nicotine-seeking behavior is likely not due to drug-induced nausea or malaise. Thus, donepezil may prevent smoking relapse within a dose range that does not induce nausea or vomiting in humans. These basic science findings, however, are limited because nausea is a subjective response in humans. Therefore, comparable clinical studies are required to uniformly rule out nausea as a potential side effect, as well as other potentially aversive effects of donepezil treatment that were not examined here.
Acetylcholinesterase inhibitors and smoking cessation
To date, there are relatively few clinical studies examining the role of acetylcholinesterase inhibitors including donepezil on smoking behavior and smoking relapse. Administration of the acetylcholinesterase inhibitors galantamine and rivastigmine effectively reduces craving and smoking rates in alcohol-dependent patients (Diehl et al. 2006, 2009). In contrast, smoking behavior was not reduced following galantamine treatment in patients with schizophrenia (Kelly et al. 2008; Sacco et al. 2008) or in methamphetamine-dependent subjects treated with rivastigmine (De la Garza & Yoon 2011). These conflicting clinical results are likely due to different patient populations (i.e. genetic variability, neuropsychiatric disorders, etc.), comorbid drug use and small sample sizes. A recent study demonstrated that, despite its pro-cognitive effects, donepezil treatment did not reduce smoking behavior in healthy, non-treatment seeking smokers who continue to smoke (Ashare et al. 2012). However, this study did not examine the effects of donepezil in treatment-seeking smokers and a reduction in smoking behavior was observed in the placebo treatment group. Short-term screening studies using non-treatment seeking smokers are limited in their clinical validity primarily because pharmacotherapies such as nicotine replacement and bupropion, which are efficacious in clinical trials for smoking cessation, are not effective in smokers unmotivated to abstain (Perkins, Stitzer & Lerman 2006). Furthermore, the dose of donepezil used in this study was relatively low compared with doses commonly prescribed for treating cognitive deficits. This study was also limited by treatment duration and its sample size. Therefore, the efficacy of acetylcholinesterase inhibitors in treating smoking relapse remains to be defined in healthy, treatment-seeking smokers. Taken together, these studies combined with the present findings suggest that donepezil may be an efficacious smoking cessation pharmacotherapy in treatment-seeking smokers.
While the present findings indicate that donepezil attenuates nicotine self-administration in rats, future clinical studies of donepezil in human smokers may reveal more complex effects of acetylcholinesterase inhibition on smoking behaviors. It is possible that donepezil treatment may reduce nicotine intake without affecting the reinforcing efficacy of nicotine. For example, donepezil may decrease the number of puffs per cigarette, but not the number of cigarettes smoked or the price that would be paid to obtain a cigarette. Thus, clinical studies will aid in delineating the effects of acetylcholinesterase inhibitors on smoking behaviors.
Cognitive deficits that are associated with smoking cessation and nicotine abstinence represent a core symptom of nicotine withdrawal that predicts smoking relapse (Patterson et al. 2010). Cognitive enhancers that target nicotine withdrawal-induced deficits may represent efficacious smoking cessation pharmacotherapies. Donepezil is a cognitive enhancer that has pro-cognitive effects in healthy smokers (Ashare et al. 2012) and therefore may attenuate drug seeking by improving cognitive deficits during periods of drug abstinence (Sofuoglu 2010). This hypothesis is supported by a recent study demonstrating that administration of an acetylcholinesterase inhibitor reverses nicotine withdrawal-induced cognitive impairments in mice (Wilkinson & Gould 2011). Future studies are required to determine if donepezil normalizes cognitive impairments during nicotine abstinence in rats with a history of nicotine self-administration.
Summary and Conclusions
The present findings indicate that acute donepezil administration attenuates nicotine taking and seeking in rats and that these effects are not due to adverse side effects such as drug-induced nausea/malaise. These results suggest that pharmacotherapies that modulate extracellular acetylcholine levels may prevent nicotine craving and relapse in abstinent human smokers. These pre-clinical findings also provide support for further clinical studies of donepezil and other acetylcholinesterase inhibitors on smoking behavior. The behaviorally relevant doses of donepezil that attenuated nicotine reinforcement and the reinstatement of nicotine seeking have been shown to dose-dependently reduce acetylcholinesterase activity approximately 15–40% in the rat brain (Giacobini et al. 1996; Liang & Tang 2004; Cerbai et al. 2007), which is comparable to levels achieved in Alzheimer's disease patients treated with donepezil (Rogers & Friedhoff 1998; Kaasinen et al. 2002; Jackisch et al. 2009; Ota et al. 2010). Therefore, the dose of donepezil that attenuates nicotine taking and seeking in rats likely represents a clinically relevant dose in humans. The effects of chronic donepezil administration remain to be determined in animal models of nicotine addiction. Since smoking cessation medications require chronic dosing, preclinical studies of chronic acetylcholinesterase inhibition are necessary in order to determine whether repeated administration produces similar effects on nicotine taking and seeking as demonstrated by the present studies of acute donepezil. Future clinical studies are required to determine the efficacy of cognitive-enhancing drugs such as donepezil as smoking-cessation pharmacotherapies in treatment-seeking smokers.
Acknowledgements
HDS was supported in part by a K01 training grant (K01 DA030445) from the National Institutes of Health (NIH), a pilot grant (P50-CA-143187) from the Center for Interdisciplinary Research on Nicotine Addiction (CIRNA) at UPENN and an Institutional Research Grant (IRG-78-002-31) from the American Cancer Society and the Abramson Cancer Center at UPenn. MRH is supported by NIH grants DK085435 and DK093874. The authors also extend a note of gratitude to Thomas J. Hopkins, Diana Olivos and Dr. Elizabeth Mietlicki-Baase for their technical assistance as well as Jill Turner and Rebecca Ashare for their critical reading of this manuscript.
Conflict of Interest
The authors declare no potential conflict of interest relating to this study.
Authors Contribution
HDS was responsible for the study concept and design, supervised and contributed to the acquisition of data, analyzed the data and drafted the manuscript. MRH was also responsible for designing the ad libitum feeding and pica experiments as well as their analysis and provided critical revision of the manuscript for important intellectual content. BAK and LER contributed to the acquisition of animal data and critical revision of the manuscript for important intellectual content. BAK also helped draft the manuscript. All authors critically reviewed content and approved final version for publication.




