Nitrogen losses from the soil/plant system: a review
Abstract
Losses of nitrogen from the soil/plant system not only reduce soil fertility and plant yield but can also create adverse impacts on the environment. Ammonia emissions into the atmosphere contribute to acid rain and represent an indirect source of nitrous oxide greenhouse gas emissions. Nitrate leaching losses into rivers and lakes can cause eutrophication resulting in excessive growth of aquatic weeds and algae, which can reduce fish populations and the recreational value of the water. Nitrate contamination of drinking water supplies can cause health risks. Legislation that is designed to limit nitrate leaching losses from land has become a constraint on agricultural land use in many countries. Nitrous oxide emissions into the atmosphere contribute to the depletion of the ozone layer and also make a significant contribution to climate change. This review describes the nitrogen cycle in temperate soil/plant systems, the processes involved in each of the individual nitrogen loss pathways, the factors affecting the amounts of losses and the methods that are available to reduce these losses. The review has shown that careful management of temperate soil/plant systems using best management practices and newly developed technologies can increase the sustainability of agriculture and reduce its impact on the environment.
Introduction
N-cycle
Soils commonly contain between 0.1% and 0.6% nitrogen (N) in the top 15 cm. This usually represents between 2000 and 12 000 kg N ha−1 depending on the soil type. Soil nitrogen is present in four major forms: (a) organic matter, such as plant material, fungi and humus; (b) soil organisms and microorganisms; (c) ammonium ions (NH4+) held by clay minerals and organic matter and (d) mineral-N forms in soil solution, including NH4+, nitrate (NO3−) and low concentrations of nitrite (NO2−). The gains, losses and transformations of nitrogen within the soil/plant system affect the availability of N to plants and the transfer of N into the wider environment (Fig. 1). In agricultural and horticultural systems, mineral-N is mainly prone to losses through: (a) ammonia volatilisation; (b) leaching (i.e. removal in drainage water) and (c) denitrification (i.e. transformations into gaseous forms), and it is these loss pathways that will be described and discussed in this review (Fig. 1).
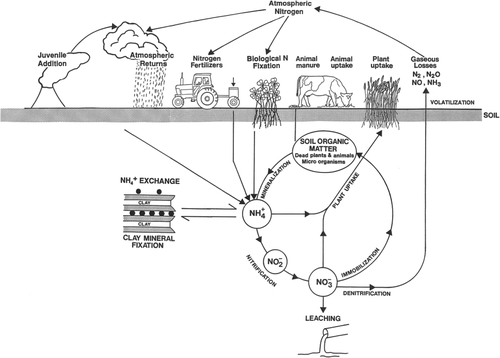
N balance equation
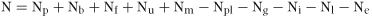 (1)
(1)where p is precipitation and dry deposition, b is biological fixation, f is fertiliser, u is urine and dung returns to the soil, m is mineralisation, pl is plant uptake, g is gaseous losses, i is immobilisation, l is leaching loss and e is erosion and surface runoff.
Ammonia volatilisation
Impacts of ammonia volatilisation
Ammonia volatilisation is the loss of gaseous ammonia from the soil surface (Fig. 1). Ammonia volatilisation is undesirable because it represents a loss of N from the soil/plant system and a threat to the wider environment. Most of the ammonia that is volatilised is returned to the earth's surface through wet deposition (i.e. dissolved in rainwater) or dry deposition (i.e. attached to particulate matter) causing acidification and eutrophication of natural ecosystems. Ammonia emissions and the subsequent deposition onto land and water also represent an indirect source of nitrous oxide greenhouse gas. Agriculture accounts for about 50% of all the ammonia that is volatilised worldwide (Sommer et al., 2004). For example, the potential risk of ammonia volatilisation from urea fertiliser can represent between 0% and 65% of the N applied, depending on soil and climatic conditions (Martens & Bremner, 1989; Watson, 1990; Bussink & Oenema, 1998; Bishop & Manning, 2010).
Processes
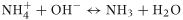 (2)
(2) (3)
(3)Factors affecting volatilisation and amounts of loss
Soil pH
Calcareous soils with a naturally high pH can lose significant amounts of ammonia gas; however, neutral or acid pH soils may also lose significant amounts of ammonia when urea or animal urine is applied, as shown in Eqn 3 (Black et al., 1985a,b).
When urea is applied to the soil surface the hydrolysis reaction produces a sharp increase in pH resulting in an increase in ammonia volatilisation. The pH of the microsite below the urea granule can increase above a value of 8 (Sommer et al., 2004). This process occurs over the first few days following application; however, once nitrification processes start to become significant then the pH of the soil is decreased, which in turn reduces the volatilisation rate.
Temperature
Soil and atmospheric temperature affect the rate of urea hydrolysis, and the rate of transfer of ammonia from soil solution into the atmosphere (Black et al., 1985a,b; McGarry et al., 1987). Under field conditions, the hydrolysis rate and the ammonia emission rate follow a diurnal sequence, with the highest emissions occurring at the time of highest daily temperature. There is also a seasonal effect with the highest losses occurring during the warmest months. For example, Ball & Keeney (1983) reported ammonia losses from animal urine patches equivalent to 5% and 16% of the N applied under cool and warm conditions, respectively. However, some studies have shown that the total amount of N lost by ammonia volatilisation may not necessarily be different at low versus high temperatures because the volatilisation may continue for longer at low temperatures (Fenn & Kissel, 1974; Harper et al., 1983).
Ammonium concentration
The amount of ammonia emitted from the soil is related to the ammonium concentration in the soil solution. In general, the higher the solution concentration of ammonium the higher the potential emission rate of ammonia gas. Thus, application of N fertiliser (especially urea) and animal urine can significantly enhance the ammonia emission rate. Nevertheless, there are many other factors that affect the concentration of ammonium in the soil solution, including nitrification rate, plant uptake rate, denitrification rate and immobilisation rate, all of which reduce the solution concentration and thus ammonia volatilisation loss potential.
Soil cation exchange capacity
Soil cation exchange reactions retain ammonium ions on the surface of clays and organic matter through electrostatic attraction. This mechanism helps to store ammonium in soil and also reduces the soil solution concentration of ammonium. The cation exchange capacity (CEC) also helps to buffer the soil against pH change; thus, when urea hydrolysis occurs in a clay soil (which has a high CEC) there is a smaller increase in pH than would occur in a sandy soil (which has a low CEC) (Daftardar & Shinde, 1980; Whitehead & Raistrick, 1993). Thus, the ammonia volatilisation potential of a clay soil (high CEC) is generally lower than that of a sandy soil (low CEC).
Soil moisture, rainfall and irrigation
Soil moisture content affects the concentration of ammonia/ammonium in solution, and low soil moisture contents therefore promote high solution concentrations and thus high ammonia emission loss. However, at very low soil moisture levels, the rate of dissolution of urea fertiliser and of urea hydrolysis will be slow and the ammonia loss will also be slow (Al-Kanani et al., 1991). Increasing the soil moisture content of a dry soil can increase the rate of urea hydrolysis and thus increase the rate of ammonia production from urea (Vlek & Carter, 1983; Reynolds & Wolf, 1987). However, significant amounts of rainfall or irrigation water applied soon after urea or urine deposition can also reduce the ammonia volatilisation loss because the rainfall or irrigation water washes the urea below the soil surface and keeps the surface solution concentration low (Black et al., 1987).
Variations in ammonia volatilisation losses are often due to a combination of factors including soil texture, soil moisture content as well as rainfall after fertiliser application. For example, Turner et al. (2012) found that small differences in weather conditions and initial soil moisture content had a significant effect on the size of ammonia volatilisation losses from fertiliser applied 1 week apart at the same location. The ammonia first lost from the application of 46 kg N ha−1 ammonium sulphate represented 12% of the applied N, whereas the loss 1 week later from a similar rate of ammonium sulphate was only 2.8% of the applied N (Turner et al., 2012).
Fertiliser use
The greatest risks of ammonia volatilisation losses occur from ammonium bicarbonate, urea and ammonium hydroxide fertilisers, with lower losses reported from ammonium sulphate or diammonium phosphate fertilisers (Black et al., 1985b; Whitehead & Raistrick, 1990; Sommer & Jensen, 1994; He et al., 1999; Sommer et al., 2004; Turner et al., 2012). Ammonia volatilisation losses from urea applied at <50 kg N ha−1 are generally in the range of 5–15% of the N applied, whereas ammonia losses from ammonium sulphate and calcium ammonium nitrate are typically less than 2–3% of the N applied at this rate (Black et al., 1984, 1985a,b). Examples of ammonia volatilisation losses from urea fertiliser are given in Table 1 (Bishop & Manning, 2010).
| Rate of N applied (kg N ha−1) | Mean % N volatilised | Location | Reference | |
|---|---|---|---|---|
| Grassland soils | 180 | 22.8 | Argentina | Barbieri et al. (2006) |
| 15–200 | 17.6 | NZ | Black et al., 1984, 1985a,b | |
| 50 | 36.0 | USA | Bowman et al. (1987) |
|
| 30–150 | 26.7 | UK | Chadwick (2005) |
|
| 25 | 7.5 | NZ | Di & Cameron (2004c) | |
| 23–536 | 11.6 | NZ | Ledgard et al. (1999) |
|
| 70–437 | 17.7 | UK | Ryden et al. (1987) |
|
| 90 | 17.8 | Denmark | Sommer & Jensen (1994) |
|
| 70–280 | 28.1 | UK | Van der Weerden & Jarvis (1997) |
|
| 80–120 | 19.3 | Netherlands | Velthof & Oenema (1990) |
|
| 150 | 4.2 | NZ | Zaman et al. (2008) |
|
| Mean | 19.0 | |||
| Arable soils | 90 | 17.8 | Denmark | Sommer & Jensen (1994) |
| 140 | 17.3 | Canada | Rochette et al. (2009) |
|
| 200 | 30.0 | India | Beyrouty et al. (1988) |
|
| 100 | 11.7 | NZ | Black et al. (1989) |
|
| 170 | 17.3 | Spain | Sanz-Cobena et al. (2008) |
|
| 80 | 9.5 | Australia | Turner et al. (2010) |
|
| 60 | 7.9 | Argentina | Palma et al. (1998) |
|
| 100 | 33.0 | Canada | Grant et al. (1996) |
|
| 46 | 5.4 | Australia | Turner et al. (2012) |
|
| 46 | 23.0 | Australia | Turner et al. (2012) |
|
| 46 | 13.0 | Australia | Turner et al. (2012) |
|
| Mean | 16.9 |
Grazing animals
It is estimated that 85–90% of the N ingested by grazing animals is excreted in urine and dung. This creates discrete patches in grazed pastures, which contain high concentrations of nitrogen. Urine is mostly a highly concentrated solution of urea (e.g. up to 10 g N L−1) that when deposited onto soil during urination can apply large amounts of N (e.g. equivalent to 1000 kg N ha−1 from a dairy cow) (Haynes and Williams, 1993; Di & Cameron, 2002a,b). The urea is quickly hydrolysed creating a temporary high pH, leading to ammonia emissions from the urine patch (Haynes et al., 1986). Ammonia volatilisation can also occur when animal manures are applied to the soil surface (Vanderholm, 1975; Bussink & Oenema, 1998).
Plants
Plant leaves can absorb ammonia directly through the stomata and can therefore reduce the amount of ammonia gas lost from the soil/plant system into the atmosphere (Schjoerring & Mattson, 2001; Denmead et al., 2008). However, plant leaves can also emit ammonia gas. For example, Wang & Schjoerring (2012) reported that green leaves and stems of ryegrass plants generally represent a sink for NH3, whereas senescent leaves have a large potential for NH3 emission. Nevertheless, research shows that in general the greater the height and density of the plant cover, the lower the ammonia loss (Denmead et al., 1982).
Methods to reduce ammonia volatilisation losses
Placement of N fertilisers 3–5 cm below the soil surface reduces the risk of ammonia volatilisation because it reduces the ammonia/ammonium solution concentration at the soil surface (Fenn & Miyamoto, 1981; Prasertsak et al., 2001; Sommer et al., 2004). Splitting fertiliser applications may also reduce volatilisation losses (Black et al., 1985b).
Application of urea before the onset of rain can significantly reduce the amount of ammonia volatilisation because it washes the urea and ammonium below the soil surface. For example, Black et al. (1987) found that 10–16 mm of rainfall soon after application of urea reduced the ammonia loss by over 80%. Applying irrigation water after the application of urea fertiliser can also reduce the risk of ammonia volatilisation (Sanz-Cobena et al., 2011).
Similarly, ammonia volatilisation from applications of anhydrous ammonia can be minimised if the fertiliser is injected to depths below 10 cm when soils are moist, generally after winter or rainy seasons (Sommer et al., 2004).
Mixing N fertilisers with acidifying agents (e.g. sulphur-coated urea) can reduce the risk of emissions in some situations (Black et al., 1985b). However, this depends on soil conditions (Stumpe et al., 1984). Coating urea with polymers can slow down the rate of dissolution of the urea and thus reduce the ammonia loss (Blaise & Prasad, 1995; Knight et al., 2007).
Probably, the most reliable way to reduce ammonia volatilisation is to coat urea fertiliser with a urease inhibitor, such as N-(n-butyl) thiophosphoric triamide (NBPT) or phenylphosphorodiamidate, because this reduces the rate that urea is converted to ammonia/ammonium in the soil by deactivating the urease enzyme (Bishop & Manning, 2010). For example, Watson et al. (1994) reported that coating urea with 0.28% NBPT reduced ammonia volatilisation by 54–95% depending on soil type. Several more recent studies carried out under field conditions in a variety of different situations have also demonstrated substantial mitigating effects of urease inhibitors on ammonia volatilisation from nitrogen fertilisers. Sanz-Cobena et al. (2008) measured a 42% reduction in ammonia emission by using NBPT-coated urea in comparison to uncoated urea in an experiment carried out on a crop of sunflower. Similarly, Turner et al. (2010) showed a dramatic reduction in ammonia volatilisation (from 9.5% to 1.0% of applied N) from the use of NBPT-coated urea in a field experiment growing winter wheat. Chambers & Dampney (2009) also showed substantial reductions in ammonia volatilisation from urea coated with urease inhibitor in 15 grassland field experiments and 13 winter cereal experiments in the UK. While noting that ammonia volatilisation losses from urea-based fertilisers are variable and unpredictable, Chambers & Dampney (2009) concluded that addition of a urease inhibitor is a potentially valuable mitigation method.
- Placing N fertilisers below the soil surface (including injection of anhydrous ammonia);
- Applying urea fertiliser before the onset of rain;
- Applying irrigation water after the application of urea fertiliser;
- Using N fertilisers with acidifying agents or polymer coatings;
- Using N fertilisers with a urease inhibitor coating and
- Dietary manipulation to reduce the amount of N deposited in animal urine patches.
Nitrate leaching
Impacts of nitrate leaching
Nitrate leaching losses from soil into water not only represent a loss of soil fertility but also represent a threat to the wider environment and to human health (Wild & Cameron, 1980; OECD, 1982; WHO, 1984; Howarth, 1988; Addiscott, 1996; Di & Cameron, 2002a; Andrews et al., 2007; Goulding et al., 2008). Nitrate that enters drinking water supplies can create a risk of methaemoglobinaemia in babies and has also been linked to cancer and heart disease (Grizzetti et al., 2011). Grizzetti et al. (2011) calculated that 50% of the European population live in areas where nitrate concentrations in water exceed 5.6 mg NO3−–N L−1 and about 20% live in areas where nitrate concentrations exceed the recommended level of 11.3 mg NO3−–N L−1, with variations between countries. It is estimated that 80% of surface water in French Brittany now exceeds 11.3 mg N L−1 (Molénat & Gascuel-Odoux, 2002). Nitrate that enters rivers or lakes can contribute to eutrophication, which may result in algae blooms and loss of fish (Smith & Schindler, 2009).
Processes
The amount of nitrate that is leached from soil depends on the concentration of nitrate present in the soil solution and the amount of drainage that occurs through the soil over a prescribed period (i.e. the solute transport flux). The amount of nitrate present in the soil solution depends on the amount of N applied, the nitrification rate and the denitrification rate etc (Fig. 1).
Nitrification
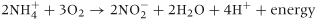 (4)
(4)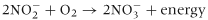 (5)
(5)The two parts of the reaction are dependent on the activity of two different genera of autotrophic bacteria. The first reaction, the oxidation of NH4+ to NO2−, is mainly conducted by the activity of soil ammonia-oxidising bacteria (AOB), such as Nitrosospira and Nitrosomonas. This occurs through oxidation reactions performed by the ammonia monooxygenase (AMO) enzyme associated with the bacteria (Ferguson et al., 2007; Prosser, 2007; Di et al., 2009a,b, 2010a,b). Ammonia-oxidising archaea (AOA) are also present in large numbers in soils, and although they may make an important contribution to nitrification in low-fertility marine and estuarine environments (Leininger et al., 2006), they do not appear to be as important as AOB in the relatively N-rich agricultural soil environment (Di et al., 2009b, 2010b) (Fig. 2).
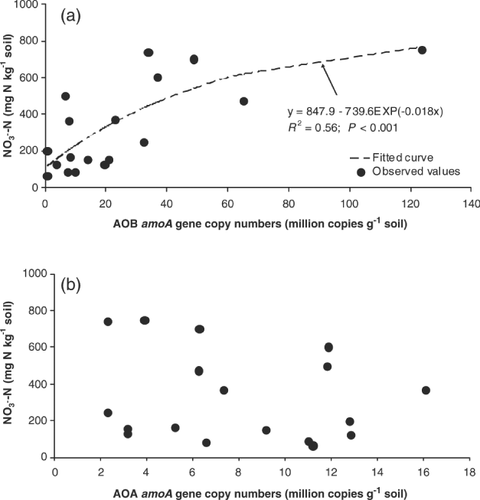
The second reaction, the oxidation of NO2− to NO3−, is conducted by Nitrobacter. The conversion of NO2− to NO3− takes place very rapidly and therefore nitrite rarely accumulates in soil.
The rate at which ammonium is converted into nitrate is sensitive to soil conditions, such as moisture content, pH and nutrient status. The maximum rate of nitrification takes place at a soil moisture content around ‘field capacity’ (i.e. at a soil matrix potential of −10 kPa) (Haynes et al., 1986). In soils that are wetter than field capacity the nitrification rate is slower. The nitrification rate is also significantly slower when the soil is dry, although it can still occur when soils are at permanent wilting point (−1500 kPa) (Malhi & McGill, 1982; Monaghan & Barraclough, 1992).
The optimum pH for nitrification to occur is between pH 4.5 and 7.5 (Haynes et al., 1986). The optimum soil temperature for nitrifying bacteria is between 25°C and 30°C (Haynes et al., 1986). Nitrification will still occur at soil temperatures below 5°C, but the rate is slower than at higher temperatures.
The amount of ammonium present in the soil also affects the nitrification rate because high concentrations of ammonium/ammonia can restrict the activity of Nitrobacter (Monaghan & Barraclough, 1992). Soil phosphate deficiency can also slow the nitrification rate as can high concentrations of heavy metals in soil (e.g. from sewage sludge applications to land) (Purchase, 1974; Ruyters et al., 2010; Griffiths & Philippot, 2012; Thavamani et al., 2012). Nitrifying bacteria are also sensitive to pesticides (Muñoz-Leoz et al., 2011).
Solute transport mechanisms
If it is assumed, for simplicity, that there are steady-state water conditions in the soil and that there is no interaction between the nitrate ion and the soil, then nitrate leaching can be described by a combination of three main mechanisms: convection, diffusion and dispersion (Hillel, 1980, 1998; Cameron & Haynes, 1986; McLaren & Cameron, 1996).
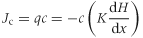 (6)
(6)where Jc is the convective nitrate flux, c is the nitrate concentration, q is the water flux, K is the hydraulic conductivity and dH/dx is the hydraulic gradient.
 (7)
(7)and θ is the volumetric water content.
Convective transport implies uniform displacement of the band of nitrate but, in reality, the band of nitrate tends to spread throughout the profile because of the processes of diffusion and hydrodynamic dispersion.
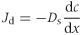 (8)
(8)where Jd is the rate of diffusion, Ds is the diffusion coefficient of the nitrate in soil and depends on the soil moisture content and dc/dx is the nitrate concentration gradient.
Hydrodynamic dispersion – This occurs because: (a) the flow velocity within a single pore is not uniform; (b) there are large variations in pore size in soil, which cause a range of pore water velocities and (c) the ‘tortuosity’ of soil pores produces a range of flow path lengths.
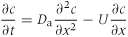 (9)
(9)where Da is the apparent diffusion coefficient and represents the sum of molecular diffusion plus hydrodynamic dispersion.
However, there are other soil factors that also affect nitrate leaching and are more difficult to describe mathematically because of their transient nature and complexity. Plant roots, earthworms, freezing and thawing cycles and wetting and drying of soil can produce surface-connected macropores (i.e. large diameter pores) in the soil profile. Water flow through these macropores can have two effects on leaching: (a) when nitrate is present in the infiltrating water then macropore flow will cause leaching to occur at a faster rate than normal, and (b) when nitrate is present within soil aggregates then the bulk of the drainage water may ‘bypass’ them leading to a slower than normal rate of leaching.
Some biological transformation processes, such as immobilisation, can remove N from the soil solution and can therefore reduce the rate of leaching. Other transformation processes, such as mineralisation, may release N into the soil solution and thus increase the rate of leaching loss. Plant uptake of nitrate decreases the concentration in soil solution and therefore reduces the rate of leaching loss (and will be discussed in more detail below).
Factors affecting nitrate leaching losses
The factors affecting nitrate leaching losses from soil have been reviewed previously (e.g. Wild & Cameron, 1980; Cameron & Haynes, 1986; Addiscott et al., 1991; Goulding, 2000; Jarvis, 2000; Di & Cameron, 2002a).
Season and climate
Nitrate leaching losses are usually greatest during late-autumn, winter and early-spring months when plant uptake of nitrate is low (because of cooler conditions) and drainage is occurring from the soil (because of rainfall inputs exceeding the evapotranspirative demand) (Wild & Cameron, 1980). For example, Di et al. (1999) compared N leaching losses from N fertiliser applied in the autumn with those from N applied in the spring and found that between 15% and 19% of the autumn-applied N (NH4Cl) was leached compared with an equivalent of 8–11% of N applied in the spring. Autumn rainfall can also leach residual fertiliser nitrate left after the crop has been harvested and nitrate released by mineralisation of soil organic nitrogen. Cameron & Wild (1984) reported that large amounts of nitrate (>100 kg N ha−1 year−1) were produced after ploughing of 3-year-old pasture soil and that this nitrate was leached down through the soil profile over the subsequent winter.
A dry summer can result in an accumulation of nitrate in the surface soil (because of low plant growth and low N uptake) and this nitrate can be leached over the subsequent winter (Scholefield et al., 1993; Goulding, 2000; Stout et al., 2000). In addition, the rewetting of soil after a dry summer can also cause a flush of mineralisation and cause more nitrate to be leached.
Rainfall soon after N fertiliser application can leach N below the active root zone, and this will be discussed in more detail below.
In tropical areas where monsoon rains occur, the nitrate leaching loss is often greatest during the summer monsoon period rather than during the winter period. Arora et al. (1980) reported greater leaching losses because 400–500 mm of rain fell during the summer monsoon compared with only 100 mm of rain during the winter.
Soil properties
Nitrate leaching losses are generally greater from poorly structured sandy soils than from clay soils because of the slower water movement and the greater potential for denitrification to occur in the clay soils. Pratt et al. (1980) presented data that gave an approximate ratio of leaching loss of 5:1 for a silt loam soil compared to a clay loam soil.
As discussed above, macropores created by earthworms, plant roots or wetting and drying cycles can allow nitrate to be leached quickly through the soil profile and can therefore have a large effect on the rate of nitrate leaching (e.g. Silva et al., 2000). Agricultural drainage systems can also have an effect on the NO3− leaching loss. For example, Scholefield et al. (1993) showed that NO3− leaching increased in the presence of an efficient drainage system, probably because of increased N mineralisation. Drainage systems also shorten the distance that NO3− travels through the soil resulting in faster transport into rivers and lakes. Drainage systems also increase the aeration status of the soil, which decreases the denitrification potential meaning that more nitrogen stays in the form of nitrate.
Soil organic matter content and mineralisation of that organic matter can affect the nitrate leaching loss. Substantial leaching losses can occur from unfertilised bare fallow soil, for example, at Rothamsted Experimental Station in the UK unfertilised bare fallow lysimeters were found to have lost 42 kg N ha−1 year−1 (Miller, 1906; Russell & Richards, 1920; Addiscott, 1988). Low & Armitage (1970) also measured large leaching losses of 102 kg N ha−1 year−1 from bare fallow soil lysimeters, emphasising the increased risk of nitrate leaching when the opportunity for plant uptake is removed.
Land use systems
It is difficult to rank land use and farming systems according to their potential leaching loss because of the differences in soil, climate, fertiliser use and experimental conditions. However, the published data indicate that N leaching losses follow the order of: forests < cut grassland < grazed grassland, arable cropping < ploughing of pasture < horticultural crops (Wild & Cameron, 1980; Di & Cameron, 2002a).
Forests
Nitrate leaching losses are generally lowest from forest systems because: (a) there is usually zero or only low rates of N fertiliser applied; (b) the N is cycled efficiently through the forest ecosystem and (c) because the soils are generally acidic and thus mostly contain ammonium-N rather than nitrate-N. Leaching losses from forest soils may range from about 1 to 15 kg N ha−1 year−1 (Gosz, 1981; Juergens-Gschwind & Follett, 1989; Kiese et al., 2011).
However, harvesting the trees can release large amounts of N that can be leached, or washed off slopes through soil erosion (Smith et al., 1994; Hazlett et al., 2007). Burning of forests can also release large amounts of N that can be leached (Stark, 1977). Hornbeck et al. (1975) reported a total N loss of over 340 kg N ha−1 over a 3-year period following the clear-cutting of a North America hardwood forest. Nevertheless, the C : N ratio of the forest litter and soil dictates the amount of N mineralisation (and thus potential risk of N leaching) that occurs from the forest residues. When the C : N ratio is high the potential leaching loss is low because net immobilisation rather than mineralisation occurs.
Grassland
In general, extensively grazed grassland systems have low nitrate leaching losses (Di & Cameron, 2002a). This is due to rapid plant uptake and immobilisation of N, which keeps the nitrate concentration in soil solution low (Woodmansee et al., 1981) and the low animal stocking density of extensive systems means that there are fewer urine patches per unit area (Moir et al., 2006). Grasslands that are mown or cut for hay or silage also have very low leaching losses (e.g. Di et al., 1998b) because grass and pasture plants are usually very efficient at capturing the nitrogen applied in fertiliser or N fixed by legumes such as clovers that are grown in the pasture sward. A large amount of the N that is applied is removed by pasture plants (300–400 kg N ha−1 year−1) (Di et al., 1998b). Our studies have shown that when 200 or 400 kg N ha−1 is applied in four split applications (either as urea fertiliser or as animal manure) the nitrate leaching losses ranged from 6 to 17 kg N ha−1 year−1 (Silva et al., 1999). However, if the N applied was only split into two rather than four applications, the nitrate leaching loss increased to 13–49 kg N ha−1 year−1 (Di et al., 1998a,b). The actual amounts leached will of course depend on the amount of fertiliser applied as well as the soil and climatic conditions (Steenvoorden et al., 1986; Addiscott, 1996; Wachendorf et al., 2004; Monaghan et al., 2005). Some examples of the amounts of nitrate leaching losses under a range of grassland systems are given in Table 2.
| N Applied (kg N ha−1 year−1) | Soil Texture (Grass Type) | Grazing Animal | Leaching Loss (kg N ha−1 year−1) | Location | Reference |
|---|---|---|---|---|---|
| 0 | Clay loam | Cattle | 30 | NZ | Monaghan et al. (2005) |
| 100 | Clay loam | Cattle | 34 | NZ | Monaghan et al. (2005) |
| 200 | Clay loam | Cattle | 46 | NZ | Monaghan et al. (2005) |
| 400 | Clay loam | Cattle | 56 | NZ | Monaghan et al. (2005) |
| 200 | Clay loam (Old grass) | Beef cattle | 39 | UK | Scholefield et al. (1993) |
| 400 | Clay loam (Old grass) | Beef cattle | 134 | UK | Scholefield et al. (1993) |
| 400 | Clay loam (New grass) | Beef cattle | 56 | UK | Scholefield et al. (1993) |
| 0 | Silt loam | Dairy cows | 25 | NZ | Ledgard et al. (1999) |
| 200 | Silt loam | Dairy cows | 59 | NZ | Ledgard et al. (1999) |
| 400 | Silt loam | Dairy cows | 100 | NZ | Ledgard et al. (1999) |
| 200 | Sandy loam | Dairy cows | 47 | NZ | Silva et al. (1999) |
| 200 | Sandy loam | Dairy cows | 54 | NZ | Silva et al. (1999) |
| 225 | Silt loam | Dairy cows | 57 | NZ | Ledgard et al. (1996) |
| 360 | Silt loam | Dairy cows | 110 | NZ | Ledgard et al. (1996) |
| 200 | Clay | Beef cattle | 39 | UK | Scholefield et al. (1993) |
| 400 | Clay | Beef cattle | 134 | UK | Scholefield et al. (1993) |
| 420 | Loam | Beef cattle | 162 | UK | Ryden et al. (1984) |
| 450 | Loam clay | Beef cattle | 11–48 | UK | Barraclough et al. (1992) |
| 0 | Sandy loam | Sheep | 6–7 | NZ | Ruz-Jerez et al. (1995) |
| 400 | Sandy loam | Sheep | 11–41 | NZ | Ruz-Jerez et al. (1995) |
| 250 | Clay loam | Cattle | 11 | UK | Hood (1976a,b) |
| 750 | Clay loam | Cattle | 54 | UK | Hood (1976a,b) |
The nitrate leaching potential increases when grassland is grazed rather than harvested (Ball & Ryden, 1984; Ryden et al., 1984; Stout et al., 1997; Monaghan et al., 2007). This is because a large proportion (60–90%) of the nitrogen ingested by the grazing animal is excreted back onto the soil in the small concentrated areas of urine and dung patches (Haynes & Williams, 1993; Jarvis et al., 1995). For example, a grazing cow may deposit the equivalent of 700–1200 kg N ha−1 (Haynes & Williams, 1993; Di & Cameron, 2002a) in each urine patch area of around 0.4 m3 (Moir et al., 2012). A cow may urinate 10–12 times per day and thus, depending on stocking rate, these concentrated urine patch areas may cover 20–30% of the grazed field each year (Moir et al., 2011). Some of the urine-N may be volatilised (Sherlock & Goh, 1983, 1984; Di & Cameron, 2000) but most of it will be nitrified into nitrate (Moir et al., 2012) and is thus at risk of leaching if it is not utilised by plants or denitrified (Fraser et al., 1994). Unfortunately, the urine-N loading rate is usually well above the ability of the pasture plants to retain the N (usually less than 600 kg N ha−1 year−1; Moir et al., 2007, 2012) and the excess nitrate remains in the soil until drainage and leaching occurs. The amount of nitrate-N leached from animal urine patches can therefore be very high and losses equivalent to over 120 kg N ha−1 year−1 have been recorded from below urine patches (Silva et al., 1999; Di & Cameron, 2004a,b).
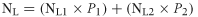 (10)
(10)where NL is the annual average NO3−–N leaching losses from a grazed field, NL1 and NL2 are the leaching losses from the urine and non-urine patch areas, respectively, and P1 and P2 are the proportion of areas covered by urine and non-urine patch areas, respectively. The values of P1 and P2 will vary depending on the stocking rate and the urine patch area coverage.
In the study by Silva et al. (1999), although the leaching loss was 124 kg N ha−1 year−1 directly under the urine patches, the leaching loss from the whole field when calculated according to Eqn 10 was equivalent to 33 kg N ha−1 year−1 (when no N fertiliser was applied). However, the addition of N fertiliser to the field increased the nitrate leaching loss to between 36 and 60 kg N ha−1 year−1, depending on the rate of fertiliser-N applied. Monaghan et al. (2005) showed that in cattle grazed pasture systems the leaching loss increased when the rate of fertiliser increased, with an average of 30, 34, 46 and 56 kg N ha−1 year−1 being leached when 0, 100, 200 and 400 kg N ha−1 year−1 of fertiliser were applied, respectively (Table 2). The effect of rate of N input (fertiliser or clover fixation) on nitrate leaching loss is illustrated in Fig. 3.
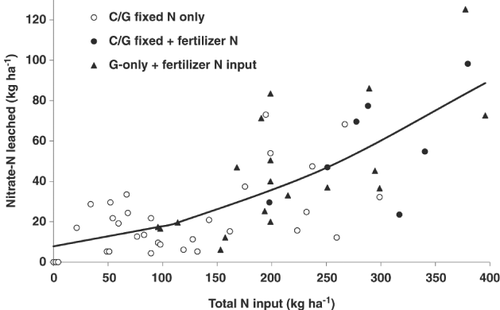
Nitrate leaching losses under sheep grazing are generally lower than under cattle grazing because sheep urine patches contain less nitrogen (equivalent to about 300–500 kg N ha−1) compared to cow urine patches (Di & Cameron, 2002a).
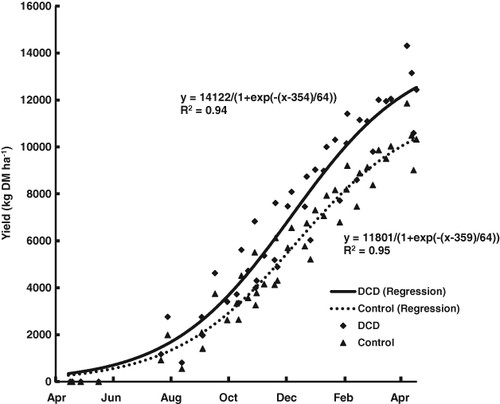
The age and thus ability of plants to capture N from urine and fertiliser has a significant influence on the leaching loss. For example, Jarvis (2000) and Scholefield et al. (1993) reported average annual leaching losses over an 8-year period to be 129 kg N ha−1 year−1 from old grazed pasture receiving 400 kg N ha−1 year−1, whereas the leaching loss was reduced to about 50 kg N ha−1 year−1 when the same rate of N fertiliser was applied to new pasture (this was owing to the greater uptake and growth of the new pasture plants) (Fig. 4).
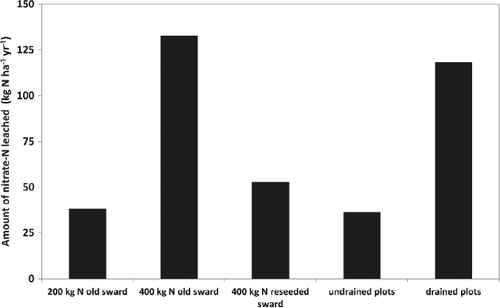
Arable and horticultural crops
There is a large amount of literature that reports leaching losses from cropping soils and examples of the losses are shown in Table 3. Nitrate leaching losses are generally greater from horticultural crops than from arable crops because of the higher rates of N fertiliser that are used in horticultural crops and the shallow root systems of horticultural plants compared to arable plants. However, the loss is also affected by the climate, soil type as well as the fertiliser rate. As shown in Table 3, leaching losses between 4 and 155 kg N ha−1 year−1 have been reported, depending on the rate of N fertiliser applied, soil type and crop rotation system.
| N Applied (kg N ha−1 year−1) | Soil Texture | Cropping Systems | Leaching Loss (kg N ha−1 year−1) | Location | Reference |
|---|---|---|---|---|---|
| 0 | Silty clay loam | Cereal rotation | 8 | UK | Goulding (2000) |
| 144 | Silty clay loam | Cereal rotation | 12 | UK | Goulding (2000) |
| 192 | Silty clay loam | Cereal rotation | 24 | UK | Goulding (2000) |
| 240 | Silty clay loam | Cereal rotation | 43 | UK | Goulding (2000) |
| 288 | Silty clay loam | Cereal rotation | 58 | UK | Goulding (2000) |
| 200 | Loamy sand | Cereal rotation: spring wheat | 17–87 | UK | Shepherd & Lord (1996) |
| 175 | Loamy sand | Cereal rotation: winter wheat | 4–45 | UK | Shepherd & Lord (1996) |
| 200 | Loam | Continuous corn | 11–107 | USA | Bjorneberg et al. (1996) |
| 170 | Loam | Corn-soybean: corn phase | 5–52 | USA | Bjorneberg et al. (1996) |
| None | Loam | Corn-soybean: soybean phase | 5–51 | USA | Bjorneberg et al. (1996) |
| 200 | Silt loam | No-till corn | 8–77 | USA | Baker & Timmons (1994) |
| 125 | Silt loam | No-till corn | 8–36 | USA | Baker & Timmons (1994) |
| 0 | Silt loam | Mixed cropping: autumn ploughing, winter wheat | 14–102 | NZ | Francis et al. (1995) |
| 26 | Sandy loam | Lemons | 46 | USA | Letey et al. (1977) |
| 149 | Sandy loam | Dates | 62 | USA | Letey et al. (1977) |
| 169 | Clay | Cotton | 35 | USA | Letey et al. (1977) |
| 396 | Sandy loam | Corn, carrots | 155 | USA | Letey et al. (1977) |
Goulding (2000) reviewed nitrate leaching losses from arable and horticultural land in the UK and reported that the greatest losses occurred from peas and potatoes as well as from rotational set-aside land (i.e. land that is left in grass and only cultivated occasionally). The lowest leaching losses were reported to occur from sugar beet, grass pasture (with low inputs of N fertiliser) and from winter cereals (Fig. 5).
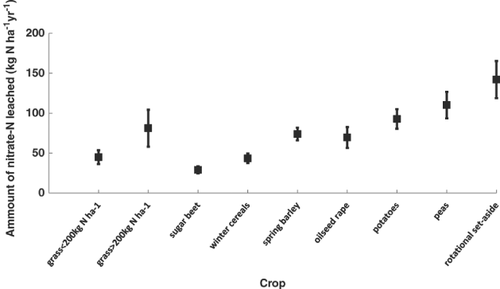
Leaching losses from cropping systems can be high when compared with pasture systems (Francis et al., 1995). Williams (1975) reported that 51 kg N ha−1 year−1 was leached from cultivated cropland compared to only 18 kg N ha−1 year−1 from grassland. In crop rotation systems where pasture is included for only a few years and then ploughed up to establish a cereal crop then leaching losses can be high (Cameron & Wild, 1984; Addiscott et al., 1991; Francis et al., 1992; Whitmore et al., 1992; Shepherd et al., 2001). Francis et al. (1992) reported losses of 60 kg N ha−1 year−1 when a 3-year grass ley was cultivated. Johnston et al. (1994) measured 126 kg N ha−1 in the top 90 cm of soil after cultivation of a 1-year grass ley and 252 kg N ha−1 after cultivation of a 6-year grass ley. Deep borehole studies in England showed that ploughing of grassland was responsible for the release of peak nitrate concentrations that are moving slowly through the unsaturated zone of chalk aquifers (Young et al., 1976).
Mineralisation of soil organic matter and nitrification of residual fertiliser can lead to large leaching losses when there is no crop to remove the nitrate from the soil solution (Cameron & Wild, 1984; Francis et al., 1992; Shepherd et al., 2001).
In organic farming systems the highest nitrate leaching losses have also been found to occur following the ploughing of grass–clover leys because of the large amounts of N that are released by mineralisation of organic-N (Stopes et al., 2002; Eriksen et al., 2004; Andrews et al., 2007). This lack of vegetation for part of the year is a key factor that affects the amount of nitrate leached from cropping soils.
Legumes such as faba beans (Vicia faba L.) can nevertheless fix large amounts of N (up to 100–200 kg N ha−1) and can thus reduce the need for N fertiliser on succeeding crops (Jensen et al., 2010). This in turn can reduce the risk of nitrate leaching as less N fertiliser may be required.
Plant uptake of N during the winter drainage period can reduce the risk of nitrate leaching loss, and cover crops can therefore reduce the risk of N leaching from ploughed or cultivated land (Shepherd & May, 1992; McLenaghen et al., 1996; Eriksen et al., 2004; Hansen et al., 2007)
Plant species can influence the amount of nitrate leaching that occurs from the soil profile. For example, alfalfa has a deep rooting system that is capable of taking up nitrate from considerable depth (up to 3 m), whereas shallow root crops such as potatoes are unable to capture nitrate that is deep in the soil profile and therefore leave large residues of N in the soil that can be leached over the drainage period (e.g. Webb et al., 1997). Brassica crops have been reported to leave more than 300 kg N ha−1 in some soils, most of which can be leached over the next winter (Rahn et al., 1992).
Studies in North America have reported annual leaching losses of 11–107 kg N ha−1 year−1 from corn production soils (Bjorneberg et al., 1996) with much of this loss being attributed to the period of bare fallow before the crop is grown (November to May). Most of the nitrate-N that is leached often comes from the mineralisation of organic-N and remineralisation of fertiliser-N that was immobilised earlier during the previous cropping phase.
Wheat crops remove a large amount of N from the soil each year. Studies in the UK have shown that when 230 kg N ha−1 year−1 of fertiliser was applied to wheat in the spring, 50–80% of the N applied was recovered in the crop, 10–25% was retained in the soil and only about 5% of the N applied was lost by leaching (Macdonald et al., 1989; Addiscott, 1996).
Biomass crops have recently been promoted as an environmentally friendly alternative to fossil fuels, and crops such as maize (Zea mays L.) have been planted for ethanol production (Dale et al., 2010). In 2008, about 30% of all maize grown in the USA was used for ethanol fuel production and this is projected to increase in the future (McIsaac et al., 2010), and the effect of this on the wider environment is now being assessed. McIsaac et al. (2010) have reported average nitrate leaching losses of 40 kg N ha−1 year−1 from a typical maize/soybean (Glycine max L.) system and compared this to losses from switchgrass of just 1.4 kg N ha−1 year−1 when grown in the same location. However, as Christian & Riche (1998) and Cadoux et al. (2012) have shown, nitrate-N losses from biofuel crops such as Miscanthus × giganteus can be significant, with losses of over 60 kg N ha−1 year−1 occurring at fertiliser rates of 120 kg N ha−1 year−1.
Intensive horticultural crop production systems have a high risk of nitrate leaching. High inputs of fertiliser-N, frequent cultivation, short periods of plant growth, high-N-containing crop residues, shallow rooted plants and low nutrient use efficiency all contribute to this high risk of nitrate leaching. Fertiliser-N rates are often as high as 600–900 kg N ha−1 year−1 with plant N recovery as low as 20% (Pionke et al., 1990; Di & Cameron, 2002a). Nitrate leaching losses of 300 kg N ha−1 year−1 have been recorded in some studies (e.g. Spiers et al., 1996) and are typically over 100 kg N ha−1 year−1 (Di & Cameron, 2002a). The nitrate concentration of water draining below market garden crops in sandy soil in Western Australia ranged from 71 to 209 mg N L−1 (Pionke et al., 1990).
There are far less data on nitrate leaching losses from orchard crops compared with vegetable crops. Kramer et al. (2006) reported 80% lower nitrate leaching losses from organically fertilised apple orchard soils compared to those that received conventional N fertilisers.
In wetland rice production systems in Asia, the risk of nitrate leaching is generally low because of the low permeability layer at about 15 cm depth, which has been formed by puddling the soil. However, in coarse textured paddy soils, nitrate leaching losses can be significant (e.g. Singh et al., 1991).
Irrigation
Irrigation water applied over summer does not generally cause leaching (unless excessive amounts of water are applied, which cause drainage events to occur). Because irrigation increases plant growth it also increases N uptake and can therefore effectively decrease the potential for nitrate leaching losses to occur. For example, Hahne et al. (1977) found that optimum irrigation and fertiliser rates reduced N leaching losses from 48% to 5% of the N applied.
However, excessive rates of irrigation can cause leaching to occur. For example, Gheysari et al. (2009) reported the effects of a range of irrigation rates on nitrate leaching and found that over irrigation of maize caused a significant increase in nitrate leaching from 25 kg N ha−1 year−1 for optimum irrigation up to 47 kg N ha−1 year−1 under an irrigation regime that applied water at 1.13 times the soil water deficit. Flood irrigation, in particular, often applies a greater amount of water than can be held in the topsoil and this can cause drainage and leaching to occur.
Fertiliser
Nitrogen fertiliser use efficiency is generally considered to be low (Goulding et al., 2008). For example, data from over 800 experiments have shown that on average only 51% of the fertiliser-N applied to cereal crops was recovered by the plant tops (Chien et al., 2009) and average N fertiliser recoveries can be even lower when applied at high rates. The recovery of fertiliser-N in livestock systems is on average lower than cropping systems with less than 25–30% of the N being recovered in the meat or milk products (Goulding et al., 2008). However, one of the most important findings in recent years is that when N fertiliser is applied at rates that match cereal plant demand there is virtually no residual mineral fertiliser-N left in the soil at harvest (Jenkinson, 2001). Studies using 15N-labelled fertilisers in Europe have shown that only 1–2% of spring-applied fertiliser remains in the soil as mineral-N at harvest (Macdonald et al., 1989). Nitrogen fertilisers applied at rates that match plant demand and at times when the risk of leaching is low can therefore minimise the direct losses of fertiliser-N leaching losses.
Despite the small amount of mineral-N remaining from spring-applied fertiliser, the amount of 15N-labelled fertiliser remaining after harvest in organic forms can represent 15–25% of the N applied (MacDonald et al., 1997). Immobilisation of N fertiliser can occur rapidly in soil (over a few weeks) but this N is released slowly. Hart et al. (1993) reported that 7% of the 15N-labelled organic nitrogen was taken up by the first cereal crop after the labelled fertiliser had been applied, 4% by the second crop, 2% by the third and fourth crops and that 55% of the immobilised 15N remained in the soil at the end of 4 years. It is often the mineralisation of this previously immobilised fertiliser nitrogen and nitrogen remaining in crop residues and plant roots that is leached from the soil over winter.
If excessive rates of fertiliser are used then the residual mineral-N may be leached over the subsequent winter. Jenkinson (2001) reported that 15N-labelled fertiliser experiments show that autumn-applied fertiliser is used far less efficiently than spring-applied fertiliser and can thus be leached more easily from the soil.
The rate of application of N fertiliser can have a direct effect on the nitrate leaching loss. Goulding (2000) has shown that when the N fertiliser rate increases above that required for optimum yield then there is a greater amount of nitrate leached from the soil (Fig. 6).
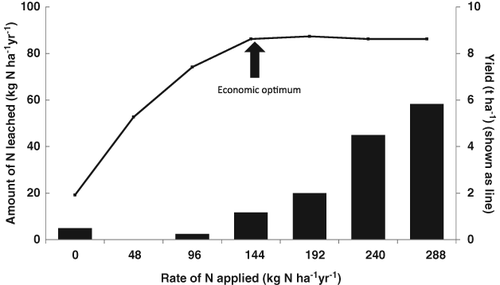
Hood (1976a,b) measured 11 and 54 kg N ha−1 year−1 leached from tile-drained pasture soils in the UK when 250 and 750 kg N ha−1 year−1 were applied to grazed pasture soils. Leaching losses can be higher than this (50–200 kg N ha−1 year−1) on intensively grazed pasture soils where high rates of N fertiliser are combined with high stocking rates (e.g. Horne, 1980; Ball & Ryden, 1984).
Research has shown that even where no N fertiliser is used, the leaching loss is unlikely to be zero as mineralisation of soil organic matter can result in losses of 10 kg N ha−1 year−1 (Goulding, 2000).
However, it is not always just the rate of fertiliser-N applied that dictates the amount of nitrate leaching. Schröder et al. (2010) reported that the ‘N surplus’ (i.e. the difference between the sum of the inputs of plant available-N and N removed in product) was a better predictor of N leaching (r2 = 0.86) compared to simply the total amount of N applied (r2 = 0.11). Our studies have found a quadratic relationship between annual leaching loss and the potentially leachable N (mineral-N and readily mineralisable-N) in the soil (Di & Cameron, 2000).
Organic manures and wastes
Agricultural wastes, industrial wastes and sewage sludge (biosolids) usually contain large amounts of nitrogen, and these wastes are increasingly being applied to land as other forms of disposal become limited (e.g. landfill or disposal into water) (Cameron et al., 1997).
During winter, grazing animals are often moved into barns or onto feed pads to reduce N leaching losses from grazed grassland. However, this creates large quantities of manure or effluent that needs to be stored and applied to land at a later date.
Much of the N in these manures and wastes can be released through mineralisation processes in the soil, which ultimately results in a risk of nitrate leaching (Cameron et al., 1996; Carey et al., 1997). However, it is very difficult to predict the mineralisation rate of these organic wastes and manures because of the variability of the constituent materials (van Kessel & Reeves, 2002). The addition of organic carbon in these wastes may also increase the rate of mineralisation/immobilisation turnover of soil N as well as the applied N (Cameron et al., 1997; Heinrich & Pettygrove, 2012).
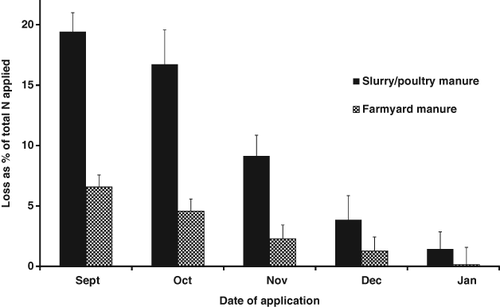
Nitrate leaching losses were reported to be significantly higher from poultry manure or slurry than from straw-based farm yard manure when applied to an arable free draining soil in the UK (Chambers et al., 2000) (Fig. 7). The date of application also affects the risk of leaching loss with the greatest losses occurring when the manure is applied in autumn because the soil is warm enough for mineralisation and there is sufficient rainfall over the autumn and winter period to leach the nitrate out of the soil profile.
Amlinger et al. (2003) reviewed the effects of land application of compost materials and concluded that high application rates should not be used on free draining soils because of the increased risk of nitrate leaching compared to control soil.
The amounts of nitrate leached also depend on the amounts of N applied. For example, Sherwood (1981) compared the leaching losses from pig slurry applied to grassland over a 4-year period at rates of nil, 400, 700 and 1400 kg N ha−1 year−1 and estimated the losses to be 0.9, 18, 77 and 162 kg N ha−1 year−1, respectively.
Nitrate leaching losses from organic farming systems have been reported to be similar, or just slightly lower, than from conventional farms that follow best practice (Kirchmann & Bergström, 2001; Stopes et al., 2002; Goh, 2011).
The amount of nitrate leaching losses from organic manures and wastes depends on: (a) the amount of readily available N in the manure; (b) timing of the application of the manure; (c) soil moisture content and amount of rainfall received soon after application of the manure and (d) N application rate of the manure relative to plant uptake (Chambers et al., 2000).
Methods to reduce nitrate leaching losses
- Applying the correct amount of N fertiliser to meet plant demand and reducing excessive rates of N input;
- Applying accurate rates of N fertiliser and using split applications of N fertiliser;
- Using a nitrification inhibitor to slow down the rate of nitrate produced in the soil from animal urine, fertilisers and manures;
- Using a spray application of nitrification inhibitor in grazed pasture systems;
- Renewing pasture swards frequently to maximise the plant uptake of N;
- Maintaining an active plant species over the drainage period (e.g. using a cover crop in arable systems);
- Sowing crops early to avoid large periods of bare fallow;
- Cultivating the soil in spring rather than autumn to avoid leaching losses of N mineralised from the soil in autumn;
- Maximising plant uptake of N, for example, by applying optimum rates of irrigation to boost plant growth, controlling disease and pest damage and applying lime and other nutrients to optimise plant growth;
- Applying nitrogen fertilisers and animal slurries at times when the risk of leaching is low and
- Applying animal manures at rates and times that match plant N demand.
Of these methods, the use of nitrification inhibitors has probably received the most attention in recent years. For example, our research has shown that spray application of nitrification inhibitors onto grazed pasture soils can significantly reduce NO3− leaching losses (and N2O emissions) (Di & Cameron, 2002b, 2004c, 2005, 2012; Cameron et al., 2007; Di et al., 2007, 2009a). Nitrification inhibitors are specific competitive enzyme inhibitors that inhibit the AMO enzyme. The nitrification inhibitor dicyandiamide (DCD) interferes with the cytochrome oxidase in the respiratory electron transport system of Nitrospira and Nitrosomonas bacteria (O'Connor et al., 2012), and this slows down the first stage of nitrification involved in the conversion of NH3 into hydroxylamine (NH2OH) and thus reduces the rate that NH4+ is converted into NO3− in the soil (Amberger, 1989; Zerulla et al., 2001; Di et al., 2009b; Andrews et al., 2011; Di & Cameron, 2011). Ammonium is a positively charged ion (cation) and is thus adsorbed onto the negatively charged cation exchange sites on soil clays and organic matter. This protects the NH4+ ions from leaching and allows it to be taken up by plants or to be immobilised into soil organic matter. Because nitrate is an anion it is not held by the negatively charged soil cation exchange complex and is therefore easily leached from the soil if it is not taken up by plants, immobilised or denitrified.
Our research using the nitrification inhibitor DCD found that nitrate leaching losses were reduced by 76%, from 85 to 20 kg N ha−1 year−1 from cow urine patches (Di & Cameron, 2004c) (Fig. 8). Research by others has confirmed that DCD can significantly reduce nitrate leaching losses from grazed pasture soils (e.g. Menneer et al., 2008a,b; Monaghan et al., 2009). Recent research in Ireland has also shown that application of DCD can reduce nitrate leaching by about 40% (Dennis et al., 2012).
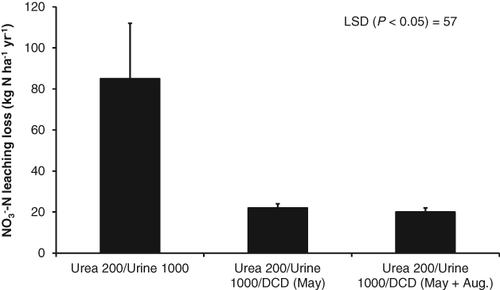
A concomitant effect of the nitrification inhibitor retaining more N in the soil/plant system is the potential to increase plant growth. Our recent research has found that application of DCD can increase pasture growth by over 10% and in some cases by over 20% per year (Di & Cameron, 2002b, 2005, 2007; Moir et al., 2007, 2012; Carey et al., 2012) (Fig. 9). Yield responses in animal urine patches from DCD application have been associated with increases in annual N uptakes of 27–37% and 46% for autumn- and spring-deposited urine, respectively (Moir et al., 2007, 2012).
Nitrification inhibitors have also been used to reduce nitrate leaching in crop production systems. For example, Cui et al. (2011) have shown that the nitrification inhibitor DCD can significantly reduce nitrate leaching losses by over 50% (and nitrous oxide emissions by over 80%) from high inputs of N fertiliser (650 kg N ha−1 year−1) applied in intensive vegetable crop production systems. Munoz et al. (2005) have also described the opportunity that nitrification inhibitor-treated fertiliser can reduce nitrate leaching losses from intensive potato growing systems. Quiñones et al. (2009) reported that the use of a nitrification inhibitor (3,4-dimethylpyrazole phosphate) in drip irrigated citrus orchard could significantly reduce the nitrate leaching loss from N fertiliser. Slow release polymer-coated urea fertilisers are also now being developed to match the N release rate with plant demand and thus reduce the potential leaching loss (Venterea et al., 2011).
Also, in recent years, the addition of ‘biochar’, from the pyrolysis of biomass, has been promoted to sequester C, improve soil productivity and reduce nitrate leaching losses (e.g. Atkinson et al., 2010; Schomberg et al., 2012). Some studies have reported that biochar can reduce nitrate leaching losses (Ding et al., 2010; Laird et al., 2010), whereas others have found little evidence of decreased nitrate leaching with the addition of biochar (Schomberg et al., 2012). The effect of biochar on N cycling in soil depends on the feedstock used to produce the biochar, the pyrolysis conditions and soil type (Chan et al., 2007; Novak et al., 2009). Thus, further work is needed before widespread use of biochars in agriculture should occur.
Emissions of nitrous oxide and dinitrogen gas
Impacts of nitrogen gas emissions
Gaseous losses of nitrogen (mainly N2 and N2O) can represent a significant loss of N from the soil/plant system (Fig. 1). The emission of nitrous oxide gas also contributes to climate change and depletion of the ozone layer. The concentration of N2O has increased from 270 parts per billion (ppb) in the preindustrial period to around 320 ppb today (IPCC, 2007). Nitrous oxide has a large radiative-forcing potential, with the long-term warming potential about 300 times greater than carbon dioxide. Approximately, 62% of total global N2O emissions are attributed to emissions from agricultural soils (4.2 Tg N year−1) and non-agricultural land (6 Tg N year−1) (Thomson et al., 2012). The remainder comes from oceans and other water bodies, power plants and vehicles. Terrestrial emissions are mainly due to the process of nitrification and denitrification. There is therefore a large scientific effort being devoted worldwide to improve our understanding of these processes and to develop methods to reduce these emissions.
Processes
Nitrification
The nitrification process described in Eqns 2 and 3 can be expanded to show that the oxidation of ammonium can also produce nitrous oxide gas (adapted from Kool et al., 2009):
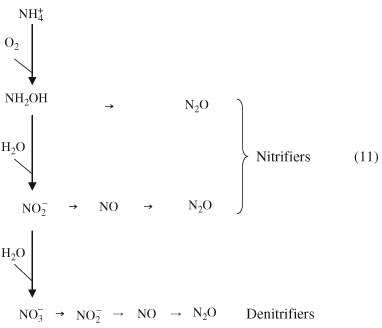
The activity of AOB such as Nitrosospira and Nitrosomonas can therefore produce N2O from NH4+ and from NH2OH (which is an intermediary compound shown in Eqn 11; Bremner, 1997; Saggar et al., 2009). It is thought that the intermediate compound nitroxyl (HNO) may dismutate chemically under low O2 concentrations to N2O, or that the nitrate reductase enzyme may produce N2O when O2 concentrations are low and NO2− replaces O2 as the terminal electron acceptor during metabolic processes (Schmidt, 1982; Haynes et al., 1986). The first article that showed that nitrification could produce N2O was published by Corbet (1935).
Denitrification
Biological denitrification mainly occurs in poorly drained soils where there are anaerobic soil conditions, low oxygen availability and low redox conditions (Firestone, 1982; Dobbie & Smith, 2001; Bateman & Baggs, 2005). Under these circumstances, facultative anaerobic bacteria can use NO3−, instead of oxygen, as the terminal electron acceptor during respiration. This causes NO3− to be reduced producing, in turn, nitrite, nitric oxide (NO), nitrous oxide and finally dinitrogen, as illustrated in the following equation:
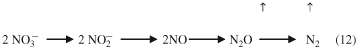
The enzymes responsible for the reduction are NO3− reductase, NO2− reductase, NO reductase and N2O reductase. These enzymes are associated with most denitrifying bacteria; however, some bacteria are only able to complete part of the reduction (i.e. they are partial denitrifiers).
The amounts of NO gas that are emitted from soil are generally low, but under field conditions significant amounts of nitrous oxide gas can be emitted before it can be converted into dinitrogen. There is currently considerable research interest in the effect of NO on plant development, metabolism and disease responses (Gupta et al., 2011). In addition to the NO produced within the plant itself, NO emissions from the soil may also affect plant functions.
Although denitrification is generally highest under anaerobic conditions it can also occur in soils that are not completely water logged and can also occur within soil aggregates, where oxygen diffusion rates are slow and the soil redox potential is low. Highest denitrification losses generally occur in situations where there are high concentrations of NO3− in soil solution, a readily available source of carbon and the soil temperature is sufficiently warm for microbial activity to occur (Firestone, 1982; Saggar et al., 2009).
Fungal denitrification can also occur in soils, and as shown by Shoun et al. (1992) and Thomson et al. (2012), although most fungal-denitrifying activity reduces only NO2− to N2O, some fungi can reduce both NO2− and NO3− to N2O (Shoun & Tanimoto, 1991). In wet soils, anaerobic ammonium oxidation, called ‘anammox’, can also occur, which involves ammonium being converted directly into N2O and N2 gas (Hayatsu et al., 2008; Thomson et al., 2012). In addition, N2 and/or N2O can also be produced through chemical reactions between nitrous acid (HNO2) in soil solution and soil organic matter, ammonium, NH2OH and compounds containing free amino groups (such as amino acids, urea and amines).
The factors that affect denitrification have been reviewed previously (e.g. Firestone, 1982; Haynes & Sherlock, 1986; Bouwman et al., 1993; Bouwman, 1996; Bremner, 1997; Freney, 1997; Mosier et al., 1998; de Klein et al., 2001; Bolan et al., 2004; Clough et al., 2005; Saggar et al., 2009; Thomson et al., 2012) and examples of N2O emissions are given in Table 4.
| N Input (kg N ha−1 year−1) | Soil Type | N Source | N2O Emission (% of N Input) | Location | Reference |
|---|---|---|---|---|---|
| 150 (3 × 50) | Silt loam | Urea | 0.56 | New Zealand | Luo et al. (2007) |
| 150 (3 × 50) | Red brown earth | Urea | 0.47 | Australia | Galbally et al. (2005) |
| 120 (4 × 30) | Sandy silt loam | Calcium ammonium nitrate | 0.3 | Germany | Anger et al. (2003) |
| 435 | Peat I | Calcium ammonium nitrate | 2.3 | Netherlands | Koops et al. (1997) |
| 1000 | Stony silt loam | Cow urine | 2.2 | New Zealand | Di & Cameron (2003) |
| 1000 | Stony silt loam | Cow urine | 1.9 | New Zealand | Di & Cameron (2006) |
| 1000 | Sandy loam | Cow urine | 3.1 | New Zealand | Di & Cameron (2006) |
| 1000 | Sandy loam | Cow urine | 2.0 | New Zealand | Di et al. (2007) |
| 1000 | Silt loam | Cow urine | 0.8 | New Zealand | Di et al. (2007) |
| 1000 | Silt loam | Cow urine | 0.6 | New Zealand | Di et al. (2007) |
| 1000 | Silt loam | Cow urine | 0.1 | New Zealand | Di et al. (2007) |
| 1000 | Sandy loam | Cow urine | 2.0 | New Zealand | Di et al. (2010b) |
| 1000 | Silt loam | Cow urine | 1.9 | New Zealand | Di et al. (2010b) |
| 1000 | Red brown earth | Cow urine | 0.5 | Australia | Galbally et al. (2005) |
| 700 (Spring) | Poorly drained clay loam | Cow urine | 0.0 | England | Allen et al. (1996) |
| 700 (Autumn) | Poorly drained clay loam | Cow urine | 1.5 | England | Allen et al. (1996) |
| 1650 | Poorly drained clay loam | Cattle dung | 0.1–0.7 | England | Yamulki et al. (1998) |
| 1000 | Clay | Synthetic urine | 1.9 | New Zealand | Clough et al. (1998) |
| 1000 | Peat | Synthetic urine | 1.9 | New Zealand | Clough et al. (1998) |
| 1000 | Sandy loam | Synthetic urine | 0.8 | New Zealand | Clough et al. (1998) |
| 1000 | Silt loam | Synthetic urine | 1.0 | New Zealand | Clough et al. (1998) |
Soil moisture and aeration
Changes in soil moisture content affect the aeration status of the soil and this influences the denitrification rate. In particular, when the moisture content of the soil is greater than field capacity there is a significant increase in the potential denitrification rate (Bremner & Shaw, 1958; Haynes et al., 1986; Mosier et al., 1986; Bouwman, 1996; de Klein & van Logtestijn, 1996; Müller & Sherlock, 2004; Saggar et al., 2009). Denitrification losses are therefore greatest during the late-autumn, winter and early-spring periods when soil moisture contents are highest. However, heavy rainfall or irrigation can also cause denitrification at other times of the year (Di & Cameron, 2003; Phillips et al., 2007). Soil texture influences the drainage rate of the soil, and thus the amount of denitrification loss is often greater in clay soils than in free draining sandy soils.
The reductase enzymes that operate in the early part of the reaction sequence shown in Eqn 12 appear to be less sensitive to the availability of O2 than the later reductase enzymes, so that when O2 concentrations are high N2O is produced, whereas at low O2 levels N2 gas is produced in greater quantities.
Nitrate and ammonium
The availability of N (NH4+ and NO3−) has a large influence on the denitrification rate with emissions generally increasing resulting in increases in mineral-N availability (Haynes & Sherlock, 1986; Ball et al., 1997; Ambus, 2005; Saggar et al., 2009). In particular, the application of N fertiliser and animal excreta (urine and dung) increases the mineral-N status of the soil and can induce very large increases in denitrification (de Klein et al., 2001; Di & Cameron, 2003; Di et al., 2007). The proportion of the N fertiliser or excreta that is emitted as N2O is called the ‘emission factor’ (IPCC, 2007) and this value is used in calculations of greenhouse gas emissions by individual countries. For fertiliser, this value is usually in the range of 0.1–2.0%, although it can be higher under some circumstances (de Klein et al., 2001). For animal manures, the emission factor value generally ranges from 0% to 5% of the amount of manure N applied (de Klein et al., 2001). Animal excreta that is deposited during grazing can apply relatively large amounts of N within discrete urine or dung patches and this can produce large amounts of N2O and N2 emissions (generally within the range of 0.1–4% of the N applied) (de Klein et al., 2001). The default IPCC emission factor for N2O emissions from urine deposited on grazed pasture soil is 2%; however, some countries such as New Zealand have a country-specific emission factor of 1% based on detailed inventory measurements (de Klein et al., 2001).
Carbon
It is well established that there is a strong relationship between readily available organic carbon in soil and the denitrification rate (Bremner & Shaw, 1958; Burford & Bremner, 1975). Thus, land management practices that increase the amount of readily available organic carbon (such as application of organic wastes or animal excreta deposited during grazing) stimulate the denitrification process (Cameron et al., 1997; Chadwick, 1997; de Klein et al., 2001; Di & Cameron, 2003). The addition of organic carbon to soil not only stimulates microbial growth and respiration but also provides the organic carbon needed by the soil denitrifiers. Taghizadeh-Toosi et al. (2011) have recently shown that the incorporation of biochar into soil can suppress in situ N2O emissions.
Soil pH
Soil pH influences both the nitrification rate and the denitrification rate and therefore has an influence on the amount of N2O and N2 gas emitted (Parkin et al., 1985; Gödde & Conrad, 2000). The N2O/N2 + N2O product ratio is influenced by soil pH and increases with acidity (Thomson et al., 2012). Therefore, acid soils with a pH <5.0 have lower denitrification rates than agricultural soils with a typical pH around 6.0 (Haynes & Sherlock, 1986). Increasing soil pH can also influence the ratio of N2O to N2 emitted (Saggar et al., 2009).
Temperature
The denitrification rate increases with temperature (Ryden, 1986; Dobbie & Smith, 2001). For example, de Klein & van Logtestijn (1996) found that the denitrification rate increased 10-fold in a grassland soil when temperature increased from 10°C to 20°C. Nommik & Larsson (1989) also found that denitrification increased 10 to 20-fold in a forest soil when soil temperatures increased from 6°C to 21°C. Dobbie & Smith (2001) as well as de Klein & van Logtestijn (1996) showed that the effect of soil temperature was greatest on non-irrigated dry soil compared to irrigated soil.
Methods to reduce N2O losses
Mitigation of nitrous oxide and dinitrogen gas emissions can be achieved by reducing the nitrification rate in the soil and reducing the potential for denitrification to occur (Smith et al., 1997; de Klein et al., 2001; Di & Cameron, 2002b, 2003; Vergé et al., 2007; de Klein & Eckard, 2008; Saggar et al., 2009; Thomson et al., 2012).
Soil physiochemical properties such as pH, organic matter content, N-content, bulk density and aeration, all influence the potential for denitrification to occur (Thomson et al., 2012). It has, therefore, been suggested that increasing the pH of agricultural soils by applying lime should reduce N2O emissions (Thomson et al., 2012). Results from Bakken et al. (2012) demonstrate that increasing soil pH could be a possible soil management option to reduce N2O emissions. However, Clough et al. (2004) found that this was highly dependent on the soil moisture content with a reduction in N2O emissions occurring when lime was applied at a soil moisture content close to field capacity and an increase in emissions when soil water content was at saturation.
It has also been suggested that increasing the amount of available copper in the soil could enhance the activity of the nitrous oxide reductase enzyme, and thus increase the relative amount of N2 emitted (Thomson et al., 2012).
Another approach that has been suggested to reduce N2O emissions is to engineer crop plants to fix nitrogen themselves and thus reduce the need for nitrogen fertilisers (Beatty & Good, 2011). It may also be possible to manipulate denitrification through inputs into the plant rhizosphere (Thomson et al., 2012).
Sustainable agricultural practices can also help to reduce the risk of N2O emissions by ensuring that the N supply is better matched to plant needs.
Slow release fertilisers and fertilisers coated with nitrification inhibitors can reduce the N2O loss (Velthof et al., 1996; McTaggart et al., 1997; Mosier et al., 1998; Shaviv, 2000; de Klein et al., 2001; Dobbie & Smith, 2001; Saggar et al., 2009; Hyatt et al., 2010). However, because these fertilisers are more expensive than regular fertilisers they have not been widely adopted in agriculture.
In grazed pastures, the vast majority of the N2O originates from animal urine patches rather than from fertilisers, making it more difficult to reduce N2O emissions. Recently, however, our research has shown that spray application of the nitrification inhibitor DCD is a very effective method to reduce N2O emissions from grazed pasture soil (Di & Cameron, 2002a,b, 2003, 2006, 2008; Di et al., 2007, 2010b). For example, Di et al. (2010b) reported that DCD reduced the N2O emission from a cow urine patch by 81% (Fig. 10). The DCD inhibited the activity of the AOB, which reduced the nitrification rate and thus reduced the potential for N2O production (Di et al., 2010b).
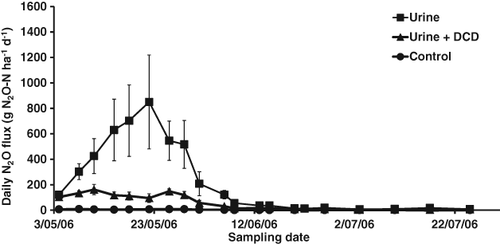
The effectiveness of this method of use of a nitrification inhibitor has been evaluated by other researchers. A recent review of published results by de Klein et al. (2011) reported that the average reduction in N2O emissions from animal urine patches, achieved by the use of the nitrification inhibitor DCD, was 59%. This nitrification inhibitor technology is now recognised by the IPCC as a viable agricultural greenhouse gas mitigation technology and its effect is included when calculating the New Zealand greenhouse gas inventory (Clough et al., 2007; Ministry for the Environment, 2009).
It has recently been reported that urease inhibitors, as well as reducing ammonia volatilisation, may also reduce N2O emissions (Zaman et al., 2008; Sanz-Cobena et al., 2012; Smith et al., 2012). The study by Smith et al. (2012) examined N2O emissions from a number of UK wheat crop and grassland sites treated with different N fertilisers including urea treated with a urease inhibitor (NBPT). Although results varied considerably between sites and seasons, N2O emissions from urea treated with urease inhibitor were lower than corresponding emissions from nitrate fertiliser forms, except under conditions where emissions were generally low. The results suggested that the use of urease inhibitors could provide some mitigation of gaseous emissions, with both reduced ammonia losses to the atmosphere compared with untreated urea, and reduced N2O emissions compared with urea or ammonium nitrate (Smith et al., 2012). However, Zaman et al. (2009) found that there was no consistent effect of a urease inhibitor on N2O emissions.
In addition to examining the effects of urease inhibitor on N2O emissions, Sanz-Cobena et al. (2012) also examined the use of combined urease and nitrification inhibitors. The results of these studies were quite variable and somewhat complex. Although treatments with urease inhibitor generally reduced N2O emissions, the extent of mitigation varied greatly with soil moisture content, climatic conditions and management factors, particularly irrigation. Effects of the combined urease and nitrification inhibitor treatments were even more variable, and the combination of inhibitors had a smaller mitigation effect on N2O emissions than urease inhibitor alone in 1 year and a much larger effect in the following year. The authors suggested that under drier nitrifying conditions there could be a negative interaction between the two inhibitors, thereby reducing the efficiency of the urease inhibitor (NBPT). Further work, therefore, appears to be needed to establish the reliability of using a urease and nitrification inhibitor combination to reduce N2O emissions.
- Improving fertiliser use efficiency to reduce excessive levels of mineral-N accumulating in the soil;
- Using lime to increase soil pH, in some soils;
- Matching fertiliser and manure applications to the rate of plant growth;
- Using optimum rates of irrigation to avoid creating anaerobic conditions;
- Using drainage to increase soil aeration;
- Avoiding compaction of the soil by animals or traffic and
- Using nitrification inhibitor technology.
Conclusions
Losses of nitrogen from the soil/plant system cause adverse impacts on the environment as well as reduce soil fertility and plant yield.
Ammonia volatilisation losses from fertiliser can range from 0% to 65% of the N applied, depending on soil and environmental conditions. Methods to reduce ammonia volatilisation include: coating the fertiliser with a urease inhibitor or polymer; applying the fertiliser during, or immediately before, rainfall or irrigation or incorporating the fertiliser into the soil.
Nitrate leaching losses into rivers, lakes and groundwater and associated legislation to reduce these losses have become a major constraint on agricultural land use in many countries. The amount of nitrate lost from soil depends on the season and climate, soil properties, farming practices and land management system. The published data indicate that N leaching losses follow the order of: forests < cut grassland < grazed grassland, arable cropping < ploughing of pasture < horticultural crops.
Methods to reduce nitrate leaching losses include: applying a nitrification inhibitor to the soil to slow down the rate of nitrate production; using a nitrification inhibitor-coated fertiliser; optimising the N application rate to meet plant demand and reduce the amount of excess N left in the soil at the end of the season; renewing pasture swards frequently to maximise plant N uptake; sowing crops early to avoid periods of bare fallow when there is no plant uptake; cultivating in spring rather than in autumn; planting a cover crop over winter and applying animal manures at times and rates that match plant demand.
Nitrous oxide emissions contribute to climate change and to the depletion of the ozone layer. The amounts of nitrous oxide emitted depend on soil and environmental conditions as well as land management. The highest emissions occur from warm, wet (anaerobic) soil that has a high content of available N and carbon. In grassland systems, the greatest emissions occur from animal urine patches and in cropping soils from excessive rates of application of N fertiliser or animal manures.
Methods to reduce nitrous oxide emissions include: applying a nitrification inhibitor to the soil to treat animal urine patches; using a nitrification or urease inhibitor-coated fertiliser; optimising the N application rate to meet plant demand; optimising application rates of irrigation water to avoid creating anaerobic conditions and avoiding compaction of the soil.
This review has shown that careful management of the soil/plant system using best management practices and newly developed technologies can increase the sustainability of the farming system and reduce the impact of agriculture on the environment.
Acknowledgements
We are grateful to Dr Barbara Brown, Prof. Ron McLaren and Amal Torky for invaluable assistance with the text and the references. We are also grateful to Stephen Moore for assistance with the figures.




