Factors affecting accuracy of clinical staging in resectable non-small cell lung cancer in a real-world study
A La Woo, Seung Hyun Yong, Youngmok Park, Song Yee Kim, Eun Young Kim, Ji Ye Jung, Young Ae Kang, and Moo Suk Park contributed equally to this work.
Seong Yong Park and Sang Hoon Lee are two corresponding authors equally contributed to this work.
Abstract
Background
The clinical staging of non-small cell lung cancer (NSCLC) is well known to be related to their prognosis. However, there is usually a discrepancy between clinical staging and pathological staging. There are few analyses of clinical staging accuracy in patients with NSCLC. We compared the concordance rate between clinical and pathological staging of NSCLC and evaluated factors affecting the accuracy in real-world data.
Methods
Altogether, 811 patients with primary NSCLC who had undergone curative lung resection surgery in Severance Hospital from January 2019 to December 2020 were retrospectively reviewed. We used the eighth edition of the American Joint Committee on Cancer TNM staging.
Results
Among 811 patients, endobronchial ultrasound (EBUS) and positron emission tomography (PET-CT) were performed in 31.6% and 96.7%, respectively. The concordance rates between clinical and pathological TNM staging, T factor, and N factor, were 68.7%, 77.7%, and 85.8%, respectively. With multivariable logistic regression analysis, current smokers (OR 0.49; 95% CI: 0.32–0.76, p = 0.001) and a higher clinical stage (p < 0.001) contributed to the clinical staging inaccuracy. Additionally, the presence of a bronchoscopy specialist was significantly associated with clinical staging accuracy (OR 1.53; 95% CI: 1.10–2.13, p = 0.011).
Conclusion
Clinical staging accuracy in NSCLC improved compared to before the widespread use of PET-CT and EBUS in clinical staging work-up. Smoking history and absence of expert bronchoscopy specialists showed a meaningful correlation with the inaccuracy of clinical staging. Thus, training more bronchoscopy experts would improve the staging accuracy of NSCLC, which could positively affect the prognosis of NSCLC.
INTRODUCTION
With an estimated 1.8 million deaths per year, lung cancer is the most frequently occurring cancer and the leading cause of cancer death worldwide.1, 2 The definitive treatment of choice for patients with early-stage lung cancer is surgery for curative aim. Meanwhile, patients with advanced-stage lung cancer are treated with systemic therapy, such as chemotherapy and/or radiotherapy for palliative aim.3 The treatment plan for non-small cell lung cancer (NSCLC) patients, especially those who are contraindicated for lung resection surgery or invasive procedures due to poor lung function, is highly dependent on the clinical stage of the disease.
Accurate clinical staging of NSCLC is crucial not only for determining the appropriate treatment but also for predicting the patient's prognosis. Depending on the extent, the 5-year survival rate for localized-stage NSCLC is 57%, while the overall 5-year survival rate for all stages of lung cancer is only 5%. The survival rate for each stage of NSCLC varies significantly, with 5-year survival rates of 54%, 35%, 10%–15%, <5%, and <2% for stages I, II, IIIA, IIIB, and IV, respectively.1, 4, 5 Therefore, precise clinical staging of NSCLC is essential for providing patients with accurate information about the disease's progression and the potential outcomes of treatment. Moreover, it can help prevent unnecessary morbidity and mortality associated with inappropriate treatment.
The TNM classification, developed by Dr Pierre Denoix, has undergone several revisions by the Union for International Cancer Control and the American Joint Committee on Cancer, with the latest being the eighth edition in 2017 based on international databases.6 According to guidelines, the clinical staging of NSCLC involves a combination of multiple imaging studies and invasive procedures. Although each diagnostic method has high sensitivity and specificity,7, 8 the overall accuracy of clinical staging has been reported to be low, ranging between 50% and 60%, compared to pathological staging as the gold standard.9-11 Moreover, no recent real-world studies have investigated the accuracy of clinical staging since the widespread use of positron emission tomography-computed tomography (PET-CT) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) for the clinical staging work-up of lung cancer.12, 13 Additionally, the expertise of the bronchoscopy specialist performing EBUS-TBNA may affect the diagnostic yield,14, 15 but no studies have explored its impact on staging accuracy.
In the present study, we examined the concordance rate between the clinical and pathological stages, with PET–CT and EBUS–TBNA in clinical staging work-up, using data from a 2500-bed tertiary hospital in South Korea. Also, we tried to evaluate the influence of a bronchoscopy specialist and clinical factors that contribute to the accuracy of clinical staging by assessing the characteristics that increase the accuracy of clinical staging in resectable NSCLC patients.
METHODS
Study design and patients
A total of 1218 patients who had undergone thoracic surgery in Severance Hospital from January 2019 to November 2020, were retrospectively reviewed. We included primary NSCLC patients with resectable clinical staging (clinical TNM stage IA–IIIA). We excluded patients with other primary cancers and those who underwent surgery for palliative aim. Among them, only 943 patients had primary NSCLC, and 132 were excluded because their pathological staging was either evaluated after neoadjuvant therapy or not thoroughly evaluated because the aim of surgery was palliative (Figure 1).
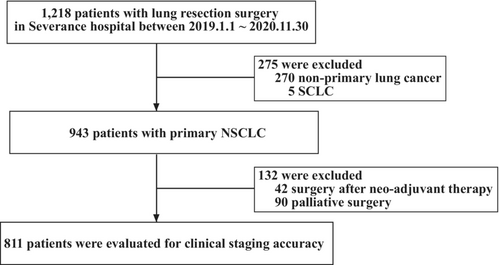
A total of 811 patients with primary NSCLC had undergone curative lung resection. Their clinical data (age; sex; smoking history; and underlying diseases such as hypertension, diabetes mellitus [DM], chronic obstructive pulmonary disease [COPD], and dyslipidemia), results of EBUS, PET, and brain magnetic resonance imaging (MRI), clinical and pathological TNM stage, and histologic subtype were retrospectively evaluated. We classified T1a, T1b, and T1c as T1, and T2a and T2b were classified separately in the accuracy analysis of the tumor (T) factor because T1a to T1c do not affect the TNM stage, while T2a and T2b affect the TNM stage.
In our study, EBUS and/or EUS were performed for invasive mediastinal lymph node staging for cN1-3 disease by CT, cN2-3 disease by PET, and central tumors with grade 1 evidence.7 Additionally, EBUS and/or EUS were not performed in patients with peripheral tumors of 3 cm with cN0 disease by CT and PET, in accordance with recommended practice guidelines for mediastinal staging in NSCLC by CHEST.7 However, patients for whom upfront surgery is deemed necessary based on the attending physician's or surgeon's decision underwent surgical mediastinal staging instead of preoperative EBUS and/or EUS, even though they are included in the aforementioned criteria.
Definitions
The current study defined a bronchoscopy specialist14 as a pulmonologist who performs more than 500 cases of flexible bronchoscopy and EBUS-TBNA annually. The bronchoscopy specialist, whose role was focused on lung cancer diagnosis and staging, started working at the institution in 2020. Before then, five pulmonologists performed a bronchoscopy, and they were not satisfied with the definition of bronchoscopy specialist described above. In this study, patients were divided into two groups according to the date of EBUS-TBNA performance: before 2020 and after 2020, when a bronchoscopy specialist was introduced. We then compared the accuracy of clinical staging between the two groups, which could reflect the influence of a bronchoscopy specialist somewhat indirectly.
Pathological staging was considered the gold standard in this study. When the clinical stage is lower than the pathological stage, it is defined as “underestimation” regardless of the extent of discrepancy. When the clinical stage is higher than the pathological stage, it is defined as “overestimation”.11
All patients underwent a uniform staging protocol (eighth edition of the AJCC TNM classification for NSCLC revised in 2018).6 The clinical diagnosis was determined based on the chest CT, PET–CT, brain MRI, and EBUS–TBNA results. The clinical N factor of patients who did not undergo EBUS–TBNA was determined based on PET–CT results, while the clinical N factor of patients who underwent EBUS–TBNA was determined based on EBUS–TBNA results regardless of PET–CT. If there was uncertainty in the clinical staging despite the imaging test and bronchoscopy results, the clinical stage was determined by a multidisciplinary team (MDT) consisting of a pulmonologist, thoracic radiologist, thoracic pathologist, thoracic surgeon, oncologist, and radiation oncologist. All patients underwent mediastinal lymph node dissection in the paratracheal, subaortic, subcarinal, hilar, lobar, and peribronchial lymph nodes, as well as lymph nodes with significant fluorodeoxyglucose (FDG) uptake in PET–CT during lung resection surgery. Patients whose clinical and pathological TNM stages were concordant were defined as a group “cTNM = pTNM” and others as a group “cTNM≠pTNM”. Those with concordant clinical and pathological TNM stage with disconcordant T factors (e.g., cT1cN1M0 [cIIB] and pT2aN1M0 [pIIB]) were classified as “cTNM = pTNM” in TNM staging analysis and “cT ≠ pT” in T factor analysis.
Statistical analysis
To evaluate the accuracy of clinical staging for NSCLC, we conducted a comparative analysis by assessing the agreement between clinical and pathological staging for TNM staging, T factor, and N factor, respectively. Categorical variables are presented as a percentage, and we used Pearson χ2 tests to compare the frequencies between the two groups. For continuous variables, the results are expressed as mean ± SD and used the student's t-test to compare the means of the two groups.
We used a univariate logistic regression model to assess whether multiple variables were correlated independently with the concordance rate of clinical and pathological staging in NSCLC. The factors significantly (i.e., p < 0.05) associated with clinical staging accuracy in univariate analysis were subsequently assessed to determine whether they independently affected the clinical staging accuracy using multivariable logistic regression analysis. An adjusted p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 26 (SPSS).
The present study protocol was reviewed and approved by the Institutional Review Board and Ethics Committee of Severance Hospital (IRB no.: 4-2022-0105).
RESULTS
Patient population
A total of 811 patients (446 men, 365 women) with a mean age of 64.2 ± 9.5 years were reviewed. Altogether, 392 patients (48.3%) were smokers whose mean smoking amount was 17.3 ± 24.1 pack–years. The number of patients who underwent EBUS, PET–CT, and brain MRI in their clinical staging work-up was 256 (31.6%), 784 (96.7%), and 525 (64.7), respectively. Adenocarcinoma (671 patients, 82.7%) was the most frequent type of tumor histology, followed by squamous cell carcinoma. Most patients had clinical stage I NSCLC (670 patients, 82.6%) (Table 1).
| All patients (N = 811) | cTNM ≠ pTNM (n = 253) | cTNM = pTNM (n = 558) | p-value | |
|---|---|---|---|---|
| Age (years) | 64.2 ± 9.5 | 63.8 ± 10.2 | 64.4 ± 9.2 | 0.432 |
| Sex | <0.001 | |||
| Male | 446 (55.0) | 162 (64.0) | 284 (50.9) | |
| Female | 365 (45.0) | 91 (50.9) | 274 (49.1) | |
| Smoking history | <0.001 | |||
| Nonsmoker | 419 (51.7) | 107 (42.3) | 312 (55.9) | |
| Smoker | 392 (48.3) | 146 (57.7) | 246 (44.1) | |
| Former | 267 (32.9) | 89 (35.2) | 178 (31.9) | |
| Current | 125 (15.4) | 57 (22.5) | 68 (12.2) | |
| Smoking amount (PY) | 17.3 ± 24.1 | 21.7 ± 25.5 | 15.3 ± 23.3 | 0.001 |
| Hypertension | 374 (46.1) | 109 (29.1) | 265 (47.5) | 0.243 |
| Diabetes mellitus | 196 (24.2) | 73 (28.9) | 13 (22.0) | 0.036 |
| Dyslipidemia | 190 (23.4) | 59 (23.3) | 131 (23.5) | 0.961 |
| COPD | 120 (14.8) | 47 (18.6) | 73 (13.1) | 0.041 |
| Clinical stage | <0.001 | |||
| Stage I | 670 (82.6) | 181 (71.5) | 489 (87.6) | |
| Stage II | 108 (13.3) | 61 (24.1) | 47 (8.4) | |
| Stage III | 33 (4.1) | 11 (4.3) | 22 (3.9) | |
| Pathological stage | <0.001 | |||
| Stage I | 723 (89.2) | 193 (76.3) | 530 (95.0) | |
| Stage II | 56 (6.9) | 37 (14.6) | 19 (3.4) | |
| Stage III | 31 (3.8) | 22 (8.7) | 9 (1.6) | |
| Stage IV | 1 (0.1) | 1 (0.4) | 0 (0) | |
| Cancer histological type | <0.001 | |||
| Adenocarcinoma | 671 (82.7) | 186 (73.5) | 485 (86.9) | |
| SCC | 117 (14.4) | 55 (21.7) | 62 (11.1) | |
| Others | 23 (2.8) | 12 (4.7) | 11 (2.0) | |
| EBUS done | 256 (31.6) | 95 (37.5) | 161 (28.9) | 0.014 |
| Brain MRI done | 525 (64.7) | 202 (79.8) | 323 (57.9) | <0.001 |
| PET–CT done | 784 (96.7) | 250 (98.8) | 534 (95.7) | 0.022 |
| PET-CT uptake | <0.001 | |||
| Negative | 541 (69.0) | 147 (58.8) | 394 (73.8) | |
| Reactive | 153 (19.5) | 51 (20.4) | 102 (19.1) | |
| Metastasis | 90 (11.5) | 52 (20.8) | 38 (7.1) | |
| Bronchoscopy specialista | 0.036 | |||
| No | 492 (60.7) | 167 (66.0) | 325 (58.2) | |
| Yes | 319 (39.3) | 86 (34.0) | 233 (41.8) |
- Note: Categorical variables are presented as numbers (percentage), and continuous variables are presented as mean ± standard deviation.
- Abbreviations: COPD, chronic obstructive pulmonary disease; EBUS–TNBA, endobronchial ultrasound-guided transbronchial needle aspiration; MRI, magnetic resonance imaging; PET–CT, positron emission tomography-computed tomography; PY, pack–years; SCC, squamous cell carcinoma.
- a Presence of a bronchoscopy specialist.
Accuracy of clinical TNM stage of NSCLC
Table 2 shows the comparison between the clinical and pathological staging according to TNM staging categories, considering the pathological stage as the gold standard. The concordance rates between the clinical and pathological stages in our study were 68.8% in the TNM stage.
| Clinical stages | |||||||
|---|---|---|---|---|---|---|---|
| Pathological stages | cIA | cIB | cIIA | cIIB | cIIIA | Total | % of total |
| pIA | 424 | 39 | 4 | 3 | 0 | 470 | 58.0 |
| pIB | 54 | 65 | 7 | 5 | 0 | 131 | 16.1 |
| pIIA | 0 | 4 | 18 | 2 | 0 | 24 | 3.0 |
| pIIB | 28 | 22 | 13 | 29 | 6 | 98 | 12.1 |
| pIIIA | 19 | 16 | 2 | 19 | 22 | 78 | 9.6 |
| pIIIB | 1 | 1 | 1 | 3 | 3 | 9 | 1.1 |
| pIV | 1 | 0 | 0 | 0 | 0 | 1 | 0.1 |
| Total | 527 | 147 | 45 | 61 | 31 | 811 | |
| % of total | 65.0 | 18.1 | 5.6 | 7.5 | 3.8 | 100 | |
| Underestimationa (%) | 19.7 | 29.3 | 35.6 | 36.1 | 19.3 | 23.2 | |
| Overestimationb (%) | – | 26.5 | 24.4 | 16.4 | 9.7 | 7.4 | |
| cTNM = pTNMc (%) | 80.3 | 44.2 | 40.0 | 47.5 | 71.0 | 68.8 (558/811) | |
- a Underestimation, When the clinical stage is lower than the pathological stage, regardless of the extent of discrepancy.
- b Overestimation, when the clinical stage is higher than the pathological stage, regardless of the extent of discrepancy.
- c cTNM ≠ pTNM, a discrepancy between clinical TNM stage and pathological TNM stage.
Clinical staging overestimated the extent of disease in 60 patients (7.4%) and underestimated it in 188 patients (23.2%). The highest concordance rate between clinical and pathological TNM staging was in clinical stage I patients (80.3%). The concordance rate of clinical IIIA, IIB, IB, and IIA patients were 71.0%, 47.5%, 44.2%, and 40.0%, respectively.
Although we included patients with curative lung resection, there was one patient with pathological stage IV due to a pathology-confirmed metastatic lesion at the diaphragm despite having a preoperative staging of IA.
Accuracy of clinical stage of NSCLC in T and N factors
Table 3 shows the concordance rate between pre- and postoperative staging in terms of tumor (T) and nodal (N) factors, respectively. The accuracy of clinical staging in the T factor was 77.7% in our study. A total of 108 patients (13.3%) were underestimated, and 73 patients (9.0%) were overestimated. The highest concordance rate was 87.5% in patients with clinical stage T4, and the lowest was 62.2% in patients with clinical stage T2a. The concordance rate of the clinical staging in the N factor was 85.8% in our study. A total of 110 patients (13.6%) were underestimated, and six patients (0.6%) were overestimated. The highest concordance rate was 87.5% in patients with clinical N0 and N2 stages.
| cT1 | cT2a | cT2b | cT3 | cT4 | Total | % of total | |
|---|---|---|---|---|---|---|---|
| pT1 | 458 | 46 | 5 | 3 | 0 | 512 | 63.1 |
| pT2a | 74 | 102 | 9 | 4 | 0 | 189 | 23.3 |
| pT2b | 0 | 6 | 29 | 4 | 0 | 39 | 4.8 |
| pT3 | 10 | 9 | 3 | 27 | 2 | 51 | 6.3 |
| pT4 | 0 | 1 | 0 | 5 | 14 | 20 | 2.5 |
| Total | 542 | 164 | 46 | 43 | 16 | 811 | |
| % of total | 66.8 | 20.2 | 5.7 | 5.3 | 2.0 | 100 | |
| Underestimationa, (%) | 15.5 | 9.8 | 6.5 | 11.6 | – | 13.3 | |
| Overestimationb, (%) | – | 28 | 30.4 | 25.6 | 12.5 | 9.0 | |
| cT = pTc (%) | 84.5 | 62.2 | 63.1 | 62.8 | 87.5 | 77.7 (630/811) | |
| cN0 | cN1 | cN2 | Total | % of total | |
|---|---|---|---|---|---|
| pN0 | 672 | 4 | 1 | 677 | 83.5 |
| pN1 | 57 | 17 | 0 | 74 | 9.1 |
| pN2 | 39 | 14 | 7 | 60 | 7.4 |
| Total | 768 | 35 | 8 | 811 | |
| % of total | 94.6 | 4.3 | 1.0 | 100 | |
| Underestimationa, (%) | 12.5 | 40.0 | – | 13.6 | |
| Overestimationb, (%) | – | 11.4 | 12.5 | 0.6 | |
| cN = pNd (%) | 87.5 | 48.6 | 87.5 | 85.8 (696/811) | |
- a Underestimation, When the clinical stage is lower than the pathological stage, regardless of the extent of discrepancy.
- b Overestimation, when the clinical stage is higher than the pathological stage, regardless of the extent of discrepancy.
- c cT = pT, concordance between clinical and pathologial T factor.
- d cN = pN, concordance between clinical and pathological N factor.
Clinical factors that contribute to the accuracy of clinical TNM stage of NSCLC
We found that several clinical factors contribute to the TNM stage concordance (Table 4). Using univariable logistic regression analysis for TNM stage concordance (cTNM = pTNM), male sex (OR 0.58; 95% CI: 0.43–0.79, p = 0.001), smokers (OR 0.58; 95% CI: 0.43–0.78, p < 0.001), use of EBUS (OR 0.67; 95% CI: 0.49–0.92, p = 0.014), positive PET uptake (p < 0.001), higher clinical stage compared to clinical stage I (p < 0.001), larger lymph node size (OR 0.12; 95% CI: 0.07–0.21, p < 0.001), higher clinical N stage compared to N0 (p = 0.005) were significant contributing factors in clinical staging inaccuracy. The introduction of a bronchoscopy specialist (OR 1.39; 95% CI: 1.02–1.90, p = 0.036) and adenocarcinoma (p < 0.001) were significant contributing factors to TNM staging accuracy.
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | ORb | 95% CI | p-value | |||
| Sex, male | 0.58 | 0.43 | 0.79 | 0.001 | 0.477 | |||
| Age | 1.01 | 0.99 | 1.02 | 0.432 | 0.059 | |||
| Smoking, PY | 0.99 | 0.98 | 1.00 | 0.001 | ||||
| Smoking, y/nc | 0.58 | 0.43 | 0.78 | <0.001 | 0.006 | |||
| Former | 0.69 | 0.49 | 0.96 | 0.028 | 0.80 | 0.56 | 1.15 | 0.226 |
| Current | 0.41 | 0.76 | 0.62 | <0.001 | 0.49 | 0.32 | 0.76 | 0.001 |
| PET uptaked | <0.001 | <0.001 | ||||||
| Reactive | 0.75 | 0.51 | 1.10 | 0.137 | 0.82 | 0.55 | 1.22 | 0.320 |
| Metastasis | 0.27 | 0.17 | 0.43 | <0.001 | 0.34 | 0.21 | 0.58 | <0.001 |
| Clinical stagee | <0.001 | <0.001 | ||||||
| Stage II | 0.29 | 0.19 | 0.43 | <0.001 | 0.36 | 0.23 | 0.57 | <0.001 |
| Stage III | 0.74 | 0.35 | 1.56 | 0.428 | 1.46 | 0.62 | 3.41 | 0.388 |
| Clinical N stagef | 0.005 | |||||||
| N1 | 0.32 | 0.16 | 0.64 | 0.001 | ||||
| N2 | 1.29 | 0.26 | 6.44 | 0.756 | ||||
| Bronchoscopy specialistg | 1.39 | 1.02 | 1.90 | 0.036 | 1.53 | 1.10 | 2.13 | 0.011 |
| Pathologyh | <0.001 | 0.101 | ||||||
| SCC | 0.43 | 0.29 | 0.65 | <0.001 | ||||
| Others | 0.35 | 0.15 | 0.81 | 0.014 | ||||
- Note: For continuous variables, OR represents the change for every 1–unit change in the independent variable.
- Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; OR, odds ratio; PET, positron emission tomography; PY, pack years; SCC, squamous cell carcinoma; y/n, yes/no.
- a cTNM = pTNM, concordance between clinical TNM stage and pathological TNM stage.
- b Adjusted odds ratios are represented by the OR.
- c Former and current smoker, reference: never–smoker.
- d Reference: PET negative.
- e Reference clinical stage I.
- f Reference: cN0.
- g Presence of bronchoscopy specialist.
- h Reference: adenocarcinoma.
With factors showing a significant correlation (p < 0.05) with an accuracy of staging, we conducted multivariable logistic regression analysis. As a result, current smokers (OR 0.49; 95% CI: 0.32–0.76, p = 0.001) and higher clinical stage (p < 0.001) were independent contributing factors in clinical staging inaccuracy. The presence of a bronchoscopy specialist (OR 1.53; 95% CI: 1.10–2.30, p = 0.011) was an independent contributing factor in TNM staging accuracy.
DISCUSSION
The current study evaluated the accuracy of clinical staging of NSCLC, based on the 8th edition TMN stage criteria using PET-CT and/or EBUS-TBNA in their clinical staging work-up, and analyzed the factors affecting the accuracy of clinical staging.
Although the treatment of NSCLC has become more complex over the past few decades, as our understanding of NSCLC heterogeneity at the genetic and cellular level progresses16 beyond histopathological descriptions, precise staging of NSCLC remains essential for high-quality care and better prognosis. The probability of concurrence between clinical and pathological stages in this study is much higher compared to previous literature conducted by Erdogan et al. published in 2004.17 The concordance rates in our study versus that of Erdogan et al. are as follows: 68.8% versus 47.7% in the TNM stage, 77.7% versus 55.5% in the T stage, and 85.8% versus 73.9% in the N stage, respectively.
The increased accuracy of clinical TNM staging compared to previous studies could be attributed to advances in diagnostic methods for clinical staging, such as EBUS and PET-CT.18, 19 The previous literature9-11 compared several studies on the clinical staging accuracy of NSCLC and showed that PET-CT and EBUS data were included since 2007 and 2013, respectively. In addition, the thoracic MDT meetings would also contribute to our study's increased clinical staging accuracy. The MDT members were composed of pulmonologists, radiologists, pathologists, thoracic surgeons, oncologists, and radiation oncologists who shared their knowledge and made collective recommendations for the diagnosis, staging, and appropriate treatment of patients. Therefore, the MDT approach allows more accurate staging of patients with ambiguous clinical stages. In fact, many current clinical guidelines recommend the MDT approach for lung cancer management despite the lack of quality evidence that MDT care contributes to the accuracy of staging and prognosis.20-22
Regarding clinical staging in the T factor, the discrepancy rate was highest between T1 and T2a (120 patients, 66.3%). Because the T2a stage includes invasion of visceral pleura regardless of size, it is challenging to distinguish cT1 from cT2a by imaging. However, this discrepancy affects the clinical staging of patients between clinical stage IA (T1N0M0) and IB (T2aN0M0), which does not affect treatment strategies because both groups are recommended under surgical treatment. Moreover, their inaccuracy of clinical staging could be corrected after confirming the pathological T staging, and the physician can determine whether to add adjuvant therapy or not, based on the pathological stage. Therefore, the majority of clinical staging discrepant groups in the T factor are not important in the real-world practice of NSCLC treatment.
In our study, the accuracy of clinical staging in the N factor was increased compared to the previous study.17 In particular, the unforeseen N2 rate was extremely decreased to 6.5% (53/811), which is under the acceptable unforeseen N2 rate, about 10% at the time of surgery defined by the European Society of Thoracic Surgery working group.23
In our study, only 31.6% used EBUS–TBNA for clinical staging work-up and 68.4% of patients used PET-CT for clinical staging work-up instead of EBUS screening. EBUS was performed only in patients where it was necessary for accurate clinical staging. Patients who were not suspected to have lymph node metastasis on PET-CT or chest CT did not undergo EBUS for their clinical staging work-up. Considering that most patients discovered that they did not have nodal metastasis after surgery (i.e., pN0), that strategy is reasonable considering cost-effectiveness. However, due to the small number of cases in which EBUS was performed, we were unable to evaluate a significant relationship showing the direct benefits of EBUS in increasing nodal staging accuracy. However, the presence of a bronchoscopy specialist showed a significant association with increased clinical TNM staging accuracy (adjusted OR 1.53; 95% CI: 1.10–2.13, p = 0.011), indirectly indicating that the bronchoscopy and EBUS contribute to increased clinical staging accuracy. A bronchoscopy specialist was introduced in 2020 to our hospital and conducted bronchoscopy and EBUS almost every day, averaging about 7 to 8 sessions over 10 sessions per week. Additionally, even bronchoscopy nonspecialists received more detailed education about EBUS skills to enhance accuracy from the bronchoscopy specialist. Therefore, the accuracy of EBUS cases after 2020, when the bronchoscopy specialist was introduced, compared to the accuracy of EBUS cases before 2020, could reflect the influence of the bronchoscopy specialist somewhat indirectly. Our institution has a bronchoscopy expert who performs bronchoscopy and EBUS in more than 1000 cases a year, so the quality of bronchoscopy examination is judged to be reliable in accurate staging. In fact, our EBUS results showed a high positive predictive value (PPV) and negative predicted value (NPV) at 100% and 82.1%, respectively, which was consistent with previous studies.7, 8 Most patients with disconcordant between clinical and pathological N factor have nodal metastasis on lymph nodes that is unable to technically access by EBUS-TBNA such as LN 5, 6 or peribronchial lymph nodes9 (Table S1).
Interestingly, a smoking history significantly contributed to clinical staging inaccuracy in multivariable logistic regression analysis. This inverse relationship between smoking and NSCLC clinical staging (i.e., TNM stage and N stage, especially N2 stage) accuracy has been proven in a previous study.24 It is explained by a pathophysiological difference in cancer between smokers and nonsmokers. Smokers have much higher background FDG tracer values,25 resulting in lower max standardized uptake values. However, there are no standardized protocols to reflect the different background FDG tracer values in interpreting PET-CT between smokers and nonsmokers. In our study, consistent with previous literature,24 the clinical staging accuracy of current smokers is lower compared to that of never-smokers (adjusted OR 0.49;95% CI 0.32–0.76, p = 0.001, Table 4). And, former smokers who quit cigarette smoking for at least 1 month showed no significant differences in clinical staging accuracy compared to never-smokers (adjusted OR 0.80; 95% CI: 0.56–1.15, p = 0.226, Table 4). Because almost 70% of patients' clinical staging in our study was determined based on PET–CT, the presence of smoking history might influence the accuracy of staging.
There were a few limitations to our study. There was selection bias because we evaluated the resectable NSCLC patients to compare the pathological staging as a gold standard. We did not aggressively perform esophageal endoscopic ultrasonography (EUS) to increase the accuracy, as more than 80% of patients were stage 1 with no metastatic lymph nodes. It is generally known that the combined use of EBUS and EUS significantly improves sensitivity in detecting mediastinal node metastasis.26, 27 However, in patients who required EUS for precise clinical staging, EUS was performed together with EBUS.
Recently, the clinical staging accuracy of NSCLC has improved compared to before the widespread use of PET-CT and EBUS in clinical staging work-up. In particular, the presence of bronchoscopy specialists was found to be one of the factors contributing to the agreement of clinical and pathological staging. Furthermore, the current study found that smokers, especially current smokers, showed more inaccuracy in clinical staging. It is known that accurate clinical staging has a definite survival benefit in individual participant data meta-analysis.28 Therefore, in conclusion, increasing the number of skilled bronchoscopy specialists for EBUS screening would be helpful in improving the clinical staging accuracy and prognosis of patients with NSCLC.
AUTHOR CONTRIBUTIONS
Study concept and design: Seong Yong Park and Sang Hoon Lee Methodology: A La Woo, Seung Hyun Yong, Youngmok Park, Song Yee Kim, Eun Young Kim, Ji Ye Jung, Young Ae Kang, and Moo Suk Park. Data curation: Hye Ran Gwon, Seong Yong Park and Sang Hoon Lee. Acquisition and analysis of data, Drafting the manuscript: Hye Ran Gwon Writing—review and editing: Seong Yong Park and Sang Hoon Lee. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2022R1F1A106878912).
CONFLICT OF INTEREST STATEMENT
There are no potential conflicts of interest to disclose.




