Predicting Geriatric Falls Following an Episode of Emergency Department Care: A Systematic Review
La Predicción de Caídas Geriátricas tras un Episodio en el Servicio de Urgencias: Una Revisión Sistemática
Abstract
enBackground
Falls are the leading cause of traumatic mortality in geriatric adults. Despite recent multispecialty guideline recommendations that advocate for proactive fall prevention protocols in the emergency department (ED), the ability of risk factors or risk stratification instruments to identify subsets of geriatric patients at increased risk for short-term falls is largely unexplored.
Objectives
This was a systematic review and meta-analysis of ED-based history, physical examination, and fall risk stratification instruments with the primary objective of providing a quantitative estimate for each risk factor's accuracy to predict future falls. A secondary objective was to quantify ED fall risk assessment test and treatment thresholds using derived estimates of sensitivity and specificity.
Methods
A medical librarian and two emergency physicians (EPs) conducted a medical literature search of PUBMED, EMBASE, CINAHL, CENTRAL, DARE, the Cochrane Registry, and Clinical Trials. Unpublished research was located by a hand search of emergency medicine (EM) research abstracts from national meetings. Inclusion criteria for original studies included ED-based assessment of pre-ED or post-ED fall risk in patients 65 years and older with sufficient detail to reproduce contingency tables for meta-analysis. Original study authors were contacted for additional details when necessary. The Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS-2) was used to assess individual study quality for those studies that met inclusion criteria. When more than one qualitatively similar study assessed the same risk factor for falls at the same interval following an ED evaluation, then meta-analysis was performed using Meta-DiSc software. The primary outcomes were sensitivity, specificity, and likelihood ratios for fall risk factors or risk stratification instruments. Secondary outcomes included estimates of test and treatment thresholds using the Pauker method based on accuracy, screening risk, and the projected benefits or harms of fall prevention interventions in the ED.
Results
A total of 608 unique and potentially relevant studies were identified, but only three met our inclusion criteria. Two studies that included 660 patients assessed 29 risk factors and two risk stratification instruments for falls in geriatric patients in the 6 months following an ED evaluation, while one study of 107 patients assessed the risk of falls in the preceding 12 months. A self-report of depression was associated with the highest positive likelihood ratio (LR) of 6.55 (95% confidence interval [CI] = 1.41 to 30.48). Six fall predictors were identified in more than one study (past falls, living alone, use of walking aid, depression, cognitive deficit, and more than six medications) and meta-analysis was performed for these risk factors. One screening instrument was sufficiently accurate to identify a subset of geriatric ED patients at low risk for falls with a negative LR of 0.11 (95% CI = 0.06 to 0.20). The test threshold was 6.6% and the treatment threshold was 27.5%.
Conclusions
This study demonstrates the paucity of evidence in the literature regarding ED-based screening for risk of future falls among older adults. The screening tools and individual characteristics identified in this study provide an evidentiary basis on which to develop screening protocols for geriatrics adults in the ED to reduce fall risk
Resumen
esIntroducción
Las caídas son la principal causa de mortalidad traumatica en los adultos geriátricos. A pesar de las recomendaciones de la reciente guía clínica multiespecialidad que propugnan protocolos de prevención de caídas en el servicio de urgencias (SU), no están ampliamente explorados la capacidad de los instrumentos para estratificar el riesgo o los factores de riesgo para identificar el conjunto de pacientes geriátricos con un riesgo incrementado de caídas a corto plazo.
Objetivos
Revisión sistemática y metanálisis de la historia clínica en el SU, la exploración física y los instrumentos de estratificación del riesgo de caída con el objetivo principal de proporcionar una estimación cuantitativa para la precisión de cada factor de riesgo para predecir futuras caídas. Un objetivo secundario fue cuantificar los umbrales de tratamiento y las pruebas de valoración del riesgo de caída en el SU mediante estimaciones derivadas de sensibilidad y especificidad.
Metodología
Un experto en biblioteconomía sanitaria y dos *urgenciólogos* llevaron a cabo una búsqueda de la literatura en PUBMED, EMBASE, CINAHL, CENTRAL, DARE, Cochrane Registry y Clinical Trials. La investigación no publicada se localizó por búsqueda manual de los resúmenes de investigaciones de medicina de urgencias y emergencias de los congresos nacionales. Los criterios de inclusión para los estudios originales incluyeron la valoración realizada en el SU del riesgo de caída pre-SU o post-SU en pacientes de 65 o más años con suficiente detalle para reproducir tablas de contingencia para metanálisis. Se contactó con los autores del estudio original para detalles adicionales cuando fue necesario. La Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS-2) se utilizó para evaluar la calidad del estudio individual en aquellos estudios que cumplieron los criterios de inclusión. Cuando más de un estudio similar cuantitativamente valoró el mismo factor de riesgo para caídas en el mismo intervalo tras una evaluación del SU, el metanálisis se realizó usando un software Meta-Disc. Los resultados principales fueron la sensibilidad, la especificidad y las razones de probabilidades para los factores de riesgo de caídas o los instrumentos de estratificación del riesgo. Los resultados secundarios incluyeron estimaciones de los umbrales de pruebas complementarias y tratamiento mediante el método de Pauker basado en la precisión, el riesgo de despistaje y los beneficios o riesgos de las intervenciones de prevención de caídas en el SU.
Resultados
Se identificaron 608 estudios originales y potencialmente relevantes, pero sólo tres cumplieron nuestros criterios de inclusión. Dos estudios que incluyeron 660 pacientes valoraron 29 factores de riesgo y dos instrumentos de estratificación del riesgo para caídas en pacientes geriátricos a los 6 meses tras una valoración en el SU, mientras que un estudio de 107 pacientes valoró el riesgo de caídas a los 12 meses posteriores. Una autoreferencia de depresión se asoció con el mayor coeficiente de probabilidad positiva, que fue de 6,55 (IC 95% = 1,41 a 30,48). Se identificaron seis factores predictivos de caídas en más de un estudio (antecedente de caídas, vivir solo, uso de andador, depresión, deterioro cognitivo y estar en tratamiento con más de seis medicaciones) y el metanálisis se realizó para estos factores de riesgo. Un instrumento de despistaje fue suficientemente preciso para identificar un conjunto de pacientes geriátricos del SU con bajo riesgo para caídas con un coeficiente de probabilidad negativo de 0,11 (IC 95% = 0,06 a 0,20). El umbral de la prueba diagnóstica fue de un 6,6% y el umbral de tratamiento de un 27,5%.
Conclusiones
Este estudio demuestra la escasez de evidencia en la literatura sobre el despistaje en el SU del riesgo de futuras caídas entre los adultos mayores. Las herramientas de despistaje y las características individuales identificadas en este estudio proporcionan una prueba básica para desarrollar protocolos de despistaje para adultos geriátricos en el SU para reducir el riesgo de caída.
Each year about 33% of community dwelling adults over age 65 years suffer standing-level falls, a percentage that increases to 50% for those over age 80 years.1 Recent falls are commonly elicited from older adults in the emergency department (ED), whether or not the fall is the reason for the current emergency evaluation.2 Geriatric fall victims frequently use health care resources, including the ED and inpatient services.3 Falling is the most common cause of traumatic injury among geriatric patients presenting to the ED.4, 5 One-fifth of falls result in injuries, and falls are the leading cause of traumatic mortality in the elderly.6-8 Older patients who suffer ground-level falls and are admitted to the hospital are subsequently readmitted to the hospital within 1 year in 44% of cases and have 33% 1-year mortality.9 Many geriatric patients with minor fall-related injuries who are discharged home from the ED experience recurrent falls, functional decline, and ED returns within 3 months.10, 11
As the number of older adult ED visits increases in coming decades,12, 13 and as EDs develop geriatric-friendly protocols,14 emergency medicine (EM) has the opportunity to prevent a first fall (primary prevention), recurrent falls (secondary prevention), or injurious falls (tertiary prevention). Although ED-based fall prevention trials are rare and yield conflicting results for effectiveness,15 one British study demonstrated impressive reductions in secondary falls prevention with a number needed to treat of five to prevent any fall at 1 year using a multidisciplinary provider team.16 Based on this study, geriatric EM experts 14 years ago recommended that geriatric patients at high risk for falls be identified and referred.17 Graduate medical education leaders more recently identified fall risk assessment as a geriatric core competency for EM residents.18 In addition, the British Geriatric Society (BGS) and American Geriatric Society (AGS) released general guidelines for fall risk assessment in 2011.19 These guidelines are not specific for the ED environment. In 2014, the American College of Emergency Physicians (ACEP), AGS, Emergency Nurses Association (ENA), and Society for Academic Emergency Medicine (SAEM) “Geriatric Emergency Department Guidelines” also recommended ED screening for fall risk.20, 21 Unfortunately, emergency physicians (EPs) rarely evaluate fall risk,22 and even those older adults who present to the ED for evaluation after falls rarely receive guideline-directed management.23, 24
Numerous fall risk factors and instruments exist.25 However, few fall risk factor studies have been conducted in ED settings.26 The lack of reliable, accurate, and feasible fall risk assessment protocols that are appropriate for ED use represents one major obstacle to implementing effective fall prevention programs in the ED.27 ED researchers, clinicians, and policy-makers therefore ranked geriatric screening and fall risk assessment studies as one of the highest priorities on which investigators and funders should focus.28, 29 The primary objective of this systematic review was to quantify the accuracy of all existing post-ED assessment fall risk factors and stratification instruments for use in ED settings. A secondary objective was to estimate test and treatment thresholds for fall risk screening and ED-based preventative interventions based on the summary estimates of predictive instruments or risk factors derived from this meta-analysis.
Methods
Search Strategy
The design of this systematic review conforms to the recommendations from the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statement and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.30, 31 In conjunction with a medical librarian (SF), two investigators (CRC, AXL) searched the medical literature from 1950 to January 2014. The published literature was searched using strategies created by a medical librarian for the concepts of ED, people 60 years and older, screening, falls, and diagnosis. These strategies were established using a combination of standardized terms and key words and were implemented in PubMed 1946-, Embase 1947-, Cumulative Index for Nursing and Allied Health (CINAHL) 1937-, Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), Cochrane Database of Systematic Reviews, and ClinicalTrials.gov. All searches were completed in January 2014 and limited to English using database-supplied limits. All results were exported to EndNote. We used the automatic duplicate finder in EndNote, and 136 duplicates were assumed to be accurately identified and removed, for a total of 560 unique citations. Seventy-three trials were located in ClinicalTrials.gov. Full search strategies are provided in Data Supplement S1 (available as supporting information in the online version of this paper).
Study Protocol
Two authors (CRC, AXL) reviewed the titles and abstracts to identify potentially relevant articles, which were then retrieved and the full manuscripts reviewed for inclusion criteria. In addition, one author (AXL) reviewed abstracts accepted for presentation at national EM conferences and published in Academic Emergency Medicine or Annals of Emergency Medicine from 1990 through April 2014.
Multiple fall risk stratification instruments and risk factor studies have been conducted in outpatient, inpatient, and rehabilitation settings.25 The ED represents a unique clinical environment with an acute illness complicating a brief encounter between health care providers and patients who usually have not previously interacted and never will again.32 Risk prediction instruments that are accurate and reliable in non-ED settings often demonstrate less impressive predictive parameters in the ED milieu.33-35 Therefore, studies and scientific research abstracts were included if they recruited a population of general geriatric adults (age ≥ 65 years) in ED settings. We sought to identify instruments to risk stratify undifferentiated older adults for subsequent falls regardless of their presenting complaints or ED diagnoses. Studies were included if they reported sufficient detail to reconstruct two-by-two tables in assessing individual fall risk factors or fall prediction instruments with an appropriate determination of standing level falls within 6 months of the index ED evaluation. We contacted the authors of studies that assessed fall risk accuracy if they did not report sufficient detail to reconstruct two-by-two tables. If the authors responded and provided the contingency tables, then their studies were included in this systematic review. A priori, we intended to include letters or scientific abstracts with original research data. We excluded non–English language manuscripts, narrative reviews, case reports, and studies focused on fall prevention interventions or therapy.
Individual Evidence Quality Appraisal
Two authors (CRC, AXL) used the revised Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS-2) for systematic reviews to evaluate the overall quality of evidence for the identified trials.36 Discrepant quality assessments were adjudicated by discussion. Statistical agreement between the two reviewers was assessed via a kappa analysis using SPSS 20. QUADAS-2 consists of nine signaling questions and four applicability questions that qualitatively assess patient selection, index test ascertainment and reproducibility, criterion standard timing and acceptability, and uniformity of obtainment and analysis, for the index and criterion standard tests. Before assessing individual study quality, the authors agreed to assess the quality of individual trials for the purposes of this systematic review by considering several pertinent study characteristics. The ideal patient population would be those admitted or discharged from an ED with predischarge risk assessment for short-term fall risk evaluated by the ED medical staff. We rated studies that recruited or obtained fall risk data from a portion of patients outside of the ED as low applicability and high risk of spectrum bias. We excluded studies that evaluated patients exclusively in the hospital or at home following an ED evaluation. If a study used research personnel to administer the prognostic screening instruments rather than standard ED clinical personnel, we rated the conduct applicability as potentially low since this administration of the instrument does not reflect real world practice. Studies that failed to explicitly mask outcome assessors to the fall risk screening instrument results or interpretation were labeled as high risk for incorporation bias.
Data Analysis
Two authors (CRC, AXL) independently abstracted data from the included studies. Information abstracted included the individual study setting, inclusion criteria, mean patient age, study design, adverse outcomes assessed, outcome prevalence, and prognostic test properties. The level of agreement between reviewers for the QUADAS-2 assessment was quantified using a kappa analysis and the qualitative level of agreement was rated as previously described by Byrt.37 The authors planned to compute meta-analytic summary estimates if more than one study assessed the same fall risk factors using comparable thresholds or definitions and using similar fall outcomes/definitions at the same follow-up interval. Meta-analysis was conducted using Meta-DiSc and a random-effects model.38 There is no consensus on whether to use a fixed-effects or a random-effects model in diagnostic meta-analyses, but recent research indicates that the majority of such studies demonstrate significant between-study heterogeneity, favoring a random-effects model.39, 40 We used a random-effects model due to the anticipation of significant between-study heterogeneity in spectrum of disease, subjective interpretation of fall predictors, and methods of ascertaining fall occurrence. Heterogeneity is the presence of variation in true effect sizes underlying the individual studies and can be assessed qualitatively and quantitatively. Interstudy heterogeneity was assessed quantitatively for pooled estimates of sensitivity and specificity using the Index of Inconsistency (I2), Cochran's Q, and tau-square.40, 41 Whereas I2 estimates the proportion of total variability in point estimates that can be attributed to heterogeneity, the square root of tau-square (tau) represents the estimated standard deviation of underlying effects across studies.42 Publication bias was not assessed because of the questionable validity of this approach when assessing diagnostic test meta-analyses.43
Test–Treatment Threshold
The Pauker and Kassirer decision threshold model is based on seven variables: false-negative proportions, false-positive proportions, sensitivity, specificity, risk of a diagnostic test, risk of treatment, and anticipated benefit of treatment.44 Evidence-based estimates for each of these variables were abstracted from our systematic review to derive theoretical test and treatment thresholds for ED management of older adults at increased risk for falls. Recognizing that these estimates are likely based on inadequate and biased research, an interactive Microsoft Excel calculator is provided in Data Supplement S2 (available as supporting information in the online version of this paper) of this article permitting readers to alter assumptions to recompute thresholds using different estimates of test performance or anticipated risks and benefits that may be more applicable to the end users’ patient populations and clinical environments.
Results
The electronic search strategy identified 601 unique manuscripts, and the hand search of research abstracts yielded another seven reports. After the titles and abstracts for inclusion criteria were reviewed, five manuscripts were obtained for full review (Figure 1). Two articles did not include data necessary to compute 2 × 2 tables and the authors were contacted to obtain additional details. The authors did not have time to provide these data so only three manuscripts are included in this systematic review.45-47
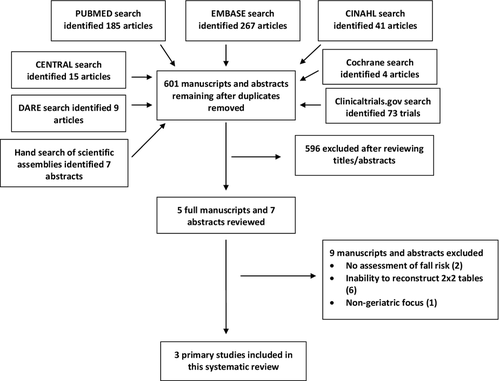
Quality Assessment
The authors’ QUADAS-2 assessment of quality had a kappa of 1.0 for exclusions and analysis of all enrolled patients, but could not be performed for the remainder of the domains due to one or both raters labeling all studies with the same level and certainty of bias (Table 1). No study used a case–control design. Two of the studies45, 46 described both individual fall risk factors and derived an instrument composed of multiple predictors, although neither instrument has been validated on different populations than those upon which they were derived. One of the studies had significant 6-month loss to follow-up.45 In two of the three studies, the predictor variables (risk factors) were obtained prospectively in the ED before fall outcomes were ascertained, so those collecting the index test were presumed to be masked to the primary outcomes. However, all studies failed to explicitly state that outcome assessors were masked to the index tests being assessed, so there was a high risk of incorporation bias.48, 49 The primary issue with incorporation bias is that knowledge of a risk factor while judging whether a fall occurred or not sways the assessor to label equivocal cases as true positives or true negatives when the predictor is being used to determine whether the outcome occurred. For example, if a noncommittal patient reports a “possible fall” to an outcome assessor who is also aware that this patient reported multiple prior falls during his or her index evaluation, and the outcome assessor believes that past falls predict future falls, then the outcome assessor is likely to label the “possible fall” as a “fall.” This decision results in past falls being in the true positive category and increases estimates of sensitivity, a well-recognized flaw in diagnostic studies.48, 49 Every study was conducted in an ED setting, so applicability bias was low. Both prospective studies45, 46 used self-reported falls as the primary outcome, but each used a different definition of falls and a different mechanism of ascertaining falls (postcards/telephone calls vs. follow-up with falls clinic), so the interstudy reproducibility of the outcome measure is questionable.
| Study | Sample | Inappropriate Exclusions? | Patients and Settings Match Study Question? | Index Test Interpretation Blinded? | Acceptable Outcome Standards? | Outcomes Assessed by Blinded Assessor? | Assessed Outcomes Pertinent? | All Patients Analyzed? |
|---|---|---|---|---|---|---|---|---|
| Carpenter 200945 | Nonconsecutive | Yes | Yes | Yes | No | No | Yes | No |
| Greenberg 201347 | Nonconsecutive | No | Yes | No | No | No | No | Yes |
| Tiedemann 201346 | Nonconsecutive | No | Yes | Yes | No | No | Yes | No |
| Kappa | N/Aa | 1.00 | N/Aa | N/Aa | N/Aa | N/Aa | N/Aa | 1.00 |
- a Kappa cannot be calculated when one value is a constant. In other words, if one or more raters assessed all three studies as having or not having inappropriate exclusions, then kappa cannot be computed.
Prevalence of Post-ED Standing-level Falls
Tiedemann et al.46 assessed 6-month falls via a monthly falls diary and follow-up telephone calls “as required.” Carpenter et al.45 assessed 6-month falls via a monthly falls calendar with affixed mandatory, addressed, and stamped postcards to self-report falls and they used follow-up telephone calls for postcard nonresponders. The study populations of Tiedemann et al.46 and Carpenter et al.45 differed in that the former recruited patients ages ≥70 who presented to the ED with falls, whereas the latter assessed community-dwelling elders ≥65 who were evaluated and discharged from the ED for any reason except a fall. Therefore, one might expect a higher baseline risk for falls in the study by Tiedemann et al. since they were older and preselected as past fallers. The 6-month fall risk in the study by Tiedemann et al. was 31%, including 17% with more than one fall. Most falls (62%) were injurious falls. In the study by Carpenter et al., 14% reported falls at 6 months. Therefore, the best-estimate 6-month fall risk for geriatric patients presenting to the ED for a fall-related complaint is 31%, whereas the general geriatric ED population risk among community dwelling elders is 14%.
Individual Risk Factors
Two studies of 660 patients assessed 29 individual risk factors with 6-month falls as the primary outcome.45, 46 These risk factors included prior ED use; sociodemographic features; subjective functional mobility; objective functional tests; and self-reported medical diagnoses, general health, and past fall history. Both studies assessed six of the risk factors (past falls, residential status, use of walking aids, consumption of more than six medications daily, self-reported dementia, and self-reported depression) permitting meta-analysis for these risk factors.
No single risk factor was an accurate predictor of 6-month fall risk (Table 2). The most accurate risk factor to increase the risk of 6-month falls was self-reported depression (positive likelihood ratio [LR] = 6.55; 95% confidence interval [CI] = 1.41 to 30.48), although meta-analysis of this risk factor yielded a summary positive LR of 2.54 (95% CI = 1.62 to 3.98). The next most accurate predictors of 6-month falls were nonhealing foot sores (positive LR = 3.20; 95% CI = 1.40 to 7.30), borderline functional mobility (positive LR = 2.52; 95% CI = 1.04 to 6.12), and previous indoor falls (positive LR = 2.16; 95% CI = 1.43 to 3.26). The lowest negative LR was 0.57 (95% CI = 0.38 to 0.86) for ability to cut one's own toenails, meaning that none of the assessed risk factors significantly decreased the risk of 6-month falls when absent. Carpenter et al. assessed the reliability of observed mobility assessments such as the chair stand in a subset of enrolled patients using five observers and noted an intraclass correlation coefficient of 0.77 (95% CI = 0.66 to 0.87) across these objective measures.45 Of note, none of the objective functional tests that were assessed (chair stand, tandem gait, raising feet while walking, turn 180 degrees, sit in chair) were predictive of a fall within 6 months (Table 3).
| Risk Factor | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive LR (95% CI) | Negative LR (95% CI) |
|---|---|---|---|---|
| Cognitive impairment | ||||
| Carpenter 2009 | 5 (1–18) | 96 (92–99) | 1.42 (0.29–7.04) | 0.98 (0.91–1.07) |
| Tiedemann Derivation 2013 | 3 (0–11) | 96 (91–98) | 0.74 (0.15–3.57) | 1.01 (0.96–1.07) |
| Tiedemann Validation 2013 | 2 (0–10) | 100 (96–100) | 5.57 (0.23–134.52) | 0.98 (0.93–1.02) |
| Pooled Accuracy | 3 (1–7) | 97 (95–99) | 1.23 (0.43–3.54) | 0.99 (0.96–1.02) |
| Depression | ||||
| Carpenter 2009 | 29 (15–46) | 88 (81–93) | 2.44 (1.24–4.81) | 0.81 (0.65–1.00) |
| Tiedemann Derivation 2013 | 23 (14–36) | 89 (83–94) | 2.22 (1.16–4.26) | 0.86 (0.74–0.99) |
| Tiedemann Validation 2013 | 13 (5–24) | 98 (93–100) | 6.55 (1.41–30.48) | 0.89 (0.80–0.99) |
| Pooled Accuracy | 21 (15–28) | 91 (88–94) | 2.54 (1.62–3.98) | 0.87 (0.80–0.94) |
| Fall in past 12 months | ||||
| Carpenter 2009 | 61 (43–76) | 76 (67–83) | 2.48 (1.67–3.67) | 0.52 (0.35–0.78) |
| Tiedemann Derivation 2013 | 69 (56–80) | 68 (60–76) | 2.17 (1.26–2.91) | 0.46 (0.31–0.67) |
| Tiedemann Validation 2013 | 53 (39–66) | 77 (67–84) | 2.26 (1.47–3.48) | 0.62 (0.46–0.83) |
| Pooled Accuracy | 61 (53–69) | 73 (68–78) | 2.27 (1.85–2.79) | 0.54 (0.44–0.67) |
| Lives alone | ||||
| Carpenter 2009 | 68 (51–82) | 35 (27–43) | 1.05 (0.82–1.35) | 0.91 (0.54–1.53) |
| Tiedemann Derivation 2013 | 56 (43–69) | 59 (51–67) | 1.38 (1.03–1.85) | 0.74 (0.54–1.01) |
| Tiedemann Validation 2013 | 67 (53–79) | 48 (38–58) | 1.28 (0.99–1.66) | 0.69 (0.45–1.06) |
| Pooled Accuracy | 63 (55–77) | 47 (42–53) | 1.21 (1.03–1.43) | 0.75 (0.60–0.94) |
| Takes at least six medications | ||||
| Carpenter 2009 | 34 (19–53) | 79 (70–86) | 1.61 (0.90–2.90) | 0.83 (0.64–1.09) |
| Tiedemann Derivation 2013 | 56 (43–69) | 58 (49–66) | 1.33 (1.00–1.78) | 0.76 (0.55–1.03) |
| Tiedemann Validation 2013 | 42 (29–56) | 76 (66–84) | 1.72 (1.09–2.73) | 0.77 (0.60–0.99) |
| Pooled Accuracy | 46 (38–55) | 70 (65–74) | 1.46 (1.16–1.83) | 0.79 (0.67–0.92) |
| Uses cane | ||||
| Carpenter 2009 | 45 (29–62) | 81 (73–87) | 2.32 (1.42–3.81) | 0.68 (0.51–0.92) |
| Tiedemann Derivation 2013 | 52 (39–64) | 64 (56–72) | 1.44 (1.04–1.98) | 0.76 (0.57–1.00) |
| Tiedemann Validation 2013 | 40 (27–54) | 60 (50–70) | 1.00 (0.67–1.50) | 1.00 (0.76–1.30) |
| Pooled Accuracy | 46 (38–54) | 69 (64–74) | 1.46 (0.96–2.24) | 0.81 (0.65–1.01) |
| Abnormal vs. normal baseline function | ||||
| Carpenter 2009 | 52 (33–71) | 67 (57–75) | 1.55 (1.00–2.41) | 0.72 (0.49–1.08) |
| Borderline vs. normal baseline function | ||||
| Carpenter 2009 | 30 (12–54) | 88 (79–94) | 2.52 (1.04–6.12) | 0.79 (0.59–1.07) |
| Inability to cut toenails | ||||
| Carpenter 2009 | 61 (43–76) | 69 (60–77) | 1.95 (1.36–2.79) | 0.57 (0.38–0.86) |
| Drives a car | ||||
| Carpenter 2009 | 53 (36–69) | 53 (45–62) | 1.13 (0.79–1.60) | 0.89 (0.61–1.29) |
| Drives only during day | ||||
| Carpenter 2009 | 24 (11–40) | 84 (76–89) | 1.45 (0.73–2.89) | 0.91 (0.75–1.10) |
| Married | ||||
| Carpenter 2009 | 56 (41–74) | 48 (40–57) | 1.12 (0.81–1.53) | 0.87 (0.58–1.32) |
| Fair/poor vs. excellent/good health rating | ||||
| Carpenter 2009 | 53 (36–69) | 65 (57–73) | 1.51 (1.03–2.21) | 0.73 (0.51–1.04) |
| Takes at least three medications | ||||
| Carpenter 2009 | 63 (44–79) | 42 (33–51) | 1.07 (0.79–1.46) | 0.90 (0.55–1.47) |
| Nonhealing foot sore | ||||
| Carpenter 2009 | 24 (11–40) | 93 (87–96) | 3.20 (1.40–7.30) | 0.82 (0.69–0.99) |
| Leg injury | ||||
| Carpenter 2009 | 3 (0–14) | 99 (95–100) | 1.78 (0.17–19.07) | 0.99 (0.93–1.05) |
| Diabetes | ||||
| Carpenter 2009 | 32 (18–49) | 81 (73–87) | 1.64 (0.92–2.93) | 0.85 (0.67–1.07) |
| Prior stroke | ||||
| Carpenter 2009 | 26 (13–43) | 82 (75–88) | 1.48 (0.78–2.82) | 0.90 (0.73–1.10) |
| Irregular heart rhythm | ||||
| Carpenter 2009 | 47 (31–64) | 59 (50–68) | 1.16 (0.79–1.72) | 0.89 (0.64–1.24) |
| Urine incontinence | ||||
| Carpenter 2009 | 32 (18–49) | 77 (69–84) | 1.38 (0.78–2.41) | 0.89 (0.70–1.12) |
| Wears eyeglasses | ||||
| Carpenter 2009 | 95 (82–99) | 8 (4–14) | 1.03 (0.94–1.13) | 0.65 (0.15–2.79) |
| Sense of imbalance | ||||
| Carpenter 2009 | 61 (43–76) | 64 (56–72) | 1.70 (1.21–2.40) | 0.61 (0.41–0.93) |
| Previous near fall | ||||
| Carpenter 2009 | 55 (38–71) | 67 (58–75) | 1.66 (1.14–2.41) | 0.67 (0.46–0.97) |
| Previous fall injury | ||||
| Carpenter 2009 | 29 (15–46) | 86 (79–91) | 2.06 (1.07–3.94) | 0.83 (0.67–1.02) |
| Sense of imbalance | ||||
| Carpenter 2009 | 61 (43–76) | 64 (56–72) | 1.70 (1.21–2.40) | 0.61 (0.41–0.93) |
| Previous 6-month ED visit | ||||
| Carpenter 2009 | 50 (3–67) | 76 (67–83) | 2.05 (1.32–3.16) | 0.66 (0.47–0.92) |
| Requires community services | ||||
| Tiedemann 2013 | 53 (39–66) | 65 (55–74) | 1.51 (1.05–2.17) | 0.73 (0.53–0.99) |
| Unable to arise after fall | ||||
| Tiedemann 2013 | 35 (22–49) | 65 (55–74) | 0.99 (0.63–1.55) | 1.01 (0.79–1.28) |
| Previous indoor fall | ||||
| Tiedemann 2013 | 55 (41–68) | 75 (65–83) | 2.16 (1.43–3.26) | 0.61 (0.45–0.83) |
- LR = likelihood ratio.
| Finding, Study | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive LR (95% CI) | Negative LR (95% CI) |
|---|---|---|---|---|
| Chair stand, Carpenter 2009 | 13 (4–28) | 87 (80–92) | 0.99 (0.39–2.48) | 1.00 (0.87–1.15) |
| Chair sit, Carpenter 2009 | 16 (6–32) | 90 (83–94) | 1.52 (0.63–3.69) | 0.94 (0.81–1.09) |
| Raise feet while walking, Carpenter 2009 | 11 (3–25) | 94 (89–97) | 1.78 (0.57–5.58) | 0.95 (0.85–1.07) |
| Turn 180°, Carpenter 2009 | 11 (3–25) | 93 (88–97) | 1.58 (0.51–4.85) | 0.96 (0.85–1.08) |
| Visual acuity < 20/20, Carpenter 2009 | 66 (49–80) | 31 (23–40) | 0.96 (0.74–1.23) | 1.10 (0.66–1.83) |
| Impaired hearing, Carpenter 2009 | 71 (54–85) | 33 (25–41) | 1.05 (0.83–1.33) | 0.89 (0.51–1.55) |
| Near tandem stand, Tiedemann 2013 | 82 (69–91) | 22 (15–32) | 1.05 (0.90–1.24) | 0.81 (0.42–1.59) |
- LR = likelihood ratio.
Meta-analysis of the six risk factors assessed by Tiedemann et al. and Carpenter et al. revealed minimal statistical heterogeneity in pooled estimates of accuracy for LRs, although pooled estimates of accuracy for sensitivity and specificity were more heterogeneous (Data Supplement S3, available as supporting information in the online version of this paper). The lack of statistical heterogeneity is notable because the patient populations were slightly different subsets of community dwelling geriatric adults. Tiedemann et al.46 enrolled patients over age 70 presenting to and discharged from one of two Australian EDs after falls. Carpenter et al.45 recruited patients over age 65 presenting to and discharged from one U. S. hospital after ED evaluations for any reason except a fall. In addition, in assessing the risk factor of “past falls” Tiedemann et al. assessed two or more falls over the preceding 12 months, whereas Carpenter et al. assessed any fall (one or more) over the past 12 months. The patients’ baseline fall risk in Tiedemann et al. should be higher than should those in the study by Carpenter et al. Despite the differences in patient population, both studies’ 6-month fall risk factor accuracy estimates are quite similar (Table 2).
Fall Risk Screening Instruments
Three studies of 767 patients derived and evaluated fall risk assessment instruments based on constellations of predictor variables (Table 4).45-47 The components and scoring of the fall risk predictor instruments are summarized in Data Supplement S4 (available as supporting information in the online version of this paper). Tiedemann et al.46 described a two-item instrument with scores ranging from 0 to 3 (a positive response to one of the items was weighted a score of two) and increasing totals calibrated with increasing fall risk ranging from 16% to 61%. A Tiedemann score of three yields a positive LR of 3.76 (95% CI = 2.45 to 5.78) and lower scores yield lower positive LRs. To define a subset of ED patients at “low risk” for falls, a Tiedemann score of zero demonstrated a negative LR ranging from 0.40 to 0.46 depending on which level (1–3 vs. 2–3 vs. 3 points) defines “high risk.” Carpenter et al.45 described a four-item instrument with scores ranging from 0 to 4. The 6-month fall risk was calibrated with increasing scores, with risk ranging from 4% to 42%. A threshold Carpenter score of >1 to define “high risk” yielded optimal predictive accuracy with positive LR of 2.40 (95% CI = 1.95 to 2.8) and negative LR of 0.11 (95% CI = 0.06 to 0.20). Unfortunately, 39% of the patients in this study did not complete the full 6-month follow-up, and those lost to follow-up were more likely to represent a frailer subset of geriatric ED patients with increased fall risk. Greenberg et al.47 evaluated a modified CAGE score to assess past fall risk in a research abstract, but report prognostic characteristics that are insufficient to increase (positive LR = 1.73, 95% CI = 1.07 to 2.81) or decrease (negative LR = 0.69, 95% CI = 0.47 to 1.01) the risk of falls in the past year.
| Finding, Study | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive LR (95% CI) | Negative LR (95% CI) |
|---|---|---|---|---|
| Carpenter score > 145 | 93 (89–96) | 61 (54–65) | 2.40 (1.95–2.80) | 0.11 (0.06–0.20) |
| Carpenter score > 245 | 100 (98–100) | 22 (18–22) | 1.30 (1.2–1.3) | 0 (0–0.14) |
| Tiedemann score > 046 | 80 (71–87) | 46 (40–53) | 1.48 (1.28–1.72) | 0.44 (0.30–0.64) |
| Tiedemann score > 146 | 75 (65–83) | 62 (55–70) | 2.00 (1.61–2.50) | 0.40 (0.28–0.57) |
| Tiedemann score > 246 | 61 (48–73) | 84 (76–89) | 3.76 (2.45–5.78) | 0.46 (0.34–0.64) |
| Modified CAGE > 047 | 52 (34–39) | 70 (59–80) | 1.73 (1.07–2.81) | 0.69 (0.47–1.01) |
- LR = likelihood ratio.
Test–Treatment Threshold
The ED management decisions for preventing future falls in most contemporary settings are limited. A patient sustaining a potentially life- or limb-threatening injury following a fall is usually admitted to the hospital for treatment of the acute injury. The fall patient presenting to the ED with noninjurious falls (or minor injuries) who suffers additional falls in the ED, dangerous gait instability, or compelling clinical concern for more falls in the near future without adequate caregiver assistance at home is also a straightforward admission decision. However, the majority of geriatric fall patients lack any of these criteria, and the EP needs to decide whether to initiate targeted falls prevention interventions after ED discharge, such as referral to a geriatrician for focused fall risk assessment or home assessment by occupational therapy.15 Although some might argue that all geriatric patients are at increased risk for falls at baseline, neither patients, payers, nor providers can afford or logistically support referral of every geriatric ED patient for further fall assessment. Therefore, it is helpful to understand evidence-based estimates at which further fall risk assessment (test threshold) or intervention (treatment threshold) are beneficial to develop meaningful ED fall-prevention management algorithms.
Multiple reports of ED-based fall prevention interventions exist, ranging from simple passive strategies like educating EPs and informing patients50, 51 to the highly structured Prevention of Falls in the Elderly Trial (PROFET) study.16 The PROFET investigators used a fall prevention team that included a geriatrician, geriatric nurse, and occupational therapist with individualized follow-up for each fall patient for at least one month after the index ED evaluation. The PROFET study reduced falls from 52% to 32% at 1 year, so we use an absolute risk reduction of 20% as the estimate of benefit in our test–treatment computation. Based on our systematic review, the most accurate fall risk prediction instrument is the Carpenter score, with a threshold of two or more abnormalities defining “high risk” (Table 4), so we used the estimates of diagnostic accuracy for this test in our test–treatment computation.
The last two elements of the test–treatment equation are difficult to surmise and our systematic review did not provide quantitative estimates for risk of the test or risk of an intervention in somebody without “disease.” In the case of falls, screening risk would be the hazard to a patient of formally assessing his or her fall risk in the ED, including patient angst, delayed disposition, or ironically, injury from a fall induced by objectively assessing risk of falls. Lacking an evidence-based estimate of this risk, we use an estimate of 0.5% to represent the constellation of all these risks. The risk of a formal, intensive intervention like the PROFET model in a patient who is not at increased risk of 6-month falls might include patient expense and time, unnecessary patient anxiety, or injury associated with the objective testing. We use an estimate of 2% to represent the constellation of these risks. The test threshold using these estimates was 6.6%, and the treatment threshold was 27.5% (Figure 2). In other words, a community dwelling geriatric adult with a 6-month fall risk less than about 7% needs no further assessment. Continuing to evaluate the risk of falls in these low-risk patients could harm more patients than it would help based on our assumptions. The treatment threshold, above which the patient merits referral for a fall prevention intervention like the PROFET model, is a 27.5% risk of falls at 6 months. By continuing to evaluate fall risk in this higher risk population (rather than initiating an intervention to reduce fall risk), more patients might be harmed than helped.
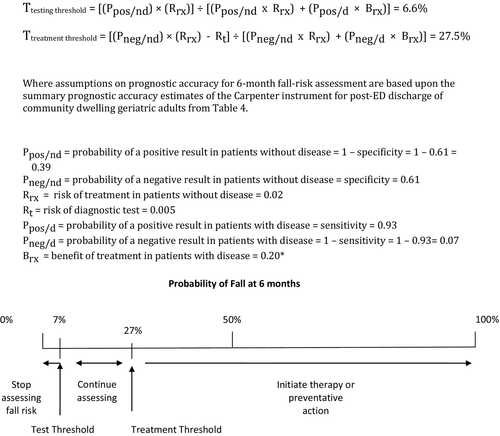
The objective of threshold estimates is neither to limit risk stratification efforts nor to define standards of care. Instead, these estimates provide a quantitative conceptual context through which to better understand effective screening protocols based on what is known, as well as to guide policy-making and future research efforts. We recognize that our estimates of prognostic accuracy, benefit of fall prevention interventions, and screening/intervention risk are based on single studies with potential biases, or no research whatsoever. Therefore, we provide an Excel file (Data Supplement S2), which readers can use to recalculate these thresholds using alternative estimates of risk, benefit, and prognostic accuracy as new research becomes available.
Discussion
The recent AGS/BGS fall risk assessment guidelines and the ACEP/AGS/ENA/SAEM Geriatric Emergency Department guidelines recommend screening older adults for short-term fall risk.21, 52 However, numerous challenges impede effective and routine fall risk screening in the contemporary ED. The lack of a sufficiently accurate risk stratification instrument or protocol is one significant barrier to implementing widespread ED-based geriatric fall assessments.27 Our systematic review demonstrates a paucity of ED-based fall research, despite the prevalence of falls and injurious falls among older patients, as well as recent multispecialty consensus-based recommendations for more research to understand strategies to accurately identify high-risk fallers.29 Although our results fail to provide a definitive fall screening strategy, the quantitative summary estimates of fall incidence and risk factor accuracy and reliability provide an evidence basis on which clinicians, nursing leaders, administrators, educators, policy-makers, and researchers can build.
No single risk factor significantly increases or decreases the risk of 6-month falls in geriatric ED patients. The ideal fall risk screening instrument would be accurate and reliable; sufficiently brief for routine ED use by clinicians, nurses, or ancillary screening staff; and not require space or equipment that is not routinely available in the average ED.11 Furthermore, implementation studies would need to demonstrate that use of the screening instrument resulted in decreased fall rates, particularly injurious falls.53 The most useful prognostic accuracy characteristics for any risk stratification instrument would be a positive LR >10 and/or a negative LR < 0.10.54 Two published instruments demonstrate potential to risk stratify geriatric patients for 6-month falls, but require further evaluation in different ED settings.45, 46 A score of 3 on the instrument of Tiedemann et al. is the most accurate predictor of increased 6-month fall risk. A score of <2 on the Carpenter et al. instrument appears to identify a non–high-risk subset of patients, but this study lost 39% of patients to 6-month follow-up, so this instrument requires further evaluation. With the exception of the PROFET study that used a preexisting fall prevention team that was not based in the ED, fall prevention research in ED settings has been disappointing and largely unsuccessful.15, 16 Effective identification of geriatric patients at increased risk of short-term falls is needed before assessing future fall prevention interventions in the ED.
Implications for Future Research
Future researchers can improve on the science of ED-based falls screening in several ways. Existing studies failed to use the Standards for Reporting of Diagnostic Accuracy (STARD) criteria or publish clinical prediction instrument derivation and validation methods.53, 55 The result is original research that is challenging to find and that does not adequately report measures of fall risk accuracy in terms of sensitivity, specificity, and LRs. Furthermore, reliability is often unreported, and design-related biases often limit the ability of clinicians to compare estimates of accuracy between studies.56 In addition, the spectrum of frailty, illness severity, and comorbidity for geriatric patients between different fall studies is often dissimilar, and validated instruments to assess for prevalent geriatric syndromes that are associated with fall risk like dementia57 and delirium58 are frequently not used. Most importantly, however, the definition of “falls” varies across studies, so the primary outcome assessed may be dissimilar. Geriatric EM researchers ought to agree upon a standard definition of falls and acceptable methods to assess falls prospectively (self-report via telephone or postcards, objective fall devices, caregiver report, etc.).59 Self-reported falls using diaries, calendars, or telephone follow-up probably underestimate the incidence of falls and confounding variables to reporting include cognitive dysfunction, education, and baseline fall risk.60, 61 Future researchers need to develop objective assessments to determine the occurrence of falls using smart phones,62 body sensors,63 or other passive monitoring systems.64
Multiple fall risk assessment instruments have been described in outpatient and inpatient settings.25 The ED represents a unique milieu and patient phenotype, so predictive instruments from other settings often fail in the chaotic environment of EM.32 Nonetheless, future investigators should assess the feasibility and prognostic accuracy for future falls of clinical gestalt,65 as well as existing instruments like the ABCS injurious fall screening tool,66, 67 CAREFALL,68 FROP-Com,69, 70 HOME FAST,71 Hendrich II Fall Risk Model,72, 73 STRATIFY,74 University of Pittsburgh Medical Center screening tool,67 New York–Presbyterian Fall and Injury Risk Assessment Tool,73, 75 Johns Hopkins Fall Risk Assessment Tool,76 Maine Medical Center Fall Risk Assessment,73 Morse Fall Scale,73, 77 Spartanburg Fall Risk Assessment Tool,78 and risk scores described by Bongue et al.79 and Stel et al.80 No study has previously evaluated these instruments in ED settings. Previous systematic reviews of fall risk factors and prediction instruments neglected ED-based studies and did not report meta-analyses or LRs, but favored the Hendrich II Fall Risk Model81 or STRATIFY instruments.82, 83
These systematic reviews did not report LRs, but we computed them from data reported in the systematic review. STRATIFY is a five-question instrument that requires approximately 5 minutes to administer. Using a threshold of ≥2 abnormal responses, STRATIFY has been evaluated in outpatients in one study (positive LR = 1.1, negative LR = 0.81) and in seven “acute care” settings that includes inpatient wards, but not specifically the ED (positive LR range from 1.2 to 5.0, negative LR range from 0.11 to 0.72).74, 84-89 The Hendrick II is a seven-question survey and the get-up-and-go functional test that requires approximately 5 to 10 minutes to administer. Using a threshold of ≥5 abnormal responses, the Hendrick II has been evaluated in two acute care settings that include inpatient wards, but not specifically the ED (positive LR range 1.5 to 1.8, negative LR range 0.33 to 0.49).86, 90
In addition, several investigators have evaluated instruments and protocols for falls that occur in the ED.91, 92 These in-ED fall instruments usually include all age groups, and the topic of falls that occur in the ED is not the focus of this geriatric systematic review. Nonetheless, falls that occur in the ED are an important safety event and merit further study. Future geriatric falls investigators should assess the predictive accuracy of in-ED fall instruments as post-ED fall risk stratification tools.
An important construct of fall risk assessment that has remained unaddressed is the fluid nature of fall vulnerability. Prior studies used a one-time fall risk assessment as the index test, but it is unlikely that fall risk remains static. Multiple intrinsic and extrinsic factors with variable day-to-day effects on fall risk for an individual patient may result in biased assessment of predicted risk if the risk effect is nonstatic. Therefore, within-ED and post-ED repeated measurements of fall risk factors might ultimately result in a more accurate predictor of future falls, but such longitudinal models transcend the traditional border between EM and primary care. Nonetheless, more reliable and efficient fall risk programs may require such atypical hybrids of geriatric emergency management and primary care.
Similarly, development of more accurate fall risk stratification instruments will have implications for interventional fall prevention trials. Most prior ED-based falls prevention efforts attempt to reduce fall rates using medical provider education or one-size-fits-all interventions.15 The exception is the PROFET study in the United Kingdom, which reduced falls using a preestablished and non–ED-based falls collaborative with resources to follow patients for a month after the ED encounter.16 Most health care systems will struggle to replicate a “falls clinic” management model, representing a pragmatic barrier to widespread implementation. However, a more accurate fall risk stratification instrument could provide more feasible, targeted prevention interventions focused on the unique risk inherent to the individual patient. Future falls prevention investigators could use evidence-based risk factors to design adaptive clinical trials in which the intervention differs for each patient and is driven by the individual patient's fall risk profile.93
Limitations
This systematic review is limited to data from three small studies. Carpenter et al. reported significant loss to follow-up and those without complete 6-month outcomes were more likely to have fallen in the past. In other words, the study population represented a healthier subset of all geriatric patients in the ED with lower baseline fall risk. Accuracy studies with healthier subject spectrum bias falsely increase estimates of specificity so future research on a more representative sampling of geriatric ED patients (i.e., with more complete follow-up of all enrolled patients) may render lower point estimates of specificity than Carpenter et al.45 reported. In addition, Carpenter et al. report a derivation study that requires validation in different settings. Prediction instrument performance characteristics in validation studies are often less impressive than in derivation trials.94
Another limitation of the included studies is that investigators relied on patient self-report of the presence or absence of both predictor variables and outcomes. Several important predictor variables like dementia and delirium are often unrecognized by EPs, nurses, and patients.22, 95, 96 However, accurate instruments validated in ED settings exist for dementia and delirium.57, 58 Furthermore, patients often forget inconsequential falls.60 To develop more accurate and reliable ED fall risk stratification instruments, future investigators should use objective, ED-validated assessment instruments to assess the presence or absence of important predictor variables. In addition, more objective assessments of actual fall events should be used in fall studies, including motion detection monitoring devices, shorter interval fall queries, and close contact informant questioning.
Finally, our systematic review is limited to 6-month fall rates because published studies did not report shorter duration fall risk or more patient-centric outcomes like injurious falls. Noninjurious falls are problematic for patients, worrisome for families, and sometimes consume ED resources. Furthermore, noninjurious falls often precede standing level falls that severely injure frail older adults, so preventing any geriatric fall is worthwhile. However, preventing injurious falls is the most compelling reason to assess fall risk and initiate multidisciplinary prevention interventions. Future fall risk assessment trials should uniformly define and report injurious results separately from all falls, while assessing the predictive accuracy of risk factors and instruments for both fall subtypes.
Conclusions
Few ED-based studies have assessed the prognostic accuracy of individual predictors or constellations of predictors for fall risk following an episode of ED care in community dwelling older adults. The pretest probability risk of falls at 6 months following an ED visit ranges from 14% in the general geriatric ED population to 31% in those evaluated for falls during the index ED episodes of care. This systematic review demonstrates that previously described predictors generally lack sufficient accuracy to increase or decrease fall risk. In a single-center trial, one instrument identified low-risk patients with sufficient accuracy (negative likelihood ratio 0.11), but significant loss to follow-up rates may skew specificity upward, so validation in other settings is needed.




