Guidelines for Reasonable and Appropriate Care in the Emergency Department (GRACE-4): Alcohol use disorder and cannabinoid hyperemesis syndrome management in the emergency department
Funding information: SAEM GRACE-4 and the overall SAEM GRACE initiative is funded and administered by the Society for Academic Emergency Medicine.
Supervising Editor: Dr. Jeffrey Kline.
Abstract
The fourth Society for Academic Emergency Medicine (SAEM) Guidelines for Reasonable and Appropriate Care in the Emergency Department (GRACE-4) is on the topic of the emergency department (ED) management of nonopioid use disorders and focuses on alcohol withdrawal syndrome (AWS), alcohol use disorder (AUD), and cannabinoid hyperemesis syndrome (CHS). The SAEM GRACE-4 Writing Team, composed of emergency physicians and experts in addiction medicine and patients with lived experience, applied the Grading of Recommendations Assessment Development and Evaluation (GRADE) approach to assess the certainty of evidence and strength of recommendations regarding six priority questions for adult ED patients with AWS, AUD, and CHS. The SAEM GRACE-4 Writing Team reached the following recommendations: (1) in adult ED patients (over the age of 18) with moderate to severe AWS who are being admitted to hospital, we suggest using phenobarbital in addition to benzodiazepines compared to using benzodiazepines alone [low to very low certainty of evidence]; (2) in adult ED patients (over the age of 18) with AUD who desire alcohol cessation, we suggest a prescription for one anticraving medication [very low certainty of evidence]; (2a) in adult ED patients (over the age of 18) with AUD, we suggest naltrexone (compared to no prescription) to prevent return to heavy drinking [low certainty of evidence]; (2b) in adult ED patients (over the age of 18) with AUD and contraindications to naltrexone, we suggest acamprosate (compared to no prescription) to prevent return to heavy drinking and/or to reduce heavy drinking [low certainty of evidence]; (2c) in adult ED patients (over the age of 18) with AUD, we suggest gabapentin (compared to no prescription) for the management of AUD to reduce heavy drinking days and improve alcohol withdrawal symptoms [very low certainty of evidence]; (3a) in adult ED patients (over the age of 18) presenting to the ED with CHS we suggest the use of haloperidol or droperidol (in addition to usual care/serotonin antagonists, e.g., ondansetron) to help with symptom management [very low certainty of evidence]; and (3b) in adult ED patients (over the age of 18) presenting to the ED with CHS, we also suggest offering the use of topical capsaicin (in addition to usual care/serotonin antagonists, e.g., ondansetron) to help with symptom management [very low certainty of evidence].
EXECUTIVE SUMMARY
The Society for Academic Emergency Medicine (SAEM) fourth Guidelines for Reasonable and Appropriate Care in the Emergency Department (GRACE-4) Writing Team developed clinically relevant questions to address the care of patients with alcohol withdrawal syndrome (AWS), alcohol use disorder (AUD), and cannabinoid hyperemesis syndrome (CHS). Five patient–intervention–comparison–outcome (PICO) questions were developed by consensus. An external group performed a systematic review of the literature and then synthesized direct evidence for each PICO question. The SAEM GRACE-4 Writing Team synthesized direct and indirect evidence following the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. Despite the relevance and commonality of these questions in everyday emergency care, the quantity and quality of the evidence were very limited. Future research opportunities include evaluating the impact of these guidelines on medical education, funding opportunities, and outcomes of clinical care in the ED environment. The development of standard terms of reporting for AWS and CHS conditions, severity assessment, and outcomes would inform discussions centered on resource utilization, costs of care, and patient and clinician preferences.
RECOMMENDATIONS BOX (RECOMMENDATION FOR EACH OF THESE MEDICATIONS)
Recommendation 1: In adult ED patients (over the age of 18) with moderate to severe alcohol withdrawal who are being admitted to hospital, we suggest using phenobarbital in addition to benzodiazepines compared to using benzodiazepines alone (conditional recommendation, FOR) [low to very low certainty of evidence].
Good practice statement: All patients treated for alcohol withdrawal should be offered follow-up treatment where such treatment is available.
Recommendation 2: In adult ED patients (over the age of 18) with AUD, we suggest a prescription for an anticraving medication for the management of AUD for patients who desire alcohol cessation (conditional recommendation, FOR) [very low to low certainty of evidence].
Good practice statement: Please see the anticraving medication algorithm (Figure 4) that was designed to help guide clinicians in the selection of anticraving medication based on patient-level factors and the strength of evidence for three medications. Dosage adjustments related to hepatic and renal function can be made at follow-up.
Good practice statement: As per American Society of Addiction Medicine Guidelines, clinicians should consider offering patients with AUD supplemental thiamine as part of their ED treatment plan and should be offered follow-up treatment where such treatment is available.
Recommendation 2a: In adult ED patients (over the age of 18) with AUD who are not taking opioids, we suggest naltrexone (compared to no prescription) for the management of AUD to prevent return to heavy drinking and/or to reduce heavy drinking (conditional recommendation, FOR) [low certainty of evidence].
Good practice statement: A bridging prescription of up to 4 weeks until follow-up with an addiction medicine physician, primary care physician, or other appropriate health care provider can take place is preferred. Monitoring of liver enzymes should be at the discretion of the provider seeing the patient in follow-up. For patients not treated with long-acting benzodiazepines for AWS in the ED, patients should be advised that sudden cessation of alcohol consumption (as a result of anticraving medication) may produce acute AWS. These patients should be counseled to slowly taper consumption and seek treatment for AWS management should symptoms occur.
Recommendation 2b: In adult ED patients (over the age of 18) with AUD, with contraindications to naltrexone, we suggest acamprosate (compared to no prescription) for the management of AUD to prevent return to heavy drinking and/or to reduce heavy drinking (conditional recommendation, FOR) [low certainty of evidence].
Good practice statement: A bridging prescription of up to 4 weeks until follow-up where renal function can be monitored with an addiction medicine physician, primary care physician, or other appropriate health care provider is preferred.
Recommendation 2c: In adult ED patients (over the age of 18) with AUD, we suggest gabapentin (compared to no prescription) for the management of AUD to reduce heavy drinking days and improve alcohol withdrawal symptoms (conditional recommendation, FOR) [very low certainty of evidence].
Good practice statement: Given the known misuse potential of gabapentin, a bridging prescription, for example, less than 2 weeks, is preferable to a long-term prescription. Patients should be cautioned about the sedative effects of gabapentin, and it should be prescribed with caution or avoided altogether in patients who use opioids. In patients with high self-reported withdrawal symptoms when they stop or reduce their alcohol intake, consider prescribing gabapentin in addition to naltrexone or acamprosate. Consider a weekly dispensing interval for gabapentin prescriptions longer than 2 weeks.
Recommendation 3a: In adult patients presenting to the ED with CHS we suggest the use of haloperidol or droperidol (in addition to usual care/serotonin antagonists, e.g., ondansetron) to help with symptom management (conditional, FOR) [very low certainty of evidence].
Good practice statement: IV fluids and nonopioid analgesics could be administered/offered to help with symptoms management.
Recommendation 3b: In patients presenting to the ED with CHS we suggest offering the use of topical capsaicin (in addition to usual care/serotonin antagonists, e.g., ondansetron) to help with symptom management (conditional, FOR) [very low certainty of evidence].
Good practice statement: One member of the SAEM GRACE-4 Writing Team emphasized the importance of recognizing that not all patients experience relief with capsaicin, and clinicians should be prompt in escalating treatment for patients whose symptoms are not alleviated promptly. This member also emphasized that capsaicin should not be used for patients for whom it had not been effective in the past (conditional, FOR) [very low level of evidence].
Good practice statement: In patients presenting to the ED with CHS, benzodiazepines and opioids should not be used as first-line treatment for CHS symptom management. In balance with the lack of evidence supporting the effectiveness of benzodiazepines and opioids in this setting, and considering prior SAEM GRACE recommendations for avoiding opioids in the management of chronic abdominal pain, opioids should be reserved for patients where pain is the primary concern and in whom haloperidol/droperidol (and if attempted, capsaicin) have not provided prompt relief. we believe the potential risks associated with administration of opioids as initial treatment for CHS outweigh any potential benefit.
Good practice statement: These interventions should be used in conjunction with anticipatory guidance on the necessity of cannabinoid abstinence for complete symptom resolution. We found no published evidence that reduction in use will prevent CHS; however, anecdotal evidence from our representative with lived experience suggests that in some cases reducing use may reduce frequency of episodes. If the health care team suspects concurrent cannabinoid use disorder based on screening with a validated tool such as the Cannabis Use Disorder Identification Test–Revised (CUDIT-R) consider referral to psychosocial interventions and/or addiction medicine specialists if available. Hydration and other supportive treatments should not be delayed to administer either haloperidol/droperidol or capsaicin (if the patient would like to try it). Clinicians should educate patients on the rationale for the use of these medications if questioned and caution them about the intensity of burning related to capsaicin application.
INTRODUCTION
Background
While the opioid epidemic has garnered much attention, other forms of substance use disorders (SUD) continue to have significant impacts on health and wellness. Globally, alcohol use disorder (AUD) is the most prevalent SUD with over 100 million estimated cases in 2016.1 Cannabis use disorder (CUD) is the third most prevalent SUD with an estimated 22 million cases worldwide (following opioid use disorder at 26 million cases).2
Conditions related to the heavy use of alcohol are common and increasingly encountered in the ED.3 Trends in the United States demonstrate a similar increase in ED presentations related to cannabis use following legalization.2, 4, 5 Unfortunately, research and education focused on the management of conditions related to the use of these substances, particularly in the ED setting, have lagged behind that which is available for opioids, and there is a paucity of evidence to guide practice in this area. Recently, the American Academy of Emergency Medicine published a white paper describing various approaches to management of AUD in ED settings.6 Similar ED-focused white papers or clinical practice guidelines do not exist for managing cannabis hyperemesis.
The objective of this guideline is to provide an evidence-based, patient-centric approach for clinicians in their evaluation and management of three conditions related to nonopioid-related SUDs commonly encountered in the adult ED setting: alcohol withdrawal syndrome (AWS), AUD, and cannabinoid hyperemesis syndrome (CHS). Members of the Society for Academic Emergency Medicine (SAEM) fourth Guidelines for Reasonable and Appropriate Care in the Emergency Department (GRACE-4) Writing Team believe that these conditions commonly occur in individuals with underlying SUDs. Management of AUD and CUD in some settings requires recognition of AUD using Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), criteria or a validated screening tool such as Cutting down, Annoyance by criticism, Guilty feeling and Eye-openers (CAGE)7 or Alcohol Use Disorders Identification Test (AUDIT-C)8 for AUD and the Cannabis Use Disorder Identification Test–Revised (CUDIT-R)9 for CUD. While AWS and CHS commonly occur in individuals with SUD, definitive diagnosis of the underlying SUD (which may require clinical information not rapidly available to emergency medicine [EM] clinicians) and grading the severity of the disorder are not necessary to treat these conditions in the ED. As such, we have chosen to adopt practical definitions based on clinical factors at the time of ED presentation. This SAEM GRACE-4 guideline is not intended to be used to guide treatment for patients with delirium tremens nor for those in whom other conditions causing intractable nausea and vomiting are being considered.
Alcohol use disorder
Medications for the management of AUD have been available for decades.10-14 For patients with moderate to severe AUD, anticraving medications (also known as relapse prevention medications or medically assisted treatment [MAT]) have been shown to be effective in reducing the amount of alcohol consumed and heavy drinking days, as well as increasing the number of days abstinent.13-15
Despite their effectiveness, uptake in prescribing these medications is low. Survey data from the United States demonstrated that the prevalence of AUD in the general population was 7.8% and that although 81.4% of individuals with AUD used health care within the previous 12 months, only 11.6% received a brief intervention, 5.1% were referred to treatment, and 5.8% received AUD treatment.15, 16 The National Institute on Alcohol Abuse and Alcoholism defines a brief intervention as a 5- to 15-min effort to reduce unhealthy alcohol consumption that can be delivered during routine visits in primary care or other health care settings and usually reinforced over another one to five sessions. In the United States, the 2021 National Survey on Drug Use and Health estimated that only 1.6% of individuals with AUD received pharmacotherapy for their condition.17, 18 Data from Canada (with a prevalence of AUD similar to the United States), demonstrates that fewer than 1% of patients with a hospital visit related to AUD were prescribed first-line anticraving medications.19
Prior research has shown that significant inequities exist in the treatment of SUD and mental health overall. Abraham et al.20 demonstrated disparities in access, with publicly funded treatment programs less likely than privately funded programs to provide access to a physician and also less likely to prescribe MAT, even when controlling for physician access. Data from the U.S. Veterans Health Administration shows higher use of MAT for AUD than in the general population (5.1%), and that Black American patients are less likely to be prescribed approved medications than Whites or Hispanics.16 As a low-barrier and crucial part of the social safety net, the ED is uniquely situated to address inequities in access to MAT for patients with AUD, by offering these medicines as part of evidence-based care guidelines.6, 21, 22
Alcohol withdrawal syndrome
There are wide variations in the way AWS is managed in hospitals.23, 24 Recent research suggests that this is also the case in the ED.25 Randomized controlled trials (RCTs) using a symptom-driven approach (using a validated tool to assess withdrawal severity as part of a standardized protocol with frequent, regular assessments and medication dosages determined by withdrawal severity) to manage AWS demonstrate both faster resolution of symptoms and lower total medication dosages being used.26-29 However, there are challenges in implementing symptom-driven approaches to managing AWS in the ED, including little consensus regarding the best tool to measure AWS severity, which protocol to use to guide treatment decisions, and the most effective medications to use.
Benzodiazepines have long been the criterion standard for treating AWS,30 and classically, phenobarbital has been reserved for severely ill patients with “benzodiazepine-resistant” alcohol withdrawal due to concerns about safety. However, recent years have seen an increase in interest regarding the use of phenobarbital either as an adjunct to benzodiazepine treatment or, in some instances, as the primary medication of choice for AWS.31, 32 Advocates of the use of phenobarbital argue that it has pharmacologic advantages over benzodiazepines (direct agonism at gamma-aminobutyric acid-A [GABAA] receptors and inhibitory effects on glutamate neurotransmission) and that the use of phenobarbital alone or in combination with benzodiazepines may be more effective than the use of benzodiazepines alone, even in patients without classic benzodiazepine-resistant withdrawal and patients earlier in their treatment course. The evidence supporting the use of phenobarbital is of mixed quality and high heterogeneity, particularly regarding the method of phenobarbital dosing and the use of phenobarbital alone or in combination with benzodiazepines. The question of the potential benefit of phenobarbital in combination with benzodiazepine treatment as part of a symptom driven approach to AWS is as yet unanswered.
Cannabinoid hyperemesis syndrome
CHS was first described in 2004 by Allen et al.33 The syndrome is characterized by episodes of frequent and severe vomiting and nausea associated with abdominal pain in patients who regularly and frequently use cannabis. In recent years, legalization of cannabis in many states has resulted in increasing numbers of people who consume cannabis regularly.34 Increased usage combined with increasing concentrations of tetrahydrocannabinol (THC) in cannabis preparations35 have both contributed to rising numbers of patients presenting to EDs experiencing CHS.2, 4
Although there is some blurring of the boundaries between cyclical vomiting syndrome and CHS, one of the hallmarks of CHS is that patients often describe trying to relieve their symptoms at home using hot showers or baths.36, 37 Patients with CHS commonly visit EDs for treatment including rehydration and symptom control.4 Since CHS is directly correlated with cannabis use, cannabis cessation is required for symptom resolution, but many patients are reluctant to discontinue cannabis given that they attribute a therapeutic benefit to cannabis and their vomiting.38, 39
The pharmacologic management of CHS has proven challenging. Routinely prescribed antiemetics such as prochlorperazine, metoclopramide, and ondansetron are typically ineffective, leaving clinicians to use off-label medications and other treatments.40 Opioids and benzodiazepines are also used in the treatment of CHS. While opioids can relieve pain, they may also be proemetic and may exacerbate one of the theoretical mechanisms of CHS (gastroparesis)40 leading some to recommend that they be avoided unless other measures are ineffective at managing CHS-related abdominal pain.41
Previous Guidelines for Reasonable and Appropriate Care in the Emergency Department discouraged opioids for recurrent abdominal pain (SAEM GRACE-2) and suggested nonopioid treatments as first-line treatments.42 In line with SAEM GRACE-2 and other guidelines encouraging opioid-sparing strategies for acute pain (where possible),43-45 there has been significant interest in the use of topical capsaicin for the management of CHS based on the observation that the skin sensations caused by the pepper extract may cause a similar effect to hot showers. Similarly, the use of antipsychotic medications that act by blocking dopaminergic receptors in the brain are known to make excellent antiemetics in low doses and have garnered off-label use interest for CHS.46, 47 To date, lack of evidence for the effectiveness of either of these investigations has left clinicians wondering if and when they should be used.
Clinical practice guidelines exist for the ambulatory and inpatient management of AUD48 and AWS49; however, similar guidance for the emergency department (ED) management of AWS, AUD, and CHS does not presently exist. For AWS and AUD the first-line medication recommendations are the same; however, there are important differences that may limit the effectiveness of these treatments in the ED setting. For example, the American Society of Addiction Medicine (ASAM) recommends long-acting benzodiazepines as first-line treatment for AWS; however, their dosage recommendations are for oral medications.49 Many ED patients will be treated using intravenous (IV) medications (unable to tolerate oral medications, need for rapid onset of action). Additionally, by necessity, the time course for managing AWS in the ED setting is very short. While local practice can differ, most ED patients with AWS are managed and discharged home and are not admitted to the hospital,50 while ASAM recommends daily monitoring for up to 5 days.49 Similarly, for the pharmacologic management of AUD, patients in ambulatory/inpatient SUD settings are typically seeking SUD care, will have ongoing relationships with their clinicians, and will be exposed to psychological counseling options and other nonpharmacologic strategies that may make them more willing to consent to pharmacotherapeutic interventions and that may also make these interventions more effective. These differences between the ED setting and ambulatory/inpatient AUD treatment sites do not mean that the ED management objectives for AUD should diverge from the holistic continuum of care advocated by ASAM. Instead, the SAEM GRACE-4 Writing Team believes that clinical practice guideline recommendations focusing on the ED as one site of frequently accessed care for AUD have been absent until now and that GRACE-4 pharmacologic and nonpharmacologic management recommendations should augment ASAM guidelines. Finally, it is unclear if initiating MAT in the ED leads to better outcomes. The purpose of this guideline is to summarize all available evidence regarding the ED management of these conditions and to provide an evidence-based framework intended to support patients, clinicians, and other health care professionals in their decisions about the evaluation and management of patients presenting in the ED setting with these conditions.
SCOPE AND PURPOSE
The target audience for GRACE-4 includes all practicing ED clinicians (physicians and advanced practice providers) responsible for the evaluation and management of undifferentiated patients with AUD, AWS, and CHS as well as health care systems and hospitals responsible for care pathways in these populations.
METHODS
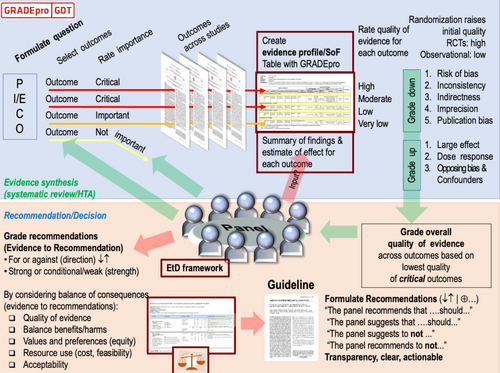
Group composition
In conjunction with the GRACE Steering Committee and GRACE methodologists, the lead author for GRACE-4 was identified based on PhD-level expertise in pharmacology and addiction medicine as well as being a practicing academic emergency physician. Subsequently, GRACE-4 Writing Team members were identified based on their scholarly efforts in the areas of SUD in EM and the interface of EM and outpatient addiction medicine. A conscious effort to include gender, ethnic, and geographic representativeness across North America was used in selecting members of the GRACE-4 Writing Team. Those SUD experts who were contacted and agreed to serve as authors became part of the GRACE-4 Writing Team. The SAEM GRACE-4 Writing Team was composed of emergency physicians, some of whom had advanced training in the management of addiction, from geographically diverse locations in the United States and Canada, including those with research methodology expertise and content expertise in the diagnosis and treatment of alcohol- and cannabinoid-related conditions, including withdrawal management and anticraving medications. Members of the SAEM GRACE-4 Writing Team also included Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodologists (4) and addiction experts (3) who were responsible for conducting the systematic reviews used to inform these guidelines, according to the clinical terms agreed upon by the larger group. The SAEM GRACE-4 Writing Team also included patient representatives with lived experience related to AUD and CHS. The patient representatives were identified by members of the GRACE-4 Writing Team using previously described methods.51 SAEM provided financial support for the development of GRACE-4.
SAEM GRACE-4 Writing Team group interaction and processes
Beginning in June 2021, the SAEM GRACE-4 Writing Team met monthly using virtual meeting software. The SAEM GRACE-4 Writing Team members were asked to review a series of instructional videos covering the use of the GRADE methodology (Figure 1) used in the development of this guideline (https://www.saem.org/publications/grace). Methodologists, experienced in the use of GRADE and who previously participated in clinical guidelines development, were assigned to support each of three independent working groups. As a quality/trustworthiness check, the final manuscript was analyzed using the recently published Agency for Healthcare Research and Quality (AHRQ) National Guideline Clearinghouse Extent of Adherence to Trustworthiness Standards (NEATS) instrument52 to ensure best possible adherence to Institute of Medicine 2011 guideline trustworthiness standards (Appendix S1).
Declaration and management of competing interests
All SAEM GRACE-4 Writing Team members disclosed potential conflicts of interest using SAEM standard methods, including review by the Academic Emergency Medicine Editor-in-Chief, SAEM Chief Executive Officer, and SAEM Executive Committee. Dr. Carpenter's roles with the American College of Emergency Physician's Clinical Policy Committee; American Board of Emergency Medicine's MyEMCert Key Advances; American Academy of Emergency Medicine Geriatrics Committee; and editorial boards of Annals of Internal Medicine, Journal of the American Geriatrics Society, and Missouri Medicine were deemed as potential conflicts to acknowledge in this GRACE-4 document. No other members of the SAEM GRACE-4 Writing Team were determined to have significant conflicts of interest related to this work.
Definitions of the intended patient population
Throughout the process of developing these guidelines, the SAEM GRACE-4 Writing Team focused on the definitions of AUD, AWS, and CHS in adults aged 18 years and older.
Alcohol use disorder
Definition
AUD is defined according to DSM-553 criteria (Figure 2).The diagnosis of AUD requires the presence of at least two of 11 individual criteria within the prior 12 months, further subdivided into mild (two or three criteria), moderate (four or five criteria), or severe (presence of six or more criteria).
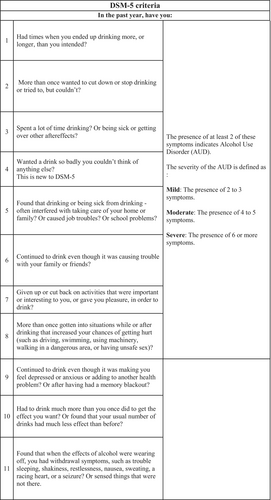
Practically speaking, making the formal diagnosis of AUD is difficult in the ED setting, and accurate use of the DSM-5 criteria relies on overcoming stigma and developing a trusting therapeutic alliance in the ED, which may be difficult in the time frame of a typical ED visit. We chose to use a pragmatic approach to the diagnosis of AUD order in the ED, which includes ED presentations related to consequences and harms related to alcohol use, which would be representative of the minimum required two criteria by DSM-5.
Alcohol withdrawal syndrome
Definition
AWS is seen in individuals with AUD who suddenly cease or reduce heavy alcohol consumption. These individuals have become tolerant to high blood alcohol concentrations, which normally produce central nervous system (CNS) depression, through adaptations of the GABA and glutamate neurotransmitter systems. Abrupt cessation in drinking disrupts this adaptation and unmasks CNS hyperactivity resulting in a constellation of clinical symptoms including nausea/vomiting, headache, diaphoresis, anxiety, auditory, visual and tactile disturbances, agitation, and tremor. In its most extreme form, patients may develop seizures and delirium tremens.54, 55
Cannabinoid hyperemesis syndrome
Definition
Different criteria for CHS have been proposed including ROME IV36 and another by Simonetto et al.,56 but no pragmatic diagnostic standard has been incorporated into practice given the requirement for resolution of symptoms with cessation of cannabis as a clinical criteria for diagnosis. Common symptoms include nausea, vomiting, and/or abdominal pain that can occur in a cyclical pattern and relief of symptoms with hot water/showers, although improvement with hot water/showers is not mandatory for the diagnosis.
Current practice
Unfortunately, there is no standardized approach to the evaluation, diagnosis and treatment of patients with CHS in the ED, often leading to high resource utilization.40, 57 Symptoms related to CHS are reported to be resistant to the usual options for management of nausea, vomiting, and abdominal pain.58 Due to prolonged duration, frequent recurrence, lack of recognition of diagnosis, and difficult-to-control symptoms, patients with CHS often receive multiple diagnostic studies including: ultrasounds, computed tomography scans, and endoscopic procedures, along with prolonged ED lengths of stay (LOSs) and high rates of hospital admissions.59, 60 Complicating this is the relative lack of efficacy of standard modalities for the treatment of CHS symptoms.41, 61
Cannabis cessation is required for complete resolution of symptoms.33, 62, 63 However, the addictive nature of cannabis, and patient awareness that cannabis may be useful for the management of nausea (specifically in the setting of oncology patients),64, 65 can make continuous abstinence difficult. Because of the onset of CHS after chronic cannabis use, and the variable and intermittent nature of CHS,33, 63 the benefits with regard to CHS symptom resolution may not be immediately obvious to most patients.
Selection of questions and outcomes of interest
The SAEM GRACE-4 Writing Team discussed the target populations and considered management challenges while keeping in mind the perspectives of EM clinicians, patients, and health systems. The goal of GRACE is to focus on conditions for which there is wide variation in treatment and ED-focused clinical practice guidelines do not already exist and for which patients have frequent ED revisits. Clinical questions were developed using the PICO format (population, intervention, comparison, outcomes) and refined by the entire team over several meetings. The GRACE-4 Writing Team prioritized questions and patient-important outcomes such as complications of alcohol withdrawal, admission to the hospital, reduction in ED visits, engagement in SUD services, and ED LOS, for each PICO question. Our lists of questions and outcomes for each question were then ranked in priority by anonymous poll, and the most important outcomes were chosen for review. The SAEM GRACE-4 Writing Team included patients with lived experience for each of the conditions under review, and our outcomes of interest were refined to include the perspective of these writing team members.
Evidence synthesis and development of clinical recommendations
The SAEM GRACE-4 Writing Team was divided into three groups composed of three clinician experts and one methodologic expert. Our patient representative members with lived experience (one with lived experience with AUD and AWS and one with lived experience with CHS) were invited to all meetings and contributed to our deliberations and discussions, including the strength and direction of GRACE-4 recommendations and the Evidence-to-Decision (EtD) framework decisions.51
The individual PICO questions included in this guideline are described below:
PICO 1: In patients 18 years of age or older receiving pharmacologic therapy for moderate to severe alcohol withdrawal in the ED, does the use of adjunctive phenobarbital by any route compared to benzodiazepines alone lead to improvement in outcomes?
| P | Adult ED patients with moderate to severe AWS as defined by the clinical judgment of the treating physician |
| I | Phenobarbital (by any route and at any dose) in addition to benzodiazepines (by any route) |
| C | Benzodiazepine (by any route) alone. |
| O* |
|
*Outcomes ranked in order of importance by the SAEM GRACE-4 Writing Team by anonymous vote.
PICO 2: In patients 18 years of age or older who present to the ED with AUD who are discharged home, does the prescription of an anticraving medication, compared to no prescription, improve outcomes?
| P | Adults older than 18 presenting to the ED with established diagnosis of, or newly recognized diagnosis of AUD |
| I | Prescription of one of the following anticraving medications Naltrexone: Oral as hydrochloride or suspension reconstituted once every 30 days (injection) Acamprosate: Oral (with reduced dosing for creatinine clearance 30–50 mL/min and contraindicated below 30 mL/min) Gabapentin: Orally three times a day with initial dose in the ED (possible reduced dosing for renal impairment) |
| C | No prescription or placebo |
| O * |
|
*Outcomes ranked in order of importance by the SAEM GRACE-4 Writing Team by anonymous vote.
PICO 3: In patients 18 years of age or older who present to the ED and are suspected to have CHS, does the use of dopamine antagonists (e.g., haloperidol, droperidol) or capsaicin compared to usual care (or no treatment) lead to improved outcomes?
| P | Adults >18 years old with suspected diagnosis of acute CHS presenting to the ED |
| I | Dopamine antagonists (e.g., haloperidol, droperidol) Capsaicin |
| C | Usual care/serotonin antagonists (e.g., ondansetron), Antihistamine/anticholinergics (e.g., diphenhydramine), (+/− metoclopramide) No active comparator |
| O* |
|
*Outcomes ranked in order of importance by the SAEM GRACE-4 Writing Team by anonymous vote.
Systematic reviews
The development of the SAEM GRACE-4 guidelines included nonauthor methodologic and content experts from the Peter Boris Center for Addictions Research at McMaster University, and the systematic reviews for each of the SAEM GRACE-4 guideline questions were led by these members and included risk of bias assessments for the individual studies of direct evidence synthesized below.51, 66-68
PICO QUESTION 166
Data sources and search strategies
Databases included in this search were OVID MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily, and OVID MEDLINE 1946 to present, APA PsycInfo 1806 to January Week 5 2022, EBM Reviews—Cochrane Central Register of Controlled Trials November 2021, EBM Reviews—Cochrane Database of Systematic Reviews, and Scopus.
Eligibility criteria and study selection
Studies were included if the study population was over 18, presenting to the ED with AWS, and given any dose of phenobarbital to manage withdrawal. No time point for the outcomes assessed were prespecified so outcome assessment could occur within the ED or after the ED episode of care.
Studies were excluded if the study population did not present to the ED or did not have a direct comparison with benzodiazepines and phenobarbital, if the article was not accessible/not accessible in English, if it was a proposed clinical trial with no public results, or if the study was a review/commentary/clinical guideline. However, relevant review articles were accessed for reference screening. Further details are available in the associated systematic review.66
PICO QUESTION 267
Data sources and search strategies
Databases included in this search were OVID MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and OVID MEDLINE 1946 to present (March 8, 2022), APA PsycInfo 1806 to February Week 4 2022, EBM Reviews—Cochrane Central Register of Controlled Trials November 2021, EBM Reviews—Cochrane Database of Systematic Reviews, and Scopus.
Eligibility criteria and study selection
Studies were included if the study population was over 18, presenting to the ED for AUD or suspected AUD treatment and were given any anticraving prescription. Studies were excluded if the study population did not present to the ED, if the anticraving medication was either topiramate or baclofen, if patients were not discharged, if the article was not accessible/not accessible in English, if it was a proposed clinical trial with no public results, or if the study was a review/commentary/clinical guideline. However, relevant review articles were accessed for reference screening. Further details are available in the associated systematic review.67
PICO QUESTION 368
Data sources and search strategies
Databases included in this search were OVID MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and OVID MEDLINE 1946 to present (March 8, 2022), APA PsycInfo 1806 to February Week 42,022, EBM Reviews—Cochrane Central Register of Controlled Trials November 2021, EBM Reviews—Cochrane Database of Systematic Reviews, and Scopus.
Eligibility criteria and study selection
Studies were included if the study population was primarily over 18 and presenting to the ED for CHS and if pharmacologic intervention was administered. Additionally, studies were included if there was a control group (e.g., usual care, no active comparator, head-to-head pharmacologic agents). Studies were excluded if the study population did not present to the ED, if the article was not accessible or did not have an English translation, if it was a proposed/registered clinical trial with no public results, or if the study was a review/commentary/clinical guideline. However, relevant review articles were accessed as full texts for reference screening. Further details are available in the associated systematic review.68
Evidence to recommendations
Core elements of the GRADE evidence were considered in the decision process, including certainty of evidence and the balance between desirable and undesirable effects as well as feasibility, resource use, and acceptability.69 For all recommendations, the expert panelists reached consensus except for the recommendation for capsaicin, where one GRACE-4 Writing Team member disagreed. Voting rules were agreed on prior to the panel meetings for situations when consensus could not be reached. The strength of a recommendation reflects the extent to which we can, across the range of patients for whom the recommendations are intended, be confident that desirable effects of a management strategy outweigh undesirable effects. As per GRADE methodology, recommendations are labeled as “strong” or “conditional.” The words “we recommend” indicate strong recommendations and “we suggest” indicate conditional recommendations (Figure 3).
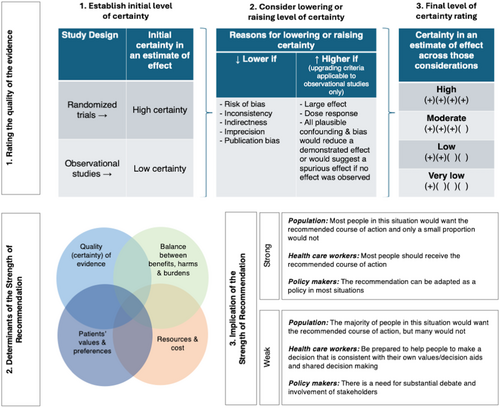
Use of indirect evidence
A recommendation associated with a treatment recommendation follows from an evaluation of the balance between the desirable and undesirable consequences of the test or treatment being considered. Inferring that a treatment or strategy improves a patient-important outcome usually requires access to effectiveness studies that evaluate the effect of that treatment or test in similar patients and in the same setting. GRADE methodology also permits the use of indirect evidence, which was important for these guidelines due to the limited quantity and quality of direct evidence upon which to base recommendations.70 The SAEM GRACE-4 Writing Team a priori determined that “direct evidence” must match each element of the PICO question, while indirect evidence deviated from one component of the PICO question. For example, in-hospital studies (non-ED setting) would be included as indirect evidence if all other components of the PICO question were matching. In the EtD analysis framing the recommendations, “indirectness” downgraded the strength of recommendations when sufficient direct evidence was lacking. No formal search strategy was devised to identify indirect evidence. Instead, the SAEM GRACE-4 Writing Team relied on their expertise and informal external stakeholders’ recommendations to develop each PICO question's EtD framework.
QUESTION 1: In patients 18 years of age or older receiving pharmacologic therapy for moderate to severe alcohol withdrawal in the ED, does the use of adjunctive phenobarbital by any route compared to benzodiazepines alone lead to improvement in outcomes?
Recommendation 1: In adult ED patients with moderate to severe alcohol withdrawal who are being admitted to the hospital, we suggest using phenobarbital in addition to benzodiazepines compared to using benzodiazepines alone (conditional recommendation, FOR) [low to very low certainty of evidence].
Good practice statement: All patients treated for alcohol withdrawal should be offered follow-up treatment where such treatment is available.
Summary of the evidence
Seventy studies were identified via database and registry searching. Of these, seven studies met inclusion criteria and were included in the systematic review of direct evidence.70-76 These included three retrospective cohort studies representing a total of 510 patients, two retrospective chart review studies representing a total of 378 patients, and two RCTs representing a total of 146 patients. Both RCTs were assessed as having low risk of bias. Serious heterogeneity in the included populations (particularly the severity of AWS), interventions (particularly the total benzodiazepine dose administered and the phenobarbital dosing strategy), and outcomes studies precluded any pooling of results or formal meta-analysis.
Rosenson and colleagues70 conducted a randomized, double-blind, placebo-controlled trial of adjunctive phenobarbital for AWS that enrolled 102 ED patients (low risk of bias). Patients were included on the basis of treating physician judgment that they had signs and symptoms of AWS that would require pharmacologic treatment and were likely to require hospital admission. All patients were treated with a standardized lorazepam-based symptom-triggered AWS protocol. Patients were randomized to receive adjunctive phenobarbital 10 mg/kg IV once or matching placebo. The primary outcome was the incidence of intensive care unit (ICU) admission; patients in the phenobarbital group were less likely to be admitted to the ICU (8% vs. 25%, difference 17%, 95% confidence interval [CI] for difference 4%–32%). There was no difference in the overall incidence of hospital admission, ICU LOS, or hospital LOS. No difference in adverse outcomes (intubation, seizure, use of restraints, or bedside sitter) was detected, although the study was likely underpowered for safety. The phenobarbital group had lower incidence of use of continuous lorazepam infusions (4% vs. 31%, difference 27%, 95% CI for difference 14%–41%) and received a lower total amount of lorazepam (26 mg vs. 49 mg, difference 23 mg, 95% CI for difference 7-40 mg).
Hendey and colleagues71 conducted a randomized, double-blind, placebo-controlled trial of phenobarbital for AWS that enrolled 44 ED patients (low risk of bias). Unlike Rosenson and colleagues, Hendey and colleagues designed their trial to identify and enroll patients who were likely to be discharged from the ED and thus recruited a population with less severe disease. Patients were randomized to treatment with lorazepam in the ED followed by an oral chlordiazepoxide taper on discharge (if applicable) or to treatment with phenobarbital in the ED followed by an oral matching placebo taper on discharge (if applicable). Phenobarbital doses in this trial were lower than in the study by Rosenson et al.—the mean total phenobarbital dose administered was 509 mg (range 260–910 mg), well under 10 mg/kg for most patients. The primary outcome was the change in withdrawal scores from ED baseline to admission or discharge. There was no significant difference between the groups regarding the primary outcome or important secondary outcomes such as ED LOS, admission rate, reported alcohol relapse, or medication compliance.
Three retrospective cohort studies were included. Lebin and colleagues73 analyzed 470 cases of AWS (285 unique patients), comparing cases receiving benzodiazepine alone (n = 235) to those receiving phenobarbital alone (n = 133) and those receiving phenobarbital plus benzodiazepine (n = 102), although total benzodiazepine doses were very low in both groups (4–6 mg lorazepam equivalents [LE]; low risk of bias). Notably, this study included a phenobarbital-alone group; the question of the effectiveness of phenobarbital alone compared to benzodiazepines alone is outside the scope of this recommendation. They found that treatment with phenobarbital alone or phenobarbital plus benzodiazepine was associated with a lower odds of return to the ED within 3 days compared to benzodiazepine alone (adjusted odds ratio [AOR] 0.45, 95% CI 0.23–0.88; and AOR 0.33, 95% CI 0.15–0.74, respectively), but this difference did not persist at 7 days after the index visit. Sullivan and colleagues76 analyzed 209 patients presenting to the ED with a primary diagnosis of AWS, comparing patients receiving phenobarbital (median cumulative dose 260 mg) to those receiving benzodiazepine alone (high risk of bias). They found no difference in rate of ICU admission, rate of ED discharge, or key complications, but did find that mean hospital LOS was higher in the benzodiazepine-only group than in the phenobarbital group (4 days vs. 3 days, p = 0.048) and that the maximum Revised Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-Ar score)55 score at 24 h was lower in the phenobarbital group than the benzodiazepine-only group (median 13 vs. 16, p = 0.045), despite indicators of higher disease severity in the phenobarbital group. It is unclear if these differences were truly clinically significant, and median total benzodiazepine doses during admission were low for both groups (14 mg LE vs. 22 mg LE). Mahmoud and colleagues74 conducted a small study of patients treated with AWS and cooccurring opioid withdrawal (n = 16), comparing those treated with buprenorphine plus phenobarbital to those treated with buprenorphine plus lorazepam (some concern for bias). They found no evidence of serious adverse events in either group but did not report any data on the efficacy of phenobarbital compared to benzodiazepine.
Two retrospective chart review studies were included. Ibarra72 analyzed 78 patients presenting to the ED with moderate to severe AWS (defined by CIWA-Ar55 score cutoffs), comparing those receiving phenobarbital plus lorazepam to those receiving lorazepam alone (high risk of bias). The authors found no significant difference in daily median lorazepam dose requirement, LOS, ICU admission, or ED discharge. Nelson and colleagues75 analyzed 300 patient encounters presenting to the ED with acute AWS, comparing those receiving phenobarbital alone (n = 100) to those receiving phenobarbital plus lorazepam (n = 100) and those receiving diazepam alone (n = 100) (high risk of bias). All patients were treated based on standardized symptom-triggered protocols; protocols changed during the study period due to drug shortages. The investigators found no difference in ICU admission rate or mechanical ventilation.
In addition to the direct evidence included in the systematic review66 and discussed above, the authors found substantial indirect evidence from the general ward and ICU settings regarding the use of phenobarbital in the treatment of AWS. Several large before-and-after ICU-based studies suggest that increased use of phenobarbital in the treatment of AWS may be associated with a reduction in the utilization of intubation and mechanical ventilation, although these results are limited by the inclusion of other changes in AWS care in addition to a shift in phenobarbital utilization.77-79 Nonrandomized observational and retrospective studies in the ward and ICU settings also suggest that phenobarbital treatment is associated with a decrease in ICU and hospital LOS,79-81 decreased utilization of continuous sedative infusions,77-79 and lower rates of delirium.82
Benefits
A modest amount of direct and indirect evidence demonstrated that the use of phenobarbital in the treatment of AWS is associated with small to moderate improvements in several outcomes of clinical importance, as identified by a survey of the SAEM GRACE-4 Writing Team as part of the guideline's development.
Reduction in intubation and mechanical ventilation
Retrospective observational data from non-ED settings demonstrate that adjunctive phenobarbital in addition to benzodiazepine is associated with reduction in intubation77 and mechanical ventilation.78, 79 Duby and colleagues77 conducted a retrospective before-and-after review of the introduction of a protocol for AWS treatment including the use of phenobarbital and demonstrated decreased rates of intubation (13/60 [22%] in the preprotocol group and 4/75 [5%] in the postprotocol group, p < 0.001, number needed to treat [NNT] 7, 95% CI 4–29 to prevent one intubation). Gold and colleagues,78 also in a retrospective cohort study before and after the introduction of a treatment guideline including phenobarbital, showed reduction in mechanical ventilation (26/54 [48.1%] in the preguideline cohort and 9/41 [21.9%] in the postguideline cohort, p = 0.008, NNT 4, 95% CI 2–22 to avoid mechanical ventilation in one individual). Tidwell and colleagues79 likewise performed a retrospective cohort analysis before and after protocol introduction including a phenobarbital pathway with benzodiazepine as needed, which showed a reduction in the need for mechanical ventilation (14/60 [23%] in the preprotocol and 1/60 [2%] in the postprotocol group, p < 0.001, NNT 5, 95% CI 4–12 to avoid mechanical ventilation in one individual).
Reduction in intensive care unit admission rates & length of stay
The decision to admit a patient with AWS to an ICU is made by considering many factors that may include subjective clinician judgments as well as hospital guidelines. Admission to an ICU and the duration of stay are markers of severity of AWS and reflect the likelihood of decompensation and level of resources required for treatment. As noted above, Rosenson and colleagues,70 in a prospective double-blind, placebo-controlled trial, demonstrated that a single dose of phenobarbital in addition to a symptom-driven lorazepam protocol for AWS in the ED led to significantly decreased number of patients admitted to an ICU (13/51 [25%] in the placebo group compared with 4/51 [8%] in the adjunctive phenobarbital group, a 17% reduction, 95% CI 4%–32%, NNT 6, 95% CI 4—82 to avoid one ICU admission). Tidwell and colleagues,79 in the retrospective before-and-after study discussed above, showed that the implementation of a phenobarbital protocol was associated with decreased ICU LOS (mean ± SD 4.4 ± 3.9 days before phenobarbital to 2.4 ± 1.5 days after the protocol was instituted, p < 0.001).
Reduction hospital length of stay
The duration of hospitalization can also be a marker of AWS severity but may also be affected by the success of treatment. Multiple retrospective studies demonstrate an association between phenobarbital use and decreased hospital LOS or increased rate of hospital discharge within 3 days.72, 79-81 Tidwell and colleagues79 reported a shorter hospital LOS (mean ± SD 4.3 ± 3.4 days vs. 6.9 ± 6.6 days, p = 0.004) in those treated with adjunctive phenobarbital. Hawa and colleagues,81 in a retrospective cohort study, showed that phenobarbital monotherapy was associated with decreased LOS compared to benzodiazepine-alone therapy (2.8 days vs. 3.6 days, p < 0.001). Bosch and colleagues80 performed a noninferiority interrupted time-series analysis to compare patients treated for AWS in a medical ICU with a benzodiazepine-based pathway to those treated with a phenobarbital-based pathway. Hospital LOS was shorter in the phenobarbital group (6.8 days vs. 8.6 days, mean difference 1.8 days, 95% CI 0.2–3.4 days). In the retrospective chart review study conducted by Ibarra72 discussed above, patients who received at least one dose of parenteral phenobarbital in the ED were more likely to be discharged from the hospital within 3 days of presentation than those who did not (9/40 [23%] vs. 2/38 [5%], p < 0.05, NNT 6, 95% CI 3–∞ to attain discharge within 3 days in one patient).
Reduction in sedation by continuous infusion
Continuous sedative infusions (such as a benzodiazepine, propofol, and/or dexmedetomidine) are sometimes used in the treatment of AWS. The logistics involved with administering a continuous sedative infusion are complex and typically require close monitoring and high resource utilization. A reduction in the use of this treatment modality is clinically significant and may help reduce nursing burden and allow for the treatment of AWS in non-ICU settings. In the trial by Rosenson et al.,70 administration of phenobarbital was associated with a significant reduction in the use of lorazepam infusions (16/51 [31%] in the placebo group vs. 2/51 [4%] in the phenobarbital group, 27% reduction, 95% CI 14%–41%, NNT 4, 95% CI 2–9). The retrospective before–after studies by Duby and colleagues77 and Tidwell and colleagues79 also demonstrate reductions in the use of continuous sedative infusions after implementation of a protocol utilizing phenobarbital.
Other benefits
Bosch and colleagues80 noted significant reduction in the use of physical restraints after the introduction of a phenobarbital-based AWS pathway (51.6% of patients before implementation vs. 32.4% after implementation, mean difference −18.0%, 95% CI −26.4% to −9.7%). Nejad and colleagues,82 in a retrospective study of AWS treatment in the context of admission for acute trauma, found that the utilization of a phenobarbital-based treatment pathway, as opposed to the utilization of a fixed-dose benzodiazepine pathway, was associated with a significant reduction in the rate of delirium associated with alcohol withdrawal (0/33 [0%] in the phenobarbital group vs. 25/52 [48.2%] in the benzodiazepine group, p = 0.001, NNT 3, 95% CI 2–4 to prevent one episode of AWS-associated delirium) as well as a reduction in the use of antipsychotics. Lebin and colleagues,73 as discussed above, found in an ED-based study that 3-day return ED visits were less common in the group treated with phenobarbital plus benzodiazepine, and Hawa and colleagues81 found that treatment with phenobarbital was associated with a reduction in 30-day ED return visits (101/543 [18.6%] vs. 7/63 [11.1%] propensity score weighted p = 0.015) and 30-day hospital readmissions (77/543 [14.2%] vs. 7/63 [11.1%] propensity score weighted p = 0.020).
Harms and burdens
There has classically been significant concern about the safety of phenobarbital in alcohol withdrawal. As noted above, historical practice has generally been to reserve phenobarbital for patients with severe or benzodiazepine-resistant alcohol withdrawal, who are generally admitted to the ICU.78 In our GRACE-4 Writing Team discussions, multiple members raised concerns about the possibility of adverse effects, particularly around the larger therapeutic index of benzodiazepines compared to barbiturates, and the risk of respiratory depression and oversedation with phenobarbital.83, 84 Previous survey-based work by Buell and colleagues23 also identified respiratory depression and oversedation as the primary concerns about phenobarbital voiced by clinicians treating alcohol withdrawal. Additional concerns included risk of hypotension, drug–drug interactions, and liver toxicity. However, our review of the direct and indirect evidence found that the preponderance of published literature assessing the use of phenobarbital did not bear out these historical concerns, suggesting that in an appropriately selected patient population, phenobarbital even in combination with benzodiazepines may be a safe therapeutic option.
The systematic review performed as part of this guideline66 did not identify any evidence of increased adverse events with phenobarbital use, although the evidence was of low quality overall. Studies specifically focusing on the question of airway intervention suggest that adjunctive phenobarbital may reduce the need for mechanical ventilation compared to benzodiazepine alone, as discussed above. The studies by Gold et al.,78 Tidwell et al.,79 and Duby et al.77 before-and-after ICU-based studies demonstrated a reduction in intubation rates and mechanical ventilation duration following the introduction of protocols incorporating phenobarbital, even in combination with benzodiazepines. The ED-based trial by Rosenson et al.70 reported no significant difference in intubation rate between the phenobarbital and placebo groups, neither did the chart review by Ibarra72 or a smaller ICU-based retrospective cohort study by Nguyen and Lam.85 Goodberlet and colleagues,86 in an ICU-based before-and-after study, found no difference in intubation rates between the benzodiazepine and phenobarbital groups, despite higher median Acute Physiology and Chronic Health Evaluation (APACHE II87) scores in the phenobarbital group. In contrast to the other studies reviewed they found significantly longer ED LOS, ICU admission, and hypotension in the group receiving benzodiazepines and phenobarbital. The methodology of this study means that it could not be controlled for unknown variables at the time of hospital presentation which may account for their findings. In general, there does not appear to be a substantial signal in the literature that the use of phenobarbital is actually associated with increased incidence of clinically relevant adverse effects. Following the completion of our systematic review, Staidle and Geier88 published a single-center self-controlled retrospective cohort study comparing patients treated with phenobarbital (with or without benzodiazepines) compared to benzodiazepines alone in 137 unique patients. They found no difference in hospital admission or return visits within 48 h; however, treatment with both benzodiazepines and phenobarbital was associated with longer ED LOS, increased ICU admission, and hypotension compared to either phenobarbital or benzodiazepine alone.
Concerns about drug–drug interactions with phenobarbital have also been reported.23 Phenobarbital is a cytochrome P450 enzyme inducer and may theoretically affect the metabolism of coadministered drugs. However, given the short duration of phenobarbital use in AWS, this is unlikely to be of serious clinical consequence, although careful attention to kinetic and dynamic interactions is always reasonable.32
The direct costs of phenobarbital are minimal.70, 79 However, multiple hospitals had policies requiring more intensive cardiac and respiratory monitoring during phenobarbital administration than during benzodiazepine administration, raising the possibility that increased phenobarbital use might lead to increased and more intensive use of scarce nursing resources and monitored beds. In contrast, the evidence outlined above demonstrated decreases in hospital LOS and duration of mechanical ventilation associated with phenobarbital use suggests the potential for substantial indirect cost savings.
Decision criteria and additional considerations
The detailed EtD framework for Question 1 is available in Appendix S2. The certainty of evidence of effect from the systematic review is low. The number of studies included is small (seven studies including 1034 patients), and only two are RCTs (n = 146). Although both RCTs are at low risk of bias, they cannot be appropriately pooled due to serious heterogeneity in their populations, particularly with regard to the severity of disease and treatment interventions, particularly the doses of phenobarbital and benzodiazepine used and the use of long-acting versus short-acting benzodiazepine. The non–randomized controlled trials suffer from significant heterogeneity in treatment interventions and outcome measures. Nevertheless, the general trend of the evidence favors the use of phenobarbital, as all included studies consistently found that phenobarbital was associated with at least one superior outcome measurement. There are some strengths, including the directness of the evidence (all derived from the ED setting) and the presence of the two well-designed RCTs. Additionally, the extensive indirect evidence from non-ED settings summarized above serves to contribute to the certainty of evidence to a limited degree.
A major limitation of the literature on alcohol withdrawal is the absence of a consensus criterion standard severity grading system. The ASAM defines alcohol withdrawal severity based on CIWA-Ar score cutoffs (with a score of 10–18 indicating moderate disease and 19 or more indicating severe disease), but acknowledges that “there is a wide variety in the literature and in practice … classification of withdrawal severity is ultimately up to the judgment of clinicians.”49 Existing literature on alcohol withdrawal uses a variety of different definitions of severity or eligibility for phenobarbital treatment, with some investigators identifying patients for phenobarbital eligibility based on physician gestalt and others using severity cutoffs based on the CIWA-Ar score or other grading instruments or the receipt of specific medications or medication doses (see Table 1 below). The SAEM GRACE-4 Writing Team felt that it was inappropriate to recommend a strict standardized definition of “moderate to severe alcohol withdrawal” because of the serious heterogeneity of the included literature on this point. The most reasonable approach at this time appears to be the definition and identification of withdrawal severity at the discretion of the treating clinician.
| Study | Design | Key inclusion criteria |
|---|---|---|
| Rosenson et al.70 | RCT | Treating physician felt that the patient would likely require inpatient treatment for alcohol withdrawal |
| Hendey et al.71 | RCT | Treating physician “considered” management with parenteral benzodiazepines or phenobarbital |
| Lebin et al.73 | Retrospective cohort study | Diagnosis of alcohol withdrawal plus administration of either benzodiazepine or phenobarbital in the ED |
| Sullivan et al.76 | Retrospective cohort study | Diagnosis of alcohol withdrawal plus administration of either benzodiazepine or phenobarbital in the ED |
| Mahmoud et al.74 | Retrospective cohort study | Diagnosis of alcohol withdrawal plus administration of either benzodiazepine or phenobarbital in the ED (in conjunction with treatment of opioid withdrawal) |
| Ibarra72 | Retrospective chart review study | Moderate or severe alcohol withdrawal by CIWA-Ar score cutoff (moderate 9–15, severe over 15) |
| Nelson et al.75 | Retrospective chart review study | Documentation of any Severity of Ethanol Withdrawal Symptoms (SEWS)89 score plus administration of either benzodiazepine or phenobarbital in the ED |
The SAEM GRACE-4 Writing Team felt that the balance between desirable and undesirable effects probably favors adjunctive phenobarbital over benzodiazepine alone for the treatment of adult ED patients with moderate to severe AWS. Although the direct prospective evidence comparing these interventions in ED patient populations is limited, indirect evidence of desirable effects of phenobarbital over benzodiazepine alone is available. Specifically, the group valued the reduction in many of our preidentified clinically relevant endpoints (need for intubation and mechanical ventilation, decreased ICU admission rate and LOS, and decreased use of continuous sedative infusions and physical restraints) and felt that it outweighed the small potential risk of harm outlined above. The weight of evidence demonstrating unchanged or reduced rates of intubation and mechanical ventilation associated with phenobarbital use was particularly impactful.
Unfortunately, there was no published evidence on the impact of phenobarbital on health equity. There was no published evidence on the acceptability of phenobarbital to patients and their families, and there was minimal published evidence on the acceptability of phenobarbital to clinicians (and none specifically including ED clinicians).
We are not able to recommend the use of phenobarbital in patients being discharged from the ED based on the existing evidence. There is minimal evidence regarding the safety and efficacy of phenobarbital in patients who are discharged from the ED, which was directly studied and reported only in the small trial by Hendey et al.71 We are unable to comment on the safety and utility of adjunctive phenobarbital for this population based on the current literature. This topic should be the focus of future high-quality studies.
Several dosing protocols for the use of phenobarbital in combination with benzodiazepines are reported in the literature, with significant heterogeneity. In several, the initial dose of phenobarbital was 130–260 mg IV, sometimes followed by additional doses based on clinical need.77, 82, 86 Some authors have reported weight-based doses between 6 and 12 mg/kg70, 75, 86 with the exact dose often guided by institutional protocol and influenced by severity of presentation and patient-specific factors. Several studies, including the ED-based RCT by Rosenson et al.,70 used a strategy of an initial 10 mg/kg IV phenobarbital load, which some experts believe is more likely to be effective than repeated smaller aliquot doses, although there is not yet sufficient evidence to directly compare the effectiveness of these approaches. While there is some variability in the dose of phenobarbital, a common theme in several published reports is an institutional protocol with guidance on initial dose, subsequent doses, and monitoring needs.
A major limitation of the current evidence base is the heterogeneity of the treatment protocols and algorithms studied. The use of protocolized, symptom-triggered therapy is often used for AWS, but the various protocols reported in this review are substantially dissimilar with respect to agent selection (e.g., short-acting vs. long-acting benzodiazepine), dosing, reassessment intervals, and choice of clinical scoring criteria to assess AWS severity (e.g., CIWA-Ar or other validated or locally developed scores). Thus, although we do recommend the use of adjunctive phenobarbital, we have no evidence on which to recommend any specific treatment protocol or algorithm incorporating phenobarbital. Local development of treatment protocols with broad support from all major clinical stakeholders is likely to be critical in the implementation of phenobarbital for AWS.
Conclusion and research needs
There is limited high-quality direct evidence supporting use of phenobarbital as an adjunct to benzodiazepine use in the ED for patients with moderate to severe AWS. Although the direct prospective evidence comparing these interventions in ED patient populations is limited, the balance between desirable and undesirable effects favors adjunctive phenobarbital over benzodiazepine alone. This is based, in large part, on indirect evidence illustrating the benefits of adjunctive phenobarbital including, but not limited to, reduction on the need for intubation, decreased hospital LOS, decreased ICU admission, and LOS.
Additional research is needed in multiple aspects of this topic. Additional high-quality prospective interventional research on adjunctive phenobarbital therapy specifically in the ED setting, and exploration of implementation considerations, is particularly important. Other important domains for future research include the identification of specific groups of ED patients who may benefit from adjunctive phenobarbital therapy, the role of AWS management with phenobarbital alone, the role of phenobarbital in patients being discharged from the ED, identification of barriers to the use of phenobarbital by emergency physicians in the management of AWS, and assessment of the cost-effectiveness and acceptability of phenobarbital to clinicians and patients. Finally, the development, testing, and validation of a specific phenobarbital-based protocol and algorithm for the management of AWS in the ED is urgently needed.
QUESTION 2: In patients 18 years of age or older who present to the ED with AUD who are discharged home, does the prescription of an anticraving medication, compared to no prescription, improve outcomes?
Recommendation 2: In adult ED patients (over the age of 18) with AUD who desire alcohol cessation, we suggest a prescription for at least one anticraving medication for the management of AUD for patients who desire alcohol cessation (conditional recommendation, FOR) [very low to low certainty of evidence].
Good practice statement: Please see the anticraving medication algorithm Figure 4 that was designed to help guide clinicians in the selection of anticraving medication based on patient-level factors and the strength of evidence for three medications. As per American Society of Addiction Medicine Guidelines49 clinicians should consider offering patients with AUD supplemental thiamine as part of their ED treatment plan and should be offered follow-up treatment where such treatment is available.
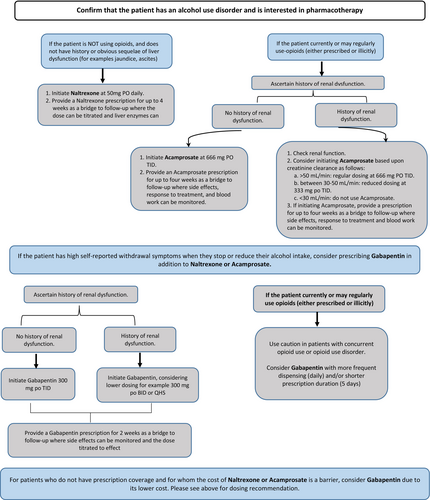
Recommendation 2a: In adult ED patients with AUD who are not taking opioids, we suggest naltrexone (compared to no prescription) for the management of AUD to prevent return to heavy drinking (conditional recommendation, FOR) [low certainty of evidence].
Good practice statement: A bridging prescription of up to 4 weeks until follow-up with an addiction medicine physician, primary care physician, or other appropriate health care provider can take place is preferred. Monitoring of liver enzymes should be at the discretion of the provider seeing the patient in follow-up. For patients not treated with long-acting benzodiazepines for AWS in the ED, patients should be advised that sudden cessation of alcohol consumption may produce acute AWS. These patients should be counseled to slowly taper consumption and seek treatment for AWS management should symptoms occur.
Recommendation 2b: In adult ED patients (over the age of 18) with AUD with contraindications to naltrexone, we suggest acamprosate (compared to no prescription) for the management of AUD to prevent return to heavy drinking (conditional recommendation, FOR) [low certainty of evidence].
Good practice statement: A bridging prescription of up to 4 weeks is reasonable until follow-up where renal function can be monitored with an addiction medicine physician, primary care physician, or other appropriate health care provider.
Recommendation 2c: In adult ED patients with AUD, we suggest gabapentin (compared to no prescription) for the management of AUD to reduce heavy drinking days and improve alcohol withdrawal symptoms (conditional recommendation, FOR) [very low certainty of evidence].
Good practice statement: Given the known misuse potential of gabapentin, a bridging prescription, for example, less than 2 weeks, is preferable to a long-term prescription. Patients should be cautioned about the sedative effects of gabapentin, and it should be prescribed with caution or avoided altogether in patients who use opioids. In patients with high self-reported withdrawal symptoms when they stop or reduce their alcohol intake, consider prescribing gabapentin in addition to naltrexone or acamprosate. Consider a weekly dispensing interval for gabapentin prescriptions longer than 2 weeks.
Summary of the evidence
Despite the high prevalence of AUD and alcohol-related presentations to the ED, only 6% of those with moderate AUD and 21% of those with severe AUD reported receiving any treatment, which includes anticraving medications such as naltrexone, acamprosate, and gabapentin.15 Specific to pharmacologic therapy for AUD, previous studies show that less than 10% receive treatment,89, 90 despite a high level of screening in health care settings.15 Several anticraving medication options exist91, 92; this guideline focuses on naltrexone and acamprosate because these agents have Food and Drug Administration (FDA) approval for this indication, and gabapentin,12, 93-95 though off label for use in patients with AUD, is very commonly used.
Naltrexone
Naltrexone is a medication approved by the FDA for the treatment of both AUD and OUD. In AUD, naltrexone acts as a “highly selective” opioid antagonist to block endogenous opioids triggered by alcohol14, 96 and is believed to thereby decrease dopaminergic activity.97 This decreased dopaminergic activity is hypothesized to reduce cravings and help prevent relapse to heavy drinking by reducing the rewarding effects of alcohol.14, 97-99
Benefits
Oral naltrexone has been shown to increase abstinence from alcohol and reduce binge drinking. Intramuscular naltrexone has been shown to reduce heavy drinking days. Oral naltrexone use results in higher follow-up rates in formal SUD treatment, while extended-release intramuscular naltrexone is associated with substantially higher follow-up rates. Naltrexone is associated with lower risks of hospitalization due to any alcohol-related causes compared with no use of AUD medication.
The COMBINE Study, a multisite RCT comparing naltrexone and acamprosate, evaluated both medications individually, in combination, and in the context of two variations of behavioral intervention, one a medical model (medical management) and the other a specialist model (combined behavioral intervention). All pill-taking groups in this study showed an increase in the percentage of days abstinent (increasing from 25.1% to 73.1%, p < 0.001) and reduction in the number of drinks per drinking day (decreasing from 12.6 to 7.1, p = 0.03) for a total 80% decrease in consumption. Naltrexone plus medical management (n = 302) resulted in a higher percentage of days abstinent (80.6%) than those receiving placebos and medical management only. Naltrexone also reduced the risk of heavy drinking days (hazard ratio 0.72, 97.5% CI 0.53–0.98, p = 0.02) over time, most evident in those receiving medical management but not combined behavioral intervention.100, 101 Unlike previous studies of acamprosate, COMBINE failed to demonstrate significant benefits from the use of that medication.
A pseudo-RCT by Anderson et al.102 evaluated oral or extended-release naltrexone in ED patients with moderate to severe AUD and their subsequent engagement with formal SUD treatment within 30 days of discharge from the ED. Among all patients in the study, 15.3% attended follow-up formal SUD treatment within 30 days of discharge, with oral naltrexone patients and extended-release naltrexone demonstrating 9.8% and 27.8% follow-up rates, respectively.
A number of reviews and meta-analysis demonstrate the benefit of naltrexone in the treatment of AUD. A 2010 Cochrane review of 50 RCTs that included 7793 patients determined that naltrexone was associated with a reduction in risk of heavy drinking to 83% of that in the placebo group, and naltrexone was also associated with an overall decrease in drinking days.103 A study by Jonas et al.104 demonstrated naltrexone's efficacy in reducing both the risk of relapse to any drinking (16 studies, N = 2347, risk decrease −0.05, 95% CI −0.10 to −0.002, NNT 20 to prevent one individual from relapsing) and a return to binge drinking (19 studies, N = 2875, risk decrease −0.09, 95% CI −0.13 to −0.04, NNT 12 to prevent one individual from returning to binge drinking). This analysis also found extended-release intramuscular naltrexone treatment was associated with fewer heavy drinking days.
In a meta-analysis by Maisel et al.,14 eight people would need to be treated with acamprosate to achieve an additional case of abstinence (NNT 7.5), and nine people would need to be treated with naltrexone to prevent an additional case of return to heavy drinking (NNT 8.6). In a 12-week double-blind RCT, Volpicelli et al.11 found that 23% of individuals receiving naltrexone relapsed compared to 54% in the placebo group. A total of 95% of individuals in the placebo group elapsed after sampling alcohol, compared to only 50% in the naltrexone group.
A subset analysis of O'Malley et al.,105 using daily diary data from their RCT, found that naltrexone, compared with placebo, was associated with lighter drinking (b = −1.27, SE = 0.39, p < 0.001, 95% CI −2.04 to −0.50) but not with craving. Within the same individuals, taking one, compared to zero, naltrexone pill was associated with lower-than-average drinking quantity (b = −0.75, SE = 0.31, p = 0.013, 95% CI −0.1.36 to −0.14), while taking two, compared to one, naltrexone pills was associated with higher-than-average drinking quantity (b = 0.82, SE = 0.17, p < 0.001, 95% CI 0.49–1.15). Weekend days were associated with heavier drinking (b = 0.96, SE = 0.15, p < 0.001, 95% CI 0.66–1.26), and days later in treatment were associated with lighter drinking (b = −0.02, SE = 0.01, p = 0.004, 95% CI −0.03 to −0.01).106
A retrospective analysis of Thompson-Reuters Market Scan Commercial Claims 6 months pre and postenrollment found that extended-release naltrexone was associated with fewer inpatient detoxification days compared to oral naltrexone or acamprosate use and was also associated with fewer inpatient days for a principal diagnosis of alcohol dependence than disulfiram or acamprosate use. A significantly higher percentage of patients receiving extended-release naltrexone (69%) had an outpatient visit for substance misuse treatment than patients receiving oral agents (38% oral naltrexone, 40% for disulfiram, and 40% for acamprosate p < 0.001).107 An RCT by O'Malley et al.105 in individuals aged 18–25 years with four or more reported heavy drinking days in the prior 4 weeks found that naltrexone significantly reduced the number of drinks per drinking day and the percentage of drinking days with an estimated blood alcohol concentration over 0.08 g/dL.
A recent feasibility study (a 12-week prospective open-label single-arm study) demonstrated that initiation of treatment of AUD with extended-release naltrexone and case management was feasible in an urban, academic ED. The authors observed significant reductions in drinking and improved quality of life in the short term. The mean baseline daily alcohol consumption was 7.6 drinks per day (interquartile range 4.5–13.4), and the median daily alcohol consumption change was −7.5 drinks per day (95% CI −8.6 to −5.9). The mean (±SD) baseline quality of life was 3.6 (±1.7) on a 7-point scale, and the mean quality-of-life change was 1.2 points (95% CI 0.5–1.9, p < 0.01).108
Harms and burden
Overall, naltrexone is well tolerated with mild side effects. Common adverse effects of oral naltrexone, compared to placebo, include somnolence (29.5% vs. 17.8%, risk difference 0.10, 95% CI 0.05–0.14), nausea (25.8% vs. 16.3%, risk difference 0.10, 95% CI 0.07–0.13), vomiting (16.9% vs. 10.4%, risk difference 0.07, 95% CI 0.04–0.09), decreased appetite (17.7% vs. 11.8%, risk difference 0.07, 95% CI 0.03–0.11), abdominal pain (15.9% vs. 7.5%, risk difference 0.08, 95% CI 0.04–0.11), insomnia (16.4% vs. 13.4%, risk difference 0.03, 95% CI 0.00–0.06), and dizziness (11.9% vs. 6.2%, risk difference 0.06, 95% CI 0.04–0.08). Long-acting naltrexone causes similar adverse events as oral naltrexone, with an additional side effect of injection site pain.103, 109
Resources—effectiveness
Gastfriend110 reviewed the economic impact of different treatments for AUD. Total health care costs, including inpatient, outpatient, and pharmacy costs, were 30% lower for patients who received a medication (naltrexone, disulfiram, acamprosate, and extended-release naltrexone) compared to nonmedicated patients. Comparing the four medication groups, baseline raw data showed the extended-release naltrexone group to be intermediate in health indices, comorbidity, utilization, and health costs; differences across the groups were subsequently controlled using propensity-score matching, which also manages differences in cohort sizes. Among the four medications, the greatest persistence with refills, fewest hospitalizations, and lowest hospital costs occurred with extended-release naltrexone. An inverse relationship emerged between refill persistence and hospitalization, which has not previously been demonstrated. Patients persisted with acamprosate for a mean of 42.6 days, with disulfiram for 45.8 days, with naltrexone for 49.8 days, and with extended-release naltrexone for 61.6 days.
As a result of this utilization pattern, inpatient costs over the next 6 months per patient for detoxification and rehabilitation were $288 for acamprosate, $203 for disulfiram, $192 for naltrexone, and $105 for extended-release naltrexone (p < 0.01 for all vs. extended-release naltrexone); for alcohol-related hospitalizations, $1166 for acamprosate, $874 for disulfiram, $618 for naltrexone, and $474 for extended-release naltrexone (p < 0.0001 for all vs. extended-release naltrexone); and for non–alcohol-related admissions, $3885 for acamprosate, $1498 for disulfiram, $1091 for naltrexone, and $730 for extended-release naltrexone (p < 0.0001 for all vs. extended-release naltrexone). Interestingly, this inverse relationship between refill persistence and hospitalization closely follows the burden of medication administration: acamprosate requires two pills three times per day, disulfiram requires one pill per day, naltrexone requires one pill per day or two pills every other day, and extended-release naltrexone involves one injection per 30 days.
Acamprosate
Acamprosate is a first-line treatment for AUD for those with a goal of abstinence and is superior to referral to psychosocial support alone. It is best started after a period of detoxification. While the mechanism of action is not fully understood, it is an N-methyl-d-aspartate (NMDA) receptor antagonist and a positive modulator of GABAA receptors.111 It is thought to be a glutamate stabilizer, resulting in decreased positive reinforcement of alcohol and decreased withdrawal cravings by alleviating both the physical and the psychological effects of alcohol cessation. The ED may be the only site of contact for patients with AUD22 and is therefore an ideal location to initiate treatment with acamprosate when there is a contraindication to naltrexone (severe liver dysfunction, ongoing or anticipated opioid treatment, known allergy).
Benefits
When combined with psychosocial treatment, acamprosate decreases alcohol intake in detoxified patients with AUD; however, no studies have been done within the ED.
Reduction of heavy drinking
A recent systematic review (122 RCTs including 22,803 participants) demonstrated that acamprosate was efficacious against placebo for a reduction in heavy drinking (RR 0.78, 95% CI 0.70–0.86).91
Reduction in any drinking (abstinence)
Compared with placebo, there is increased probability of abstinence at 12 months with acamprosate (OR 1.86, 95% CI 1.49–2.33, corresponding to an absolute probability of 38% or NNT 3 to promote abstinence in one individual who otherwise would not have remained abstinent). Treatment was started after an unknown period of detoxification, although detoxification had to occur less than 4 weeks prior to initiation of acamprosate.92
The 2010 Cochrane systematic review shows that compared to placebo, acamprosate was shown to significantly reduce the risk of any drinking RR 0.86 (95% CI 0.81–0.91); NNT 9 (95% CI 6.7 to 14.3) to reduce any drinking in one individual. It is important to note that the effect was seen when acamprosate was added to psychosocial treatment strategies. Most (23/24) of the studies in the Cochrane review required at least a 3-day period of abstinence prior to the initiation of acamprosate.103
Reduction in return to use
The 2010 Cochrane systematic review shows that compared to placebo, acamprosate was shown to significantly increase the cumulative abstinence duration from alcohol (95% CI 5.08–16.81).103
Harms and burden
Adverse events of acamprosate tend to be mild and transient in nature. They are predominantly gastrointestinal or dermatologic.103, 112 In the 2010 Cochrane systematic review of 24 RCTs with 6894 patients, among 38 side effects, only diarrhea was more frequently reported with acamprosate compared with placebo, RR 0.11 (95% CI 0.10–0.13). The dose of acamprosate varied from 1332 to 3000 mg per day. Treatment duration varied from 8 weeks, with a duration of 6 months being most common.104 Although diarrhea can occur in up to 16% of patients, it usually resolves within a few days.113, 114
Resources and cost-effectiveness
To our knowledge, there are no studies outlining the resources required for acamprosate with regard to training and education of patients and prescribers. A 2012 Italian cost-effectiveness study used a reference population of almost 110,000 patients and demonstrated that increasing the acamprosate use was associated with a progressive decrease in total direct costs, calculated as the sum of the diagnosis-related groups, rehabilitation, and drug expenses. The increasing use of acamprosate over usual care generated cost savings up 44€6.5 million ($7M USD) over 10 years.115
Short-term managed AUD therapies and counseling were found to be extremely cost-effective in a USD 2021 hypothetical study using the Markov simulation model on patients with compensated alcohol-related cirrhosis.116 The study projected the lifetime costs and health benefits of different alcohol use treatments. To estimate treatment effects, they used unpublished, corrected results of a retrospective cohort study that compared the rate of decompensation between compensated alcohol-related cirrhosis patients who received different alcohol use treatments versus compensated alcohol-related cirrhosis patients who did not receive an intervention. Calculated health care costs include health care service delivery (e.g., physician and facility fees) and drug costs. Compared to other interventions, acamprosate and oral naltrexone cost the least and provided the most quality-adjusted life-years, reported as quality-adjusted life-years gained and the incremental cost-effectiveness ratio.
Another modeling study from Germany found acamprosate to be cost effective based on data from the 1996 Prevention of Relapse with Acamprosate in the Management of Alcoholism (PRAMA study). The model included 500,000 patients and demonstrated a net savings in direct medical costs of Deutsche Mark 2600 (about $1400 US$ in 2023) per additional abstinent patient being treated with acamprosate as an anticraving medication.117
Gabapentin
Gabapentin is an anticonvulsant that indirectly influences GABA and glutamate activity.100, 118 It is approved by the FDA for the treatment of postherpetic neuralgia and as an adjunctive treatment for seizure disorders. It has also been shown to be effective for relieving acute AWS symptoms.119 It is thought that gabapentin may be effective as an off-label treatment for AUD, especially in individuals whose alcohol use is triggered by withdrawal symptoms. Gabapentin dosing varies widely, with total daily doses ranging from 300 mg to 3600 mg/day depending on the study.
Benefits
Gabapentin has been studied off-label for the treatment of AUD; however, none of the studies have been based in the ED, and therefore all evidence is indirect. A 2020 meta-analysis of 16 studies on gabapentin for AUD explored its effect on multiple outcomes including cumulative days of abstinence, abstinence rate at study endpoint, time to relapse, percentage of heavy drinking days, number of heavy drinking days per week, and amount of alcohol consumption.12 While the meta-analysis did not show a significant effect on its composite outcome, it did find that gabapentin had a significant benefit compared to placebo or control treatment on the percentage of heavy drinking days (Hedges' g = 0.5478, 95% CI 0.0145–1.0812, p = 0.0441) and AWS symptoms (Hedges' g = 0.2475, 95% CI 0.0286–0.5483, p = 0.0425).120 Hedge's g is a bias-corrected form of Cohen's d and effect size interpretation conventions for both are: small ~0.2, medium ~0.4, and large ~0.8+, although caution should be used for all effect size benchmarking.121 The meta-analysis found a large amount of heterogeneity with respect to the percentage of heavy drinking days (I2 = 89.0%), while it found a low amount of heterogeneity for the effect on AWS symptoms (I2 = 0%).
A 2020 double-blind RCT, not included in the meta-analysis above, evaluated gabapentin versus placebo for the treatment of AUD.100 Patients were treated with gabapentin doses up to 1200 mg/day. More gabapentin-treated individuals had no heavy drinking days (12 of 44 participants [27%]) compared with placebo (four of 46 participants [9%]), a difference of 18.6% (95% CI 3.1–34.1, p = 0.02, NNT 5.4), and more total abstinence (eight of 44 [18%]) compared with placebo (two of 46 [4%]), a difference of 13.8% (95% CI 1.0–26.7, p = 0.04, NNT 6.2 to have total abstinence in one individual who otherwise would not have had total abstinence).
The study specifically explored the impact of high versus low self-report alcohol withdrawal. Patients were required to be abstinent from alcohol for at least 3 days prior to randomization, and they were asked to self-report how bothered they were by various symptoms of alcohol withdrawal. Those that reported high AWS had larger gabapentin effects on no heavy drinking days (p < 0.02, NNT 4) and total abstinence (p = 0.003, NNT 3) compared with placebo, while those that reported low AWS symptoms had no significant differences when treated with gabapentin versus placebo.
Harms and burden
Overall, gabapentin is well tolerated with minimal side effects. The 2020 meta-analysis12 found that overall, the OR for all adverse events was 1.09 (95% CI 0.98–1.21). They found a significant effect of gabapentin on somnolence (OR 1.79, 95% CI 1.16–2.76, NNH 18) and dizziness (OR 1.54, 95% CI 1.05–2.24, NNH 19). There was no comment made on the relationship between dose and side effects.122
Some recent evidence suggests that gabapentin is increasingly used as a drug of misuse and has been associated with overdose deaths. A review of unintentional overdose deaths from 23 states and the District of Columbia found that toxicology tests were able to detect gabapentin in 10% of all overdose deaths between 2019 and 2020. The risk is likely highest in those who use gabapentin in combination with opioids, as 90% of overdose deaths where gabapentin was detected also had significant blood levels of opioids.123, 124
To our knowledge, no studies exist that examine the cost-effectiveness of prescribing gabapentin in the ED for AUD. A simulation study on the cost-effectiveness of pharmacotherapy interventions for AUD in a population of patients with compensated alcohol-related cirrhosis found that gabapentin would be cost saving (but less cost saving than acamprosate or naltrexone).116
Conclusion and research needs
There is limited high-quality direct evidence on the use of anticraving medications in the ED for the treatment of AUD. Despite this limitation, the balance of desirable and undesirable effects favors prescribing anticraving medications in the ED for people with AUD. This is based on indirect evidence demonstrating the effectiveness of naltrexone, acamprosate, and gabapentin in reducing heavy drinking days and increasing abstinence. In addition, these medications are well tolerated with mild side effects. To help ED clinicians determine which anticraving medications to offer, please refer to Figure 4.
Additional research is needed on the treatment of AUD in the ED. ED-based effectiveness trials for naltrexone, acamprosate, and gabapentin would improve the directness of the evidence for prescribing these medications in the ED. In addition, it would be helpful to understand the barriers and facilitators for ED clinicians prescribing anticraving medications to help with knowledge translation efforts. Finally, cost-effectiveness studies of ED-based interventions would be helpful in determining the resources that should be mobilized in increasing the comfort and availability of anticraving medications in the ED.
Decision criteria and additional considerations
The detailed EtD frameworks for each medication covered in Question 2 are available in Appendix S2.
Prescribing algorithm
A prescribing algorithm that can be used to guide treatment decisions is shown in Figure 4.
QUESTION 3: In adult ED patients (>18 years old) who are suspected to have CHS, does the use of dopamine antagonists (e.g., haloperidol, droperidol) or capsaicin compared to usual care (or no treatment) lead to improved outcomes?
Recommendation 3a: In adult patients presenting to the ED with CHS, we suggest the use of haloperidol or droperidol in addition to usual care to help with symptom management (conditional, FOR) [very low level of evidence].
Good practice statement: IV fluids and nonopioid analgesics could be administered/offered to help with symptom management.
Recommendation 3b: In patients presenting to the ED with CHS, we suggest offering the use of topical capsaicin to help with symptom management (conditional, FOR) [very low level of evidence].
Good practice statement: One member of the GRACE-4 Writing Team emphasized the importance of recognizing that not all patients experience relief with capsaicin, and clinicians should be prompt in escalating treatment for patients whose symptoms are not alleviated promptly. This member also emphasized that capsaicin should not be used for patients for whom it had not been effective in the past.
Good practice statement: In patients presenting to the ED with CHS, benzodiazepines and opioids should not be used as first-line treatment for CHS symptom management. In balance with the lack of evidence supporting the effectiveness of benzodiazepines and opioids in this setting and considering prior SAEM GRACE of chronic abdominal pain, opioids should be reserved for patients where pain is the primary concern and in whom haloperidol/droperidol (and if attempted, capsaicin) have not provided prompt relief. We believe the potential risks associated with administration of opioids as initial treatment for CHS outweigh outweigh any potential benefit.
Good practice statement: These interventions should be used in conjunction with anticipatory guidance on the necessity of cannabis abstinence for complete symptom resolution. We found no published evidence that reduction in use will prevent CHS; however, anecdotal evidence from our representative with lived experience suggests that in some cases reducing use may reduce frequency of episodes. If the health care team suspects concurrent CUD (based on screening with a validated tool such as CUDIT-R), consider referral to psychosocial interventions and/or addiction medicine specialists, if available.
Hydration and other supportive treatments should not be delayed to administer either haloperidol/droperidol or capsaicin. Clinicians should educate patients on the rationale for the use of these medications if questioned and caution them about the intensity of burning related to capsaicin application.
Summary of the evidence
Haloperidol/Droperidol
A total of seven studies were identified, two for haloperidol and droperidol (one retrospective study and one RCT) and five for capsaicin (four retrospective studies and one RCT).47, 122, 125-129 Most studies provided direct evidence for ED care but were retrospective and had limitations in terms of significant variability in identifications of CHS patients, dosing of medications and outcomes.
A retrospective review evaluated the use of droperidol in patients with CHS as defined by a history of long-term cannabis use, recurrent vomiting, and no other diagnosis to explain the patient's symptoms.47 Seventy-six patients with CHS were included with 37 receiving droperidol while the rest did not. The primary outcome was hospital LOS. Median LOS in the droperidol group was 6.7 h (IQR 4.7–11.9 h) compared to 13.9 h (IQR 5.2–57.3 h; p = 0.14) in the group that did not receive droperidol. Median LOS to discharge was also shorter in the group that received droperidol (137 min vs. 185 min, p = 0.002). The median number of antiemetics used after droperidol was 0 (IQR 0–1) with 54% in the droperidol group not requiring further medications.
A randomized crossover trial with up to three treatments per subject compared haloperidol at two different dosages (0.05 or 0.1 mg/kg) to ondansetron in the treatment of abdominal pain and nausea in patients with CHS.128 Patients were included if they presented with symptoms consistent with CHS for at least 2 h and excluded if they received an antiemetic (including antimuscarinic or antipsychotic medications) intravenously in the prior 24 h. After adjustment for demographics, change on the visual analog scale between ondansetron and haloperidol was 2.3 cm (95% CI 0.6–4.0 cm, p = 0.01) favoring haloperidol. Haloperidol resulted in higher treatment success (54% vs. 29%, difference 24%, 95% CI 16%–59%), reduced use of any medications (including rescue antiemetics) and a shorter time to discharge (3.1 h vs. 5.6 h, difference 2.5 h, 95% CI 0.1–5 h, p = 0.03). Most patients dropped out between Phase I and Phase II of the study, so they only participated in a single treatment arm and therefore did not receive ondansetron and both doses of haloperidol.
Capsaicin
A retrospective cohort study evaluated capsaicin in patients with CHS.122 All patients had prior ED evaluations for CHS where they did not receive capsaicin and were discharged from the ED on the study visit. The comparison was the same patient's prior visit. Forty-three patients were included. Median (IQR) LOS after the administration of capsaicin (179 [147–270] min) compared with 201 (168–310 min, p = 0.33) for those not treated with capsaicin. Median time to discharge following the last medication administered was less in the capsaicin group, but the difference was not statistically significant. However, fewer additional medications were administered with the use of capsaicin (four doses vs. three doses, p = 0.015), including opioid use as measured by oral morphine equivalents (OME; 166.5 mg vs. 69 mg OME). Eighteen patients (42%) who received capsaicin did not have a repeat ED presentation within 30 days of receiving capsaicin for an additional 3 months after the study ended.
A single-center retrospective cohort study included both adolescents and adults who presented with suspected CHS (reoccurring, unexplained vomiting in the context of cannabis use).126 A total of 149 patients who received capsaicin were compared to 52 who did not. A significantly greater number of patients who received capsaicin only required zero or one additional dose of medication compared to the control group (n = 84/149, 56.4% vs. 11/52, 21%). Time to discharge or admission from receipt of reference medication (capsaicin vs. first medication) was also significantly shorter in the group that received capsaicin (3.72 h vs. 6.11 h, p = 001, 95% CI 2.80–3.50). However, total ED LOS and admission rates were not significantly different due to delays in obtaining capsaicin. Pain scores and the number of patients who returned to the ED within 24 h were similar between groups. Predictors for efficacy included symptom duration of greater than 1 month and presence of nausea. However, symptoms of vomiting, abdominal pain, and relief with hot showers did not predict the efficacy of capsaicin.
Another retrospective study was conducted to determine if capsaicin reduced ED LOS in patients with CHS.129 To be included, capsaicin must have been ordered for the treatment of CHS for patients who subsequently were discharged from the ED. As capsaicin was not on the ED formulary, a clinical pharmacist had to be available to administer it. As such, capsaicin could be ordered but not administered if the pharmacist was not available. Any patients who were admitted to the hospital were also excluded. No measures of symptom reduction were included in the analysis. Capsaicin was ordered in 55 patients but only administered to 35. There was no significant difference from when capsaicin was ordered to patient discharge between the two groups. Additionally, there was no difference in rescue medications administered, return ED visits within 24 or 72 h, and hospital admission rates. Similar percentages of patients in each group received benzodiazepines, while more patients that had capsaicin administered received opioids (31% vs. 20%; comparative statistics were not given). Timing of receipt of additional medications relative to capsaicin administration was not described.
A retrospective chart review included patients with an index ED visit with symptoms of abdominal pain, nausea, and vomiting and with a history of cannabis consumption that received capsaicin 0.025%.127 Fifty-seven patients were included, but many data points were missing around assessment of pain and symptom relief (only 34 patients had paired pre- and postcapsaicin pain scores documented). Median (IQR) pain scores appeared similar pre- and postadministration of capsaicin (8 [0–10] vs. 5 [0–8]). However, 45% of patients did not need any further rescue medications postcapsaicin.
An RCT pilot study compared capsaicin 0.1% to placebo in 30 patients presenting with suspected exacerbation of CHS.125 Initially patients were excluded if they received antiemetics in the ED prior to randomization, but this exclusion was later removed. At 60 min using a visual analog score, nausea in the treatment group was significantly improved compared to the placebo (−3.2 cm, 95% CI −0.9 to −5.4) and a higher proportion had complete resolution of nausea (RR 3.4, 95% CI 1.6–7.1). Vomiting was improved but the difference was not statistically significant. The total numbers of patients requiring rescue medications or admission were similar in both groups.
Indirect evidence
Haloperidol/Droperidol
A systematic review evaluated multiple medications in the treatment of CHS.61 The review included two case series and five case reports. In one of the case series, symptoms recalcitrant to other medications improved with haloperidol. In the other, symptoms improved after haloperidol and olanzapine were administered. In three of the case reports, haloperidol was reportedly effective, while it was reported as ineffective in one and its effectiveness was not reported in the other.
A retrospective chart review identified four patients with CHS who received haloperidol.130 A 34-year-old male who did not improve after receiving multiple antiemetics was able to be discharged 1 h after receiving haloperidol 5 mg IV. A 28-year-old male with recurrent presentations for CHS stated that prior medical therapies never improved his symptoms and requested admission. He received haloperidol 5 mg IV and diphenhydramine, improved within 1 h, and was discharged 6 h later. A 48-year-old also reported that he had not previously improved with other medications. His initial treatment was haloperidol 5 mg IV. He improved within 1 h and was discharged 8 h later. Finally, a 22-year-old male received haloperidol 5 mg IV as his initial medication with symptom improvement in 2 h and was discharged 6 h later.
Capsaicin
Indirect evidence included a review and meta-analysis on the use of capsaicin for CHS in the ED that combined four cases series and three cohorts,131 including two studies described above, to assess a primary outcome of hospital admissions. Most of the included studies were of low quality and included no comparison group for the primary outcome. The overall rate of admission was 15% (95% CI 6%–32%) in patients treated with capsaicin. Secondary outcomes, including time to symptom relief and ED LOS, were not reported in most studies. The meta-analysis demonstrated a low admission rate.
Capsaicin as a treatment for CHS and has been documented in multiple case reports.132-135 A case series from two academic institutions demonstrated an improvement in 13 patients after receiving capsaicin.136 However, patients also received a broad range of other medications. For two patients, capsaicin was the first medication administered but both needed other rescue medications. Only one patient returned to the ED within 72 h for similar gastrointestinal symptoms after receiving capsaicin.
Benefits
An ideal agent would improve the patient's symptoms, reduce the need for rescue medications while reducing LOS in the ED and ED return visits, and prevent hospital admissions. Unfortunately, the studies identified had heterogeneous outcomes, and there was variability in the data that was reported limiting comparisons between studies.
Haloperidol and droperidol
Benefits of haloperidol and droperidol include symptom relief including improvement of nausea, vomiting, and abdominal pain.128, 137 Patients who received droperidol or haloperidol for nausea were less likely to require rescue analgesics or antiemetics and opioids.137 One study demonstrated decreased ED LOS.47
Capsaicin
Benefits include symptom relief. Patients who received capsaicin were less likely to receive rescue analgesics (including opioids) and antiemetics. Median LOS or time to discharge was not reduced with use of capsaicin, but noted delays from ordering to administration of capsaicin may have contributed to this.68
Overall, existing research may demonstrate some improvement following the administration of dopamine antagonists or capsaicin. No study specifically compared using both agents. However, the studies were of very low methodologic quality with many being observational and retrospective in nature, used different criteria for identification of CHS patients, and included different outcomes. One member of the GRACE-4 Writing Team emphasized the importance of recognizing that not all patients experience relief with capsaicin and worried that the potential benefit was too heterogeneous among patients to demonstrate any improvement for it to be offered as a treatment in the ED. This opinion diverged from consensus by the GRACE-4 Writing Team, who felt the low cost and ease of administration of this treatment warranted an attempt to use it.
Harms and burden
Harms associated with the use of haloperidol, droperidol, or capsaicin are minimal.
Haloperidol and droperidol
Haloperidol and droperidol can cause extrapyramidal symptoms such as dystonia47, 128; however, these events were infrequent, easily treated, and usually only occurred with administration of doses greater than 5 mg. Indirect studies mirrored this finding, with the use of droperidol and haloperidol for non–CHS-related presentations demonstrating a higher number of dystonic reactions only with administration of higher doses than those typically used for CHS management (<5 mg).137-140 Significant rates of CNS depression were not reported. Both have been implicated with causing QTc prolongation, which has led to a black box warning for their use. Since then, multiple indirect studies demonstrated the absence of any significant adverse effects of droperidol on the QTc interval in doses typically used for the treatment of CHS.40, 137, 141, 142
Rural or smaller EDs, or those that are overcrowded, may experience difficulty in using either haloperidol or droperidol if there is a requirement for patients to be on cardiac monitors. However, GRACE-4 Writing Team consensus supported that current evidence dictates that cardiac monitoring is not required for administration of this medication at doses typically administered for CHS unless other risk factors are present.
Capsaicin
Capsaicin may cause a burning sensation. Indirect, small retrospective studies reported rates between 4.8% and 17.8%.122, 125, 129 Of those experiencing this, most will require removal of the cream but do not suffer any long-term complications. No other significant adverse effects are reported following the topical application of capsaicin. One member of GRACE-4 Writing Team was worried the use of capsaicin could cause more harm if it did not work and led to delays in the administration of other treatments that might be more effective. Capsaicin may not be available in all EDs, is often stored in a central pharmacy leading to delays in administration, and requires proper application.
Decision criteria and additional considerations
Haloperidol and droperidol
The detailed EtD frameworks for both dopamine antagonists (haloperidol/droperidol) and capsaicin in Question 3 are available in Appendix S2. Evidence of the efficacy of haloperidol and droperidol in the treatment of CHS is limited by the low quality of many of the included studies. However, minimal adverse effects are associated with either of these agents at doses used in the treatment of CHS. Additionally, many other standard treatments with similar side effect concerns (e.g., ondansetron and QT prolongation) have demonstrated limited to no efficacy in CHS. Given this, we believe the balance favors the use of haloperidol and droperidol in the treatment of CHS.
Capsaicin
Studies involving the administration of capsaicin in the treatment of CHS were of very low quality and demonstrated small but mixed results in terms of benefit. A similar recommendation to that of haloperidol and droperidol was proposed for capsaicin in terms of use in the management of CHS in the ED. While the group acknowledges very limited evidence demonstrating capsaicin's efficacy, this was balanced against the very limited evidence of adverse effects, generally low cost, and the ability of patients to self-administer it at home following the ED evaluation. After careful consideration and anonymous voting, the SAEM GRACE-4 Writing Team determined that the balance of effects probably favored the use of capsaicin.
One member of GRACE-4 Writing Team was concerned about the recommendation to use capsaicin in the management of CHS in the ED. The concerns centered around experiences with lack of efficacy in symptom relief and belief that many patients with CHS would have already found hot showers not to be effective controlling symptoms prior to presenting to the ED, decreasing the likelihood that capsaicin would provide significant benefit. There was also concern that recommending capsaicin instead of other treatments would cause increased patient discomfort, increase ED LOS, and hospital admission rates.
These recommendations focused on managing symptoms of CHS in the ED. It must be acknowledged that abstinence from cannabis is required for complete resolution of symptoms and should be advised in conjunction with acute symptom management. We found no published evidence that reduction in use will prevent CHS, however anecdotal evidence from our representative with lived experience suggests that in some cases reducing use may reduce frequency of episodes.
Conclusion and research needs
There is limited, low-quality evidence evaluating the use of either droperidol or haloperidol in the treatment of CHS in the ED. However, the balance between desirable and undesirable effects supports the recommendation of these agents, in comparison to the lack of effectiveness of standard therapies for symptoms related to CHS (e.g., abdominal pain, nausea, and vomiting). Indirect evidence supports its use in nausea and vomiting for other non–CHS-related presentations with low rates of adverse effects.
Capsaicin also received a conditional recommendation. The evidence supportive of capsaicin was even more limited with the recommendation mainly based on the lack of significant adverse effects, low cost of capsaicin, and the ability for the patient to apply the medication at home after discharge. Improvements in prompt access to capsaicin in the ED and support in removing the necessity of cardiac monitoring for haloperidol and droperidol in appropriate patients will ease concerns regarding the feasibility of implementation. In conjunction with treatment of acute symptoms in the ED, abstinence from cannabis is required for complete resolution of symptoms and prevention of recurrence, and referral for additional treatment is recommended for patients with a suspected CUD.
Further research is needed to standardize screening and diagnosis of CHS in the ED. Presently, there are no standard diagnostic criteria—consistent and early patient identification is difficult and current studies have significant heterogeneity. More randomized trials with better methodology that compare droperidol, haloperidol, and/or capsaicin to either standard care or each other in the treatment of CHS are needed to better quantify effectiveness and monitor safety to provide more definitive recommendations. Finally, research focused on cost-effectiveness and exploring outcomes stratified across different patient socioeconomic and demographic subgroups is needed.
GENERAL ISSUES NECESSARY FOR CORRECT INTERPRETATION AND IMPLEMENTATION OF RECOMMENDATIONS
Limitations
Topic selection and lack of direct evidence
The SAEM GRACE Steering Committee selected the topic of nonopioid use disorder based on consensus agreement on the clinical importance, without feasibility assessment to determine the availability of definitions to address the topic. The GRACE Steering Committee felt that generating a guideline even in the absence of strong evidence fills an important gap for clinicians. Identifying the paucity of evidence has its own value in directing future research efforts.
Within the topic chosen by the SAEM GRACE Steering Committee, the SAEM GRACE-4 Writing Team generated outcomes of interest prospectively, before performing a literature search, and chose to abide by these decisions even when it was discovered that there was an absence of direct evidence to address these. Ongoing surveys of topics of importance to patients and clinicians should be incorporated for future SAEM GRACE projects as the writing group represents a small sample, though with specific topic expertise.
SAEM GRACE-4 Writing Team composition
The SAEM GRACE-4 Writing Team was selected for topic expertise related to SUD, specifically alcohol and CUDs. The size of the committee was limited by practical constraints and funding availability. A priori, efforts were made to include diverse representation; however, the majority of our members consisted of emergency physicians practicing in large suburban or urban centers (without rural or critical access hospital practitioners). The SAEM GRACE-4 Writing Team did include two patient representatives, one with a history of AUD, and a second with a history of CHS. Our patient representatives were included and participated in all phases of the guideline development, but physicians comprised the majority of the GRACE-4 Writing Team, meaning that topics of importance to treating physicians could have been favored in the selection process. The patient representatives influenced the discussion before voting occurred. In accordance with Guidance for Reporting Involvement of Patients and the Public (GRIPP-2) reporting standards, the patient representatives were engaged in setting the aims of the guideline project, providing insights on values and preferences for clinical outcomes, reviewing and interpreting evidence reviews in PICO subgroups, and contextualizing results during the EtD framework discussions based on lived experiences.51, 143
Future GRACE guidelines should continue to recruit diverse teams (including patient representatives) across many different factors, including gender, race, ethnicity, sexual orientation, and academic or community practice settings. Early attention to diversity could improve the applicability to clinicians caring for diverse patient populations in diverse settings, including care outside of North America. The GRACE-4 Writing Team did not include experts in primary care, medical/legal considerations, health economists, government or hospital decision makers, or specialist groups who may ultimately admit these patients to the hospital. The addition of other multidisciplinary writing team members to represent stakeholders such as physician assistants, nurses, clinical pharmacists, social workers, and family members could also be considered, especially for conditions which are treated in multiple settings.
Assumed values and preferences
The SAEM GRACE-4 Writing Team attempted to incorporate values such as prompt and sustained symptom relief, medication safety, costs, feasibility and access to various pharmaceutical alternatives as well as operational issues such as LOS, admission rates, and ED returns. Nonetheless, shared decision making with individual patients is essential to align treatment options with patient's priorities and unique values. Patients and physicians may differ regarding their values and preferences for different outcomes. These guidelines should be applied in the context of local policies and resources and in conjunction with clinical pharmacists and addiction specialists monitoring criteria for audit/feedback to facilitate implementation.
Plans for updating these guidelines
We suggest that GRACE-4 be updated at an interval of approximately 3 to 5 years or when significant new evidence emerges for the pharmacologic or nonpharmacologic management of AWS or CHS in ED settings. The relative absence of high-quality direct evidence identified by the SAEM GRACE-4 Writing Team implies that new research targeting the specific populations using appropriately rigorous and pragmatic methodology could substantially alter the direction and/or strength of recommendations.
Monitoring criteria for audit/feedback of implementation
Acknowledging that SAEM GRACE-4 recommendations are conditional (or good practice statements) and supported by very-low- or low-level evidence, an audit mechanism is inappropriate, other than to accumulate additional health outcomes data to support future research. There are potential disparities in care for persons with AUD, which may also exist for those with CHS. Since medical rationale for these differences may exist limiting our ability to understand the unknown unknowns of AUD and CHS medical management in ED settings, audit mechanisms should be used cautiously to identify potential foci for future research and not to judge the quality of care.
CONCLUSIONS
There is no high-quality evidence to inform the care of patients with alcohol withdrawal syndrome, alcohol use disorder, and cannabinoid hyperemesis syndrome in the ED setting. Nonetheless, low- to very-low-quality evidence does exist upon which SAEM GRACE-4 bases recommendations for management of alcohol withdrawal syndrome, alcohol use disorder, and cannabinoid hyperemesis syndrome in ED settings. Development and standardization of key outcomes of interest will inform improvements in the care of these patients in the ED. Research that rigorously revaluates the recommendations provided in this article are required to direct care in the ED. While we await high-quality evidence to guide practice, the GRACE-4 Writing Team strongly recommends that all ED patients with substance use disorders be offered brief ED substance use disorder interventions and referral to psychosocial support services/outpatient referrals to community services where such services are available.
AUTHOR CONTRIBUTIONS
All authors participated in the writing and review of this manuscript.
CONFLICT OF INTEREST STATEMENT
All GRACE-4 Writing Team members disclosed conflicts of interest using SAEM's standard methods. All members were able to participate as voting members with the following disclosures and management. Fernanda Bellolio receives grant funding from the Agency for Healthcare Research and Quality for the Study of Diagnostic Errors, the National Institutes of Health, the Food and Drug Administration, the Mayo Clinic Robert and Patricia Kern Center for the Science of the Healthcare Delivery, and the Kogod Center on Aging. Bjug Borgundvaag serves on the Board of Directors of Stella's Place. Christopher Carpenter is the Deputy Editor-in-Chief of Academic Emergency Medicine; Associate Editor, Annals of Internal Medicine's ACP Journal Club; and Associate Editor, Journal of the American Geriatrics Society. Dr. Carpenter serves on the American College of Emergency Physicians Clinical Policy Committee and the American Board of Emergency Medicine, as an MyEMCert Editor. Dr. Carpenter serves on the American Academy of Emergency Medicine Geriatrics Committee and the Missouri Medicine Editorial Board. Dr. Carpenter recused himself from all conversations with the above organizations related to the same content being discussed in GRACE-4. David Liss serves on the Science Committee for the American Academy of Clinical Toxicology, Research Committee for the American College of Medical Toxicology, and on the Clinical Protocols Working Group for the Behavioral Health Network of Greater St. Louis, Regional Sobering Center. Suneel Upadhye is a curator for the EmergencyGuidelines website. Jody A. Vogel serves on the Society for Academic Emergency Medicine Board of Directors as a Member-at-Large. Dr. Vogel was recused from voting on or approving the content of GRACE-4. The other authors declare no conflicts of interest.




