Mitochondrial Respiratory Dysfunction Is Not Correlated With Mitochondrial Genotype in Premature Aging Mice
Funding: This work was supported by the Grant-in-Aid for Scientific Research B [22H02536 and 23 K23801 to K.N.] from the Japan Society for the Promotion of Science (JSPS), by the AMED-CREST [JP23gm1110006 to K.N.] from the Japan Agency for Medical Research and Development (AMED), by the JST FOREST Program [JPMJFR204M to K.I.] and by JST SPRING [JPMJS2124 to H.T.] from the Japan Science and Technology Agency (JST). This work was partly supported by the Grant-in-Aid for Scientific Research C [JP21441138 and JP24008885 to E.O.] from JSPS.
ABSTRACT
mtDNA mutator mice (Polgmut/mut mice) have reinforced the mitochondrial theory of aging. These mice accumulate multiple mutations in mtDNA with age due to a homozygous proofreading-deficient mutation in mtDNA polymerase gamma (Polg), resulting in mitochondrial respiratory dysfunction and premature aging phenotypes. However, whether the accumulation of multiple mutations in Polgmut/mut mice induces mitochondrial respiratory dysfunction remains unclear. Here, we determined the accurate mtDNA genotype, including the frequency of total mutations and the number of non-synonymous substitutions and pathogenic mutations, using next-generation sequencing in the progeny of all three genotypes obtained from the mating of heterozygous mtDNA mutator mice (Polg+/mut mice) and examined their correlation with mitochondrial respiratory activity. Although Polg+/mut mice showed equivalent mtDNA genotype to Polg+/+ (wild-type) mice, the mitochondrial respiratory activity in the Polg+/mut mice was mildly reduced. To further investigate the causal relationship between mtDNA genotype and mitochondrial respiratory activity, we experimentally varied the mtDNA genotype in Polg mice. However, mitochondrial respiratory activity was mildly reduced in Polg+/mut mice and severely reduced in Polgmut/mut mice, regardless of the mtDNA genotype. Moreover, by varying the mtDNA genotype, some Polg+/+ mice showed mtDNA genotype equivalent to those of Polgmut/mut mice, but mitochondrial respiratory activity in Polg+/+ mice was normal. These results indicate that the mitochondrial respiratory dysfunction observed in mice with proofreading-deficient mutation in Polg is correlated with the nuclear genotype of Polg rather than the mtDNA genotype. Thus, the mitochondrial theory of aging in Polgmut/mut mice needs further re-examination.
1 Introduction
Point mutations and deletions in mtDNA can reduce the translation of respiratory complex subunits, leading to mitochondrial respiratory dysfunction and mitochondrial diseases, as well as other diseases such as diabetes and cancer, and contributing to aging (Wallace 1999). Because mtDNA is 5–10 times more prone to accumulating mutations than nuclear DNA (Ames et al. 1993; Brown et al. 1979), the mitochondrial theory of aging has been proposed, which suggests that the accumulation of multiple mutations in mtDNA with age induces mitochondrial respiratory dysfunction, ultimately leading to aging (Harman 1956). mtDNA mutator mice (Polgmut/mut mice) with a homozygous proofreading-deficient mutation in the Polg gene accumulate multiple mutations in mtDNA, resulting in mitochondrial respiratory dysfunction and premature aging phenotypes (Kujoth et al. 2005; Trifunovic et al. 2004). These mice have been considered model organisms supporting the mitochondrial theory of aging. However, previous studies have reported that multiple deletions and specific pathogenic mutations in mtDNA do not result in premature aging phenotypes (Fan et al. 2008; Hashizume et al. 2012; Inoue et al. 2000; Kasahara et al. 2006; Lin et al. 2012; Shimizu et al. 2014; Tani et al. 2022; Tyynismaa et al. 2005; Yokota et al. 2010; Zhang et al. 2025). In addition to mitochondrial respiratory dysfunction, somatic stem cell dysfunction (Ahlqvist et al. 2012; Hämäläinen et al. 2015) and immunometabolism dysfunction (Lei et al. 2021) have been proposed as factors for premature aging in Polgmut/mut mice. However, the causal relationship between random point mutations accumulating in mtDNA and mitochondrial respiratory dysfunction in Polgmut/mut mice remains unclear.
Previous studies have reported that neither random point mutations nor deletions in mtDNA are pathogenic (Edgar et al. 2009; Vermulst et al. 2007), and the accumulation of random non-synonymous substitutions in mtDNA, among other things, may induce mitochondrial respiratory dysfunction in Polgmut/mut mice (Edgar et al. 2009). However, the pathogenicity of random non-synonymous substitutions in Polgmut/mut mice may be suppressed due to the presence of inter-mitochondrial complementation and a threshold effect (Nakada et al. 2001; Ono et al. 2001). Thus, it has been hypothesized that mtDNA molecules with a low frequency of pathogenic mutations clonally expand above a threshold, leading to mitochondrial respiratory dysfunction in Polgmut/mut mice (Trifunovic et al. 2004; Vermulst et al. 2008). However, to date, experimental evidence for clonal expansion of mtDNA molecules with pathogenic mutations causing mitochondrial respiratory dysfunction in these mice is lacking.
Therefore, the causal relationship between the accumulation of random point mutations in mtDNA and mitochondrial respiratory dysfunction, as proposed in the mitochondrial theory of aging, remains unclear. Contradicting this theory, a previous study has suggested that the accumulation of random point mutations in mtDNA cannot be pathogenic and has focused on not only Polgmut/mut mice but also on Polg+/mut mice with a heterozygous proofreading-deficient mutation in Polg (Vermulst et al. 2007). However, no detailed investigations have explored the correlation between mtDNA genotype (including Polg+/mut mice), such as the frequency of total mutations, the number of non-synonymous substitutions, and pathogenic mutations, and mitochondrial respiratory dysfunction.
Accurate detection of mtDNA mutations is essential for examining the causal relationship between mtDNA genotype and reduced mitochondrial respiratory function in Polg mice. However, PCR amplification of partial regions of mtDNA or full-length mtDNA for DNA sequencing (Ma et al. 2018; Ni et al. 2015) could amplify nuclear mitochondrial segments (NUMTs) (Goios et al. 2009, 2008) and fragmented mtDNA, which are increased in Polgmut/mut mice (Edgar et al. 2009), making accurate detection of mtDNA challenging. In fact, previous reports on mtDNA mutation frequency in Polgmut/mut mice, compared with wild-type mice, vary from 3 to 8-fold (Kujoth et al. 2005; Trifunovic et al. 2004) to 2500-fold (Vermulst et al. 2007) increase.
In this study, we investigated the causal relationship between mtDNA genotype and mitochondrial respiratory function in Polg+/mut mice, in addition to Polg+/+ and Polgmut/mut mice. To accurately detect mtDNA mutations, we used enriched circular mtDNA from organs of Polg mice without PCR amplification for next-generation sequencing (Bagge et al. 2020). Furthermore, to examine this relationship in more detail, we generated Polg mice with the same nuclear genotype as Polg but varying mtDNA genotype and compared their mitochondrial respiratory activity. Our findings demonstrate that mitochondrial respiratory dysfunction is not due to mtDNA genotype in Polgmut/mut mice, suggesting that there is no causal relationship between the accumulation of random point mutations in mtDNA and mitochondrial respiratory dysfunction in mice, in contrast to the mitochondrial theory of aging.
2 Materials and Methods
2.1 G ≥ 10 and G1 Polg Mice
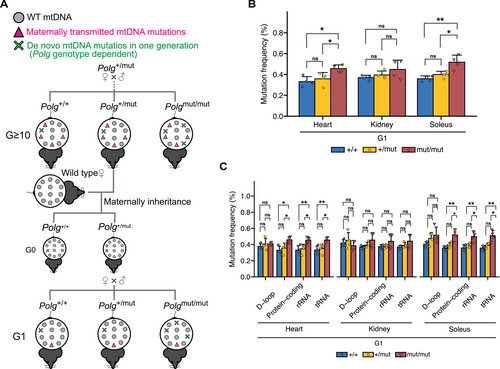
Based on prior research (Trifunovic et al. 2004; Kujoth et al. 2005), we generated Polg+/mut and Polgmut/mut mice with a C57BL/6J nuclear background (Mito et al. 2013) as the 0th generation (G0) of the mouse strain in our previous study. G0 Polg+/mut mice were crossed to generate G1 Polg mice, and G1 Polg+/mut mice were then crossed to generate G2 Polg mice. Thus, we utilized G ≥ 10 (including G10–G13) Polg+/+, Polg+/mut, and Polgmut/mut mice in this study. In the strains maintained by crossing Polg+/mut mice, it was assumed that random point mutations occurring in mtDNA during oocyte maturation are maternally inherited, leading to the accumulation of random point mutations in mtDNA through the maternal germ line in G ≥ 10 Polg mice (Ross et al. 2013; Yang et al. 2020). To eliminate maternally inherited random point mutations in mtDNA of G ≥ 10 Polg mice, G0 Polg+/mut mice were generated by crossing male G ≥ 10 Polg+/mut mice with female B6 (wild-type) mice. Then, by crossing G0 Polg+/mut mice, we obtained G1 Polg+/+, Polg+/mut, and Polgmut/mut mice (Figure 2A), which were used in this study. Male G ≥ 10 and G1 Polg mice at 8–10 months of age were used for the analysis. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Tsukuba.
2.2 mtDNA Enrichment From Mouse Tissue
To accurately detect mutations in mtDNA, we enriched circular mtDNA by treating total DNA obtained from mouse organs with Exonuclease V (Exo V), which degrades linear DNA, based on a previous report (Bagge et al. 2020). The restriction enzyme reaction solution consisted of 2 μL of Exo V (#M0345S, NEB), 10 μL of NEB buffer 4 (10×), and 10 μL of ATP (10 mM). Approximately 5000 ng of total DNA was added to the solution, adjusted to a final volume of 100 μL with distilled water, and incubated at 37°C overnight. The solution was further treated with 2 μL of NEB buffer 4 (10×), 8 μL of ATP (10 mM), 8 μL of Exo V, and 2 μL of distilled water at 37°C overnight to fully degrade the linear DNA. The remaining circular mtDNA was extracted using 0.4 vol AMPure XP beads (#A63881, Beckman).
2.3 Next-Generation Sequencing of mtDNA
Following library preparation from enriched mtDNA using the TruePrep DNA library prep kit (Vazyme # TD501-503), deep sequencing was conducted using Hiseq 2000 (Illumina), with chrM of the UCSC mm10 as a reference sequence. Total read numbers, average depth, and coverage rate for each sample are indicated in Table S1. mtDNA mutation frequency (%) indicates the probability that a mutation occurs in each base of mtDNA (mtDNA mutation frequency = number of bases differing from the reference sequence × 100/total number of bases). Non-synonymous substitutions and pathogenic mutations were quantified for positions where the mutation frequency exceeded 1%. Alignments for nucleic acid and amino acid sequences from mice and humans were performed for every gene and region using T-COFFEE.
2.4 COX/SDH Staining
COX/SDH staining is a histochemical method used to assess mitochondrial respiratory activity (Trifunovic et al. 2004; Wai et al. 2008; Wang et al. 1999), with COX staining for detecting the activity of respiratory enzyme complex IV (COX) and SDH staining for detecting the activity of respiratory enzyme complex II (SDH). When the mitochondrial respiratory function is reduced, COX staining (brown) based on the activity of respiratory enzyme complex IV is reduced, and SDH compensates by facilitating redox reactions, resulting in blue-stained cells (COX−/SDH+). In cells with normal mitochondrial respiratory function, the activity of respiratory enzyme complex IV is maintained, resulting in brown cells (COX+/SDH−).
Using these techniques, mitochondrial respiratory activity was assessed in fresh-frozen sections (10 μm thick) prepared from the heart, kidney, and soleus of G ≥ 10 and G1 Polg mice. The cryosections were reacted with 2 mg/mL diaminobenzidine tetrahydrochloride (DOJINDO, #349–00903) in 0.1 M acetate buffer (pH 5.5) containing 0.1% MnCl2 and 0.1% H2O2, followed by incubation with 1 mg/mL of NBT (Wako, #144–01993) in 0.2 M phosphate buffer (pH 7.4) with sodium succinate. Images were acquired using a microscope camera (DFC310FC, Leica) after observation with a microscope (DMRE, Leica). To quantify cells with reduced mitochondrial respiratory activity, the percentage of COX−/SDH+ cells in each organ was calculated based on the stained images.
2.5 Statistical Analysis
The sample size of each test and statistical method used for data analysis are stated in each figure legend. Statistical analyses were conducted using R and RStudio. A p-value of less than 0.05 was considered to indicate statistically significant differences between samples.
3 Results
3.1 mtDNA Mutation Frequency Is Not Correlated With Mitochondrial Respiratory Activity in G ≥ 10 Polg Mice
To investigate the correlation between mtDNA genotype and mitochondrial respiratory function, we enriched mtDNA in the heart, kidney, and soleus of G ≥ 10 Polg mice, followed by next-generation sequencing (see Material and Methods). Our analysis revealed that, compared with G ≥ 10 Polg+/+ mice, G ≥ 10 Polgmut/mut mice exhibited a significantly a higher mutation accumulation in mtDNA, with the mutation frequency reaching up to approximately 1.7 times higher (Figure 1A). Conversely, G ≥ 10 Polg+/mut mice showed no statistically significant difference in the mutation frequency compared with Polg+/+ mice across all organs (Figure 1A). We further examined whether mtDNA mutations caused by the proofreading deficiency of Polg were influenced by gene function (Figure 1B). The results indicated that G ≥ 10 Polgmut/mut mice exhibited a higher mutation frequency than G ≥ 10 Polg+/+ mice across all gene regions, with significant differences in all gene regions except for the D-loop (Figure 1B). In contrast, compared with G ≥ 10 Polg+/+ mice, G ≥ 10 Polg+/mut mice exhibited a similar mutation frequency in all gene regions (Figure 1B). These results suggest that random point mutations significantly accumulate in the mtDNA of mice only when the proofreading capacity of Polg is homozygously defective, with mutations occurring randomly across all gene regions of the mtDNA.
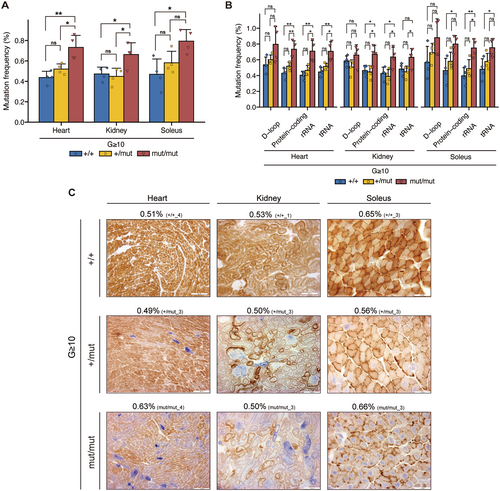
Next, to examine the correlation between the mutation frequency and mitochondrial respiratory function, we assessed mitochondrial respiratory activity in each organ of G ≥ 10 Polg mice by COX/SDH staining. Our results revealed that mitochondrial respiratory activity was mildly reduced in Polg+/mut mice and severely reduced in Polgmut/mut mice across all organs (heart, kidney, and soleus) (Figure S1A–C). Notably, some G ≥ 10 Polg mice exhibited similar mutation frequency across the organs despite having different nuclear Polg genotypes (Figure 1C; red frame in Figure S1A–C). Regardless of the mutation frequency, mitochondrial respiratory activity in these organs was mildly reduced in Polg+/mut mice and severely reduced in Polgmut/mut mice (Figure 1C; red frame in Figure S1A–C). For example, in the soleus, the mutation frequency of Polg+/mut mice was 0.56%, which was lower than that of Polg+/+ mice; however, mitochondrial respiratory activity in Polg+/mut mice was mildly reduced (Figure 1C). Similarly, although the mutation frequency of Polgmut/mut mice was comparable to that of Polg+/+ mice (0.66%), their mitochondrial respiratory activity was severely reduced (Figure 1C).
3.2 Generation of Mice With Experimentally Reduced mtDNA Mutation Frequency
To further investigate the causal relationship between random point mutations in mtDNA and mitochondrial respiratory function, we attempted to vary mtDNA genotype in Polg mice. Polg mice are assumed to accumulate random point mutations in mtDNA through the maternal germline (see Material and Methods). Indeed, mtDNA mutations accumulating in the organs of G ≥ 10 Polg mice showed a high mutation frequency (Figure S2A–C), which remained consistent across all organs in each mouse (Figure S2D–I). These results indicate that random point mutations in mtDNA occurring in the oocyte are inherited maternally and accumulate at similar levels across organs in mice.
Thus, to eliminate random point mutations in mtDNA accumulated through the germline in G ≥ 10 Polg mice, we generated G1 Polg (Figure 2A, see Material and Methods) and conducted mtDNA analysis using next-generation sequencing. The accumulation of mtDNA mutations with a higher frequency observed in G ≥ 10 Polg mice (Figure S2A–C) was rare in G1 Polg mice (Figure S3A–C), and a mutation frequency in G1 Polg mice was lower than that in G ≥ 10 Polg mice (Figures 1A and 2B). Furthermore, G ≥ 10 Polg mice exhibited greater variation in mutation frequency compared to G1 Polg mice (Figures 1A and 2B). This variability may reflect differences in the number of generations, as G ≥ 10 Polg mice include G10-13 Polg mice. In G1 Polg mice, a mutation frequency was significantly higher in the heart and soleus of Polgmut/mut mice than in those of Polg+/+ mice, whereas the mutation frequency of Polg+/mut mice was approximately equal to that of Polg+/+ mice (Figure 2B). This pattern was further confirmed by analyzing a mutation frequency across gene regions, revealing that Polgmut/mut mice accumulated significantly more mtDNA mutations than Polg+/+ mice, except for the D-loop region, whereas Polg+/mut mice showed a similar mutation frequency to Polg+/+ mice across all regions (Figure 2C).
3.3 Non-Synonymous Substitution and Pathogenic Mutations Accumulate in mtDNA Through Maternal Germline in Polg Mice
A previous study has demonstrated purifying selection against non-synonymous substitution in mtDNA in the germline (Stewart et al. 2008). This finding suggests that random point mutations in mtDNA accumulated through the germline in G ≥ 10 Polg mice may be neutral point mutations, unlike somatic mutations in mtDNA. If so, variation in mtDNA genotype through the germline is not an appropriate approach to examine the causal relationship between random point mutations in mtDNA and mitochondrial respiratory dysfunction, which is the basis of the mitochondrial theory of aging.
Thus, to assess whether the accumulation of non-synonymous substitutions in mtDNA varied between generations of mouse strains, non-synonymous substitutions that accumulated in each organ of G ≥ 10 and G1 Polg mice at mutation frequency over 1% were quantified. G ≥ 10 Polgmut/mut mice tended to accumulate more non-synonymous substitutions in mtDNA than G1 Polgmut/mut mice, with significant differences identified in the heart and soleus (Figure 3A). A similar pattern was also observed in Polg+/+ mice and Polg+/mut mice without significant differences (Figure 3A). In G ≥ 10 Polg mice, mtDNA mutations with a high mutation frequency assumed to be maternally inherited were observed (Figure S2A–C). To determine whether non-synonymous substitutions are present in these maternally inherited mutations, a mutation frequency of non-synonymous substitutions in the organs of G ≥ 10 and G1 Polg mice was analyzed. Compared with G1 Polg mice, G ≥ 10 Polg mice accumulated more non-synonymous substitutions, with more than 10% mutation frequency (Figures 3B and S4A–B). For example, in the heart, G ≥ 10 Polg mice accumulated non-synonymous substitutions with more than 10% mutation frequency, whereas such non-synonymous substitutions were rare in G1 Polg mice (Figure 3B).
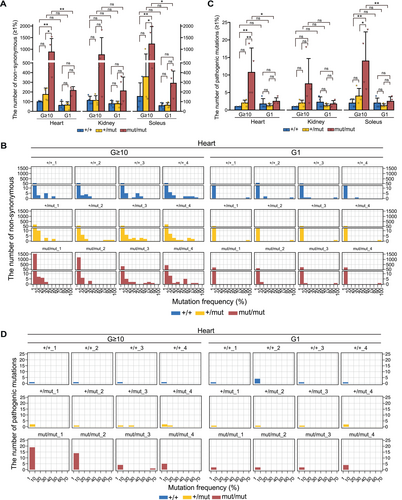
When pathogenic mutations accumulate above a threshold in mtDNA molecules, they cause structural abnormalities in the subunits of the respiratory enzyme complex and impaired translational function, resulting in mitochondrial respiratory dysfunction. Thus, we assessed the mutations homologous to confirmed pathogenic mutations in human mtDNA among the mutations that accumulated in G ≥ 10 and G1 Polg mice at a mutation frequency over 1%. All confirmed pathogenic mutations (125 in total, last accessed: October 8, 2024) are listed in MITOMAP (https://mitomap.org). G ≥ 10 Polg+/+ and Polg+/mut mice accumulated as many pathogenic mutations as G1 Polg+/+ and Polg+/mut mice, respectively (Figure 3C). In contrast, G ≥ 10 Polgmut/mut mice accumulated significantly higher pathogenic mutations in mtDNA than G1 Polgmut/mut mice (Figure 3C). Additionally, pathogenic mutations with more than 10% mutation frequency were observed to accumulate in G ≥ 10 Polg+/mut and Polgmut/mut mice, but no such mutations were present in G1 Polg mice (Figures 3D and S5A,B). For example, in the heart of a G ≥ 10 Polg+/mut mouse (Mouse ID: +/mut_3) and G ≥ 10 Polgmut/mut mouse (Mouse ID: mut/mut_3), pathogenic mutations with 20%–30% and 60%–70% mutation frequency were observed to accumulate, respectively (Figure 3D).
These results indicate that non-synonymous substitutions and pathogenic mutations with more than 1% mutation frequency could accumulate in the mtDNA beyond purifying selection in the germline of the crossed Polg+/mut mice.
3.4 Reduced Mitochondrial Respiratory Activity Observed in Polg Mice Is Not due to mtDNA Mutation Frequency
If the accumulation of random point mutations in mtDNA leads to reduced mitochondrial respiratory function, mitochondrial respiratory activity in G1 Polg mice would have improved or normalized compared with that in G ≥ 10 Polg mice. To investigate this, we assessed mitochondrial respiratory activity in each organ of G1 Polg mice using COX/SDH staining (Figure S6A–C). The results indicated that mitochondrial respiratory activity was mildly reduced in G1 Polg+/mut mice and severely reduced in G1 Polgmut/mut mice (Figure S6A–C). However, a comparison between G ≥ 10 and G1 Polg mice revealed that mitochondrial respiratory activity in G1 Polg remained equivalent to that in G ≥ 10 Polg mice, despite a lower mutation frequency in the G1 Polg mice (Figure 4A; blue box in Figures S1A–C and S6A–C). For instance, G1 Polgmut/mut mice exhibited a severe reduction in mitochondrial respiratory activity as G ≥ 10 Polgmut/mut mice, even though the mutation frequency in G1 Polgmut/mut mice was 0.36%, approximately half of the mutation frequency (0.69%) observed in G ≥ 10 Polgmut/mut mice (Figure 4A).
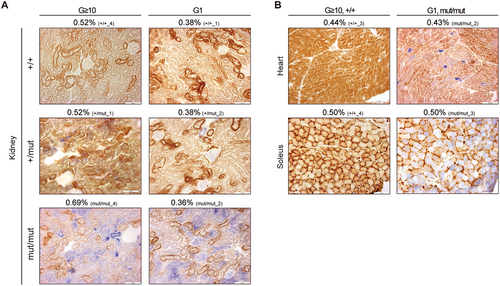
Next, we focused on G1 Polgmut/mut mice that exhibited the same mutation frequency as G ≥ 10 Polg+/+ mice (Figures 1A and 2B). If the accumulation of random point mutations in mtDNA causes reduced mitochondrial respiratory activity, G1 Polgmut/mut mice would be expected to exhibit normal mitochondrial respiratory activity, similar to G ≥ 10 Polg+/+ mice. However, as mentioned, mitochondrial respiratory activity was severely reduced in G1 Polgmut/mut mice (Figure S6A–C). A detailed comparison of COX/SDH staining between G ≥ 10 Polg+/+ and G1 Polgmut/mut mice revealed significant differences in mitochondrial respiratory activity (Figure 4B; green box in Figures S1A–C and S6A–C). For example, in the heart, G1 Polgmut/mut mice exhibited severely reduced mitochondrial respiratory activity (Figure 4B) despite showing the same mutation frequency (0.43%) as G ≥ 10 Polg+/+ mice. In the kidney, mitochondrial respiratory activity was severely reduced in G1 Polgmut/mut mice, even though their mutation frequency was 0.36%, which was lower than that of G ≥ 10 Polg+/+ mice (0.52%) (Figure 4A).
3.5 Reduced Mitochondrial Respiratory Activity Observed in Polg Mice Is Not due to the Accumulation of Non-Synonymous Substitutions and Pathogenic Mutations in mtDNA
A previous study has indicated that, contrary to the presence of inter-mitochondrial complementation and the threshold effect (Nakada et al. 2001; Ono et al. 2001), the random accumulation of non-synonymous substitutions in mtDNA causes instability of respiratory enzyme complexes, resulting in mitochondrial respiratory dysfunction in Polgmut/mut mice (Edgar et al. 2009). Consequently, we hypothesized that the reduced mitochondrial respiratory activity observed in Polg mice was due to non-synonymous substitutions (quality of mutations that accumulate in mtDNA) rather than mtDNA mutation frequency (number and ratio of all mtDNA mutations that accumulate). To investigate this hypothesis, we examined G ≥ 10 Polg+/+ and Polg+/mut mice, which had comparable numbers of non-synonymous substitutions to G1 Polgmut/mut mice, and assessed their mitochondrial respiratory activity (Figure 5A,B; yellow frame in Figures S1A–C and S6A–C). Our findings revealed that G ≥ 10 Polg+/+ mice maintained normal mitochondrial respiratory activity despite accumulating 360 non-synonymous substitutions, which was more than that observed in G1 Polgmut/mut mice (Figure 5A). G ≥ 10 Polg+/mut mice had fewer non-synonymous substitutions in the heart than G ≥ 10 Polg+/+ mice, but their mitochondrial respiratory activity was mildly reduced (Figure 5B).
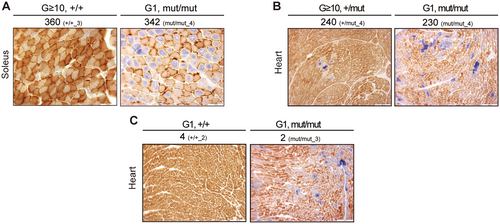
As previously discussed, the accumulation of pathogenic mutations in mtDNA causes structural abnormalities in the subunits of the respiratory enzyme complex and impaired translational function, resulting in reduced mitochondrial respiratory activity. A previous study hypothesized that mtDNA molecules with a low frequency of pathogenic mutations clonally expand beyond a threshold with age, resulting in mitochondrial respiratory dysfunction in Polgmut/mut mice (Trifunovic et al. 2004; Vermulst et al. 2008). To examine the causal relationship between mitochondrial respiratory dysfunction and the accumulation of pathogenic mutations in mtDNA, we analyzed G1 Polg+/+ mice, which exhibited a comparable number of non-synonymous substitutions to G1 Polgmut/mut mice, and assessed their mitochondrial respiratory activity (Figure 5C; purple frame in Figures S6A). Despite G1 Polg+/+ mice and Polgmut/mut mice accumulating 3 and 2 pathogenic mutations, respectively, only the G1 Polgmut/mut mice exhibited cells with reduced mitochondrial respiratory activity (Figure 5C). This indicates that the reduced mitochondrial respiratory activity in Polgmut/mut mice is not due to the accumulation of pathogenic mutations exceeding 1% mutation frequency. However, a pathogenic mutation (T8393C, homologous to T8993C in humans) with a mutation frequency of 60%–70% accumulated in the protein-coding (ATP6) region in the heart of a G ≥ 10 Polgmut/mut mouse (Mouse ID: mut/mut_3) (Figure 3D). Thus, it is possible that the reduced mitochondrial respiratory activity observed in this individual (Figure S1A, Mouse ID: mut/mut_3) was caused by the accumulation of this pathogenic mutation.
3.6 Reduced Mitochondrial Respiratory Activity in Polg Mice Is Not due to the Accumulation of Small Deletions and Insertions in mtDNA
Small insertions, deletions, and point mutations can occur in mtDNA. To investigate whether the accumulation of small deletions and insertions contributes to reduced mitochondrial respiratory activity in Polg mice, we analyzed their frequency in different genotypes.
Our results showed that the frequency of small deletions and insertions in G ≥ 10 and G1 Polgmut/mut mice tended to be higher compared to that in G ≥ 10 and G1 Polg+/+ mice, respectively (Figure S7A,B). In contrast, the frequency of small deletions and insertions in G ≥ 10 and G1 Polg+/mut mice, which exhibited mildly reduced mitochondrial respiratory activity in COX/SDH staining, was comparable to that in G ≥ 10 and G1 Polg+/+ mice, respectively (Figure S7). Additionally, the frequency of small deletions and insertions in G1 Polg mice was lower than that in G ≥ 10 Polg mice (Figure S7A,B). For example, the frequency of small deletions in G1 Polgmut/mut mice was approximately 0.01%, which was comparable to that in G ≥ 10 Polg+/mut mice (Figure S7A,B). However, no individuals in the G ≥ 10 Polg+/mut group exhibited severely reduced mitochondrial respiratory activity, similar to that observed in G1 Polgmut/mut mice, as assessed by COX/SDH staining (Figures S1A–C and S6A–C). Furthermore, the frequency of small deletions and insertions in G ≥ 10 and G1 Polg mice was more than 40 times lower than that of point mutations (Figure S7A,B). These findings suggest that point mutations in mtDNA have a greater impact on mitochondrial respiratory function than small deletions and insertions.
3.7 Reduced Mitochondrial Respiratory Activity Observed in Polg Mice Is Correlated With the Nuclear Genotype of Polg
Finally, to investigate the correlation between the nuclear genotype of Polg and mitochondrial respiratory activity, COX/SDH staining results from the organs of G ≥ 10 and G1 Polg mice were quantified (Figure 6A–C). The results revealed a correlation between the nuclear genotype of Polg and the proportion of cells with reduced mitochondrial respiratory activity in the organs of G1 and G ≥ 10 Polg mice, with significant differences across all genotypes (Figure 6A–C). Furthermore, despite variations in mtDNA genotype between G ≥ 10 and G1 Polg mice (Figures 1A, 2B, 3A,C), no significant differences were observed in quantitative COX/SDH staining results (Figure 6A–C).
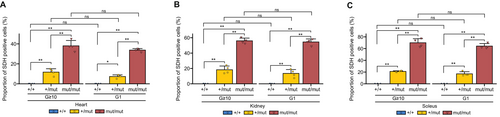
Overall, these findings indicate that reduced mitochondrial respiratory activity in Polg mice is closely associated with the nuclear genotype of Polg rather than the mtDNA genotype.
4 Discussion
Previous studies have suggested that the accumulation of random point mutations in mtDNA, particularly non-synonymous substitutions, leads to mitochondrial respiratory dysfunction and have mostly made comparisons between Polg+/+ and Polgmut/mut mice (Edgar et al. 2009; Trifunovic et al. 2004). In contrast, a study focusing on Polg+/mut mice suggested that the accumulation of random point mutations in mtDNA did not induce aging (Vermulst et al. 2007). However, these previous studies have not examined the correlation between mtDNA genotype and mitochondrial respiratory dysfunction in Polg mice, including Polg+/mut mice. In this study, mtDNA genotype was determined in various organs of Polg mice using NGS analysis, and their correlation with mitochondrial respiratory activity was assessed using COX/SDH staining. The present study showed that compared with G ≥ 10 Polg+/+ mice, G ≥ 10 Polgmut/mut mice exhibited significantly increased mtDNA mutation frequency (Figures 1A and S7A) and a higher number of non-synonymous substitutions (Figure 3A), along with reduced mitochondrial respiratory activity (Figure 6A–C). These findings, consistent with previous research (Edgar et al. 2009; Trifunovic et al. 2004), reinforce the hypothesis that random point mutations and non-synonymous substitutions in mtDNA contribute to mitochondrial dysfunction. However, G ≥ 10 Polg+/mut mice exhibited mildly reduced mitochondrial activity despite having a similar mtDNA genotype to those of G > 10 Polg+/+ mice (Figures 1C, 2A, 3A,C). Since this result suggests that the reduced mitochondrial respiratory activity in Polg mice was not caused by the accumulation of random point mutations or small deletions and insertions in mtDNA, we generated G1 Polg mice, which have a lower mutation frequency compared to G ≥ 10 Polg mice (Figures 1A, 2A,B, S7A,B). Notably, even when the mutation frequency was reduced, mitochondrial respiratory activity did not improve (Figure 4A). Additionally, mitochondrial respiratory activity was severely reduced in G1 Polgmut/mut mice despite having mutation frequency and non-synonymous substitutions similar to G ≥ 10 Polg+/+ mice (Figures 4B and 5A). Moreover, the number of pathogenic mutations in G1 Polgmut/mut mice was the same as that in G1 Polg+/+ mice (Figure 5C). Collectively, these results suggest that the accumulation of random point mutations and small deletions and insertions in mtDNA, i.e., mtDNA genotype, may not induce mitochondrial respiratory dysfunction in Polgmut/mut mice.
A previous study suggested that non-synonymous substitutions occurring in mtDNA are less likely to be maternally transmitted due to purifying selection in Polg mice (Stewart et al. 2008). However, our study demonstrated that G ≥ 10 Polg mice accumulated more non-synonymous substitutions than G1 Polg mice (Figure 3A,B). This discrepancy may be attributed to the presence of the proofreading-deficient Polg allele in female mice during mating. The previous study tested germline selection pressure by crossing wild-type male mice with wild-type Polg females carrying maternally inherited mtDNA mutations randomly accumulated in Polgmut/mut mice (Stewart et al. 2008). In this context, no new non-synonymous substitutions were expected to occur in mtDNA due to the presence of proofreading-deficient Polg in the female germline (Stewart et al. 2008). However, in this study, the maintenance of mouse strains by mating Polg+/mut mice always made the mtDNA molecule in female mouse germ cells prone to new non-synonymous substitutions through proofreading-deficient mutations in Polg. Consequently, non-synonymous substitutions may have been maternally inherited by subsequent generations beyond the purifying selection observed in prior studies. This allowed us to explore the causal relationship between the accumulation of non-synonymous substitutions in mtDNA and reduced mitochondrial respiratory function in Polg mice.
When pathogenic mutations accumulate over a threshold in mtDNA molecules, mitochondrial respiratory function declines and can be detected as COX-/SDH+ cells in COX/SDH staining (Baines et al. 2014; Greaves et al. 2010). Previous research has indicated that mtDNA molecules with low-frequency pathogenic mutations clonally expand over a threshold with age, resulting in mitochondrial respiratory dysfunction in Polgmut/mut mice (Trifunovic et al. 2004; Vermulst et al. 2008). In the present study, COX-/SDH+ cells were identified in Polg+/mut and Polgmut/mut mice (Figure 5A–C). If non-synonymous substitutions and pathogenic mutations clonally accumulate in COX-negative cells, these mutations should be detected at low mutation frequencies in NGS results from bulk tissue. For example, in the heart of a G1 Polgmut/mut mouse, approximately 38% of cells were COX-/SDH+ (Figure 6A), and if these COX-/SDH+ cells had accumulated 50% of certain non-synonymous substitutions and pathogenic mutations, the mutation frequency would be detected as approximately 19% (= 50% × 38%) in the NGS analysis from the bulk tissue. In other words, if the clonal accumulation of non-synonymous substitutions and pathogenic mutations occurs in COX-/SDH+ cells in Polgmut/mut mice, the number of non-synonymous substitutions and pathogenic mutations with low mutation frequency should be higher in Polgmut/mut mice than in Polg+/+ mice. Therefore, the number of non-synonymous substitutions and pathogenic mutations with low mutation frequency (> 1%) was examined in relation to mitochondrial respiratory activity (COX-/SDH+ staining). The results showed that G ≥ 10 Polg+/+ mice had a similar number of non-synonymous substitutions as G1 Polgmut/mut mice, in which COX-/SDH+ cells were observed, but no COX-/SDH+ cells were identified in the former (Figure 5A). Similarly, G1 Polg+/+ mice exhibited a comparable number of pathogenic mutations to G1 Polgmut/mut mice, but no COX-/SDH+ cells were identified (Figure 5C). These findings suggest that the COX-/SDH+ cells observed in Polgmut/mut mice are not due to clonal accumulation of non-synonymous substitutions or pathogenic mutations, at least at a mutation frequency of 1%.
In previous studies, PCR-amplified fragments of mtDNA were used to analyze mtDNA mutation frequency in Polg mice (Kujoth et al. 2005; Trifunovic et al. 2004; Vermulst et al. 2007). In this study, next-generation sequencing was conducted to accurately detect mtDNA mutations using enriched circular mtDNA without PCR amplification, as referenced in prior research (Bagge et al. 2020). The findings, consistent with previous studies, demonstrated that significant accumulation of mtDNA mutations occurred in all organs of G1 and G ≥ 10 Polgmut/mut mice compared with G1 and G ≥ 10 Polg+/+ mice, although the mutation frequency was at most 1.7 times higher than that in Polg+/+ mice (Figures 1A and 2B). Previous studies reported that compared with those in Polg+/+ mice, a mutation frequency in Polgmut/mut mice could increase 3-8-fold (Kujoth et al. 2005; Trifunovic et al. 2004) or up to 2500-fold (Vermulst et al. 2007). In contrast, the relative mutation frequency calculated in this study was lower (Figures 1A and 2B). The proofreading function of Polg, through its exonuclease activity, facilitates ligation during mtDNA replication (Macao et al. 2015) and degrades fragmented mtDNA (Nissanka et al. 2018; Peeva et al. 2018). Consequently, fragmented linear mtDNA has been observed to increase in cells and mice with Polg-deficient proofreading (Nissanka et al. 2018; Peeva et al. 2018; Trifunovic et al. 2004). NUMTs, sequences within nuclear DNA that resemble mtDNA, may be misidentified during mtDNA sequencing (Goios et al. 2009; Hazkani-Covo et al. 2010). Therefore, previous studies that relied on sequencing PCR-amplified fragments of specific regions or the full length of mtDNA may have amplified fragmented linear mtDNA and NUMTs. However, no significant increase in NUMTs has been reported in Polgmut/mut mice. Hence, previous studies may have overestimated mutation frequency in Polgmut/mut mice by including fragmented linear mtDNA in their sequencing data.
Mitochondrial respiratory dysfunction is one of the contributing factors to aging (López-Otín et al. 2023; Wallace 1999). Polgmut/mut mice exhibit severely reduced mitochondrial respiratory activity (Figures 1C, 4A,B and 6A–C) and premature aging phenotypes (Kujoth et al. 2005; Trifunovic et al. 2004). Conversely, mice with pathogenic mutations in mtDNA show reduced mitochondrial respiratory function but mitochondrial diseases and diseases associated with mitochondria rather than a premature aging phenotype (Fan et al. 2008; Hashizume et al. 2012; Inoue et al. 2000; Kasahara et al. 2006; Lin et al. 2012; Shimizu et al. 2014; Tani et al. 2022; Tyynismaa et al. 2005; Yokota et al. 2010; Zhang et al. 2025). This raises the question of why Polgmut/mut mice exhibit a premature aging phenotype, whereas mice with pathogenic mutations in mtDNA exhibit mitochondrial diseases despite both exhibiting mitochondrial respiratory dysfunction. Previous studies have shown that Polgmut/mut mice exhibit somatic stem cell dysfunction (Ahlqvist et al. 2012) and immunometabolism dysfunction (Lei et al. 2021), which could be key driving forces behind premature aging phenotypes. Therefore, to understand the mechanisms underlying premature aging in Polgmut/mut mice, it will be necessary to investigate various aspects of the aging process beyond mitochondrial respiratory dysfunction.
In this study, G ≥ 10 and G1 Polg+/mut mice exhibited a similar mtDNA mutation frequency as G ≥ 10 and G1 Polg+/+ mice, respectively, despite carrying a proofreading-deficient mutation in the Polg gene (Figures 1A and 2B). The reason for this remains unclear. One possibility is that, in Polg+/mut mice, mutant mtDNA is selectively degraded through mechanisms such as mitophagy. Alternatively, only mutations that can be repaired may have occurred. Future studies should focus on Polg+/mut mice to elucidate the mechanisms that prevent the accumulation of mtDNA mutations in these mice.
In conclusion, this study provides evidence that mitochondrial respiratory dysfunction in Polgmut/mut mice is more likely associated with the nuclear genotype of the Polg gene rather than the accumulation of point mutations in mtDNA (Figure 6A–C). Previous findings have suggested that the exonuclease activity of Polg may play roles beyond mtDNA proofreading, including the degradation of linear mtDNA fragments (Nissanka et al. 2018; Peeva et al. 2018) and the ligation process during mtDNA replication (Macao et al. 2015). Additionally, studies have suggested the interactions between POLG and other factors unrelated to mtDNA replication and proofreading (Liyanage et al. 2017). Thus, the reduced mitochondrial respiratory function in Polgmut/mut mice may be caused by a disruption in unknown functions of Polg. Therefore, it is crucial to reconsider the mitochondrial theory of aging in Polgmut/mut mice with a focus on these unidentified functions of Polg.
Author Contributions
Hiroaki Tamashiro: writing – original draft, writing – review and editing, investigation, data curation, visualization, methodology, resources, formal analysis, funding acquisition. Kaori Ishikawa: writing – original draft, writing – review and editing, investigation, validation, resources, methodology, funding acquisition. Koichi Sadotomo: resources. Emi Ogasawara: investigation, resources, funding acquisition. Kazuto Nakada: writing – original draft, writing – review and editing, funding acquisition, conceptualization, project administration, supervision.
Acknowledgments
We thank Emilie Kristine Bagge at the RIKEN for assisting us with mtDNA enrichment. A professional English-speaking editor from Editage (www.editage.jp) has proofread and edited the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Sequencing data has been deposited to the Sequencing Read Archive at NCBI under accession number PRJNA1195219 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA1195219?reviewer=lvlgfilldugpen0bt0bjd982em). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.