Clinical application of photodynamic therapy for malignant airway tumors in China
Abstract
With the development of interventional pulmonology, photodynamic therapy (PDT) is gradually being used in the treatment of respiratory malignant tumors because of its low level of trauma, high specificity, and compatibility with traditional or common therapies. However, at present, the data of clinical evidence-based medicine for PDT applied in central airway tumors is very limited, and derives mainly from case reports or series of case studies which lack consensus on clinical diagnosis and treatment. In order to further disseminate China's experience, the Tumor Photodynamic Therapy Committee of China Anti-Cancer Association and the World Endoscopy Association-Respiratory Endoscopy Association invited experts from relevant fields to form an expert committee. After several rounds of discussion and revision by this committee, and following a vote, the consensus was formulated for reference by physicians in respiratory, oncology and other related disciplines to refer to the practice of tumor photodynamic therapy.
Introduction
Photodynamic therapy (PDT) is an ancient and modern technology. More than 4000 years ago, it was recorded that Psoralea corylifolia L. was used to treat skin diseases.1 However, the scientific exploration of PDT began in the mid-19th century. In the late 1970s, PDT gradually became a new technology for the treatment of tumors, and was approved by many countries such as the United States, UK, France, Germany, and Japan for the treatment of malignant tumors. In 1998, the US FDA approved Photofrin for the treatment of both early and obstructive bronchogenic carcinoma.2 The domestic hematoporphyrin (currently known as HiPorfin) was approved by the National Medical Products Administration for cancer treatment in 2001.
With the development of interventional pulmonology, PDT is gradually being used in the treatment of respiratory malignant tumors because of its low level of trauma, high specificity, and compatibility with traditional or common therapies.3-6 However, at present, the data of clinical evidence-based medicine for PDT applied in central airway tumors is very limited, arising mainly from case reports or series of case studies, which lack consensus on clinical diagnosis and treatment. In 2010, academician Gu Ying recruited domestic experts to publish the “Standard Clinical Operating Procedures” for photodynamic therapy, which laid a solid foundation for promoting the clinical application of PDT.7
In order to further disseminate China's experience, the Tumor Photodynamic Therapy Committee of China Anti-Cancer Association and the World Endoscopy Association-Respiratory Endoscopy Association invited experts from relevant fields to form an expert committee, and develop an expert consensus based on international research progress, Chinese clinical experience and research status by searching PubMed, Embase, Cochrane Library, and Chinese Journal Full-text Database (CJFD), China Science and Technology Journal Database and WanFang database, etc. After several rounds of discussion and revision by the expert committee, a consensus was formulated after the vote, for reference by physicians in respiratory, oncology and other related disciplines to refer to the practice of tumor photodynamic therapy.
Principle of PDT
PDT is the combination of drugs and devices which kill tumors and other pathologically proliferating tissues through local selective photosensitization of the lesions. After the photosensitizer is injected into the bloodstream, it has a high affinity with the tumor tissue and will form a relatively high accumulation in the tumor tissue. At this time, light of a suitable wavelength is irradiated to the lesion, and the energy of the photosensitizer absorbs the photon transitions to the excited state. The excited photosensitizer transfers energy to oxygen, producing some radical oxygen species (ROS), which are the main killers of target damage, acting through both free radicals (also known as type I mechanism) and singlet oxygen (also called type II mechanism), causing tumor cell apoptosis or death.
Furthermore, the photosensitizer has a high concentration in the neovascular endothelial cells of the tumor tissue, and thus the vascular injury caused by the irradiation and the resulting local ischemia and hypoxia of the lesion tissues, which make a difference in the clinical therapeutic mechanism of PDT and determines the selective cytotoxic activity toward malignant cells.
The characteristics of photosensitization, which selectively accumulates in tumor tissue and selectively irradiates the lesion tissue to form a dual targeting of photodynamic therapy, constitutes dual targeting of photodynamic therapy; that is, drug targeted enrichment and light-targeted activation.8 PDT can also induce anti-tumor immune effects, enhance the anti-tumor effect of various immune cells of the body, and cause local inflammatory reactions, activate various immune molecules such as chemotactic cytokines and activated complement, thereby effectively removing tumor cells and inhibiting tumor recurrence.
Drugs and equipment needed for photodynamic therapy
1. Photosensitizers9
Photosensitizers currently used in lung cancer include the first generation of photosensitizers which are derivatives of hematoporphyrin. These have a definite curative effect and complex composition. However, the killing depth of these drugs appears to be shallow, and the retention time in the skin is several weeks, and it is therefore easy to cause a skin photosensitive reaction. A longer light protection time is therefore required. Representative drugs include: HiPorfin (China), Photofrin (Canada), Photosan (Germany), and Photogem (Russia).
The second generation of photosensitizers are mostly derivatives of porphyrin compounds, and include porphyrins, porphins, purpurin, endogenous porphyrins, and metal phthalocyanines, fused ring quinone compounds, etc., which are improved on photodynamically activity, the absorption spectrum and selectivity to the tissue. They have a stronger affinity with tumor cells, a shorter body retention time, can be removed quickly, and almost no skin photosensitive reaction is induced. The representative drug is Laserphyrin (Japan), although this has not yet been introduced to the Chinese market.
Other photosensitizers such as Fudasaiyin (phthalocyanine) and hypocrellin are in the domestic clinical trial stage, and it is hoped that they will be available in the near future.
2. Types of photodynamic therapeutic apparatus
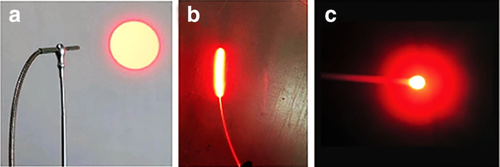
Oxygen, photosensitizers and visible light are the basic requirements under which photodynamic reactions occur. Among these, photosensitizers and specific wavelengths of light are the two key factors in photodynamic reaction. At present, the photodynamic laser therapeutic apparatus for clinical use is mainly a semiconductor laser (power is 0.1–2 W), which is popular because of its small size and power stability; and a high-power helium-neon laser tumor treatment apparatus, which is relatively inexpensive (Fig 1). There are two main types of optical fibers for treatment. One is a flat optical fiber, which is used for lesions with a range of less than 0.5 cm and can be directly irradiated to the lesion. The other is a cylindrical optical fiber, which is most commonly used in therapy. The length of the diffusion section is 2–6 cm, and the fiber of the appropriate diffusion length is selected according to the length of the lesion. At present, there are also optical fibers with balloon catheters in use overseas, which inject normal saline into the balloon, and the balloon is then expanded. The light emitted by the cylindrical optical fiber in the central part of the balloon can be uniformly irradiated to the lesion by the expanded balloon (Fig 2).

Indications
Treatment of early airway malignant tumors
Treatment of early airway malignant tumors: These patients are expected to achieve a radical cure after photodynamic therapy.2, 10
(i) Early central lung cancer
(ii) primary tracheal malignancy
(iii) severe atypical hyperplasia of the trachea and bronchus
The following conditions should be met: pathological confirmation that the tumour is malignant or the lesion is precancerous, or the lesions confirmed by CT, endobronchial ultrasound (EBUS) or optical coherence tomography (OCT), narrow-band imaging (NBI) or autofluorescence bronchoscopy (AFB), involving the mucosa, submucosa, without involving the cartilage and outer layer, and the length of lesion is <1 cm and within the visual range of the bronchoscope, the depth of invasion is <1 cm, no lymph nodes and distant metastasis, the patient cannot tolerate surgery or chooses not to accept surgical treatment.
- Primary or metastatic tracheobronchial malignancies
- Multiple primary central lung cancer
- Local recurrence of stump after lung cancer surgery
- Local recurrence of central lung cancer after radiotherapy.
The following conditions should be met: there is a tracheal and bronchial obstruction, and the tumor is of endotracheal type or endotracheal combined with wall type.
Contraindications
- Hematoporphyrin and other diseases exacerbated by light.
- Those known to be allergic to porphyrins or to any excipients.
- Photosensitizers are already being used for treatment.
- Those who plan to undergo surgery within 30 days.
- In the presence of an ophthalmic condition, a light examination will be required within 30 days.
- Severe cardiopulmonary dysfunction, liver and kidney dysfunction, and unable totolerate bronchoscopy.
- Obvious coagulopathy.
- The tumor has invaded large blood vessels and tracheoesophageal tumor with penetrating infiltration.
- Tracheoesophageal, tracheal mediastinal, or bronchopleural fistula, bronchial wall structure is destroyed.
- Patients with severe luminal stenosis caused by tracheal tumors (>75%) are prohibited from direct photodynamic therapy.19, 20
- Mixed lesions dominated by extratracheal tumors.
- Precautions for pregnant women: photofrin is considered to be a C-grade (toxic, nonteratogenic) drug for pregnancy risk, with nondialysis.21 The risk of HiPorfin for pregnant women is not clear, and it should therefore be used with caution.
Preoperative examination and preparation
- Laboratory examination: this includes routine blood, liver and kidney function, coagulation function, hepatitis B, anti-HCV, and sexually transmitted diseases test.
- Pulmonary function test, electrocardiogram (ECG), ultrasound cardiogram (UCG).
- Plain and contrast-enhanced chest CT scan, tracheal tree three-dimensional reconstruction: define the thickness of the wall, whether it penetrates the whole layer, is infiltrated with adjacent organs, whether there is infiltration with adjacent blood vessels, and adjacent lymph node metastasis.
- Bronchoscopy: observe the location, number, thickness of the lesion, degree of clogging of the lumen. If conditions permit, endobronchial ultrasound and autofluorescence bronchoscopy should be performed simultaneously to determine the extent and thickness of the lesion. ]
- Informed consent and notification: inform patients and their families of the course of PDT treatment, intra- and postoperative risks and complications, prognosis and follow-up, and the advantages and disadvantages of the treatment and other alternative treatment options.
- Ward requirements: the doors and windows of the ward must be protected from light curtains, using milky white lighting (<60 W).
- Patients should wear sunglasses and stay in the darkroom after injection of the photosensitizer. Doctors should pay close attention to any changes in the condition.
Operation process and skills
- Photosensitizer skin testing: The photosensitizer (Hipo) is diluted to 0.01 mg/mL, and 0.1 mL intradermally injected, and the injection area is protected from glare. Judgment of the results: A local reaction is observed after 15 minutes, only those who have a negative skin test can use the drug.
- Method of administration of photosensitizer: The medicine is taken out from the constant temperature refrigerator and placed in a place where sunlight cannot be illuminated. After standing and rewarming until the medicine recovers from the state of the ice water mixture to the liquid state, the medicine is dissolved in 250 mL of normal saline, and infused with a light-proof infusion device. Close attention should be paid during the infusion process to prevent extravasation of liquid medicine.
- Dosage of the drug: HiPorfin 2–3 mg/kg.
Light source selection
The light source used for the HiPorfin-PDT is a semiconductor laser with an emission wavelength of 630 ± 3 nm and a power of 0.1–2 W. The cylindrical optical fiber is mostly used for irradiation, and the fiber of different length of the diffusion section is selected according to the length of the lesion (2–6 cm).
Lighting parameters
Irradiation parameters are extremely important when applied clinically. Energy density, power density, and irradiation time are the three parameters of irradiation. Irradiation time (s) is achieved by dividing energy density (J/cm2) by power density (W/cm2).22 Irradiation time and power density are two irradiation parameters that can be adjusted during clinical application. The longer the irradiation time, the greater the power density, the greater the energy density, the better the efficacy, but the more serious the adverse reactions.
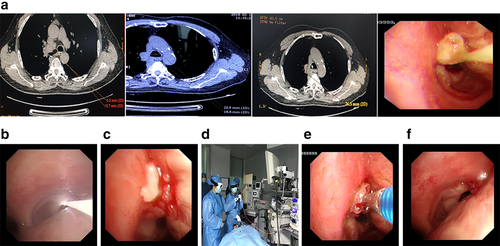
- Develop a treatment plan: Evaluate the length of the tumor to be treated by a flexible bronchoscope, determine the irradiation range, and calculate the power density and irradiation time. Conventional application of light with 630 nm wavelength, power density of 100 mW/cm2, total energy density of 150–200 J/cm2 can alleviate the obstructive symptoms of bronchogenic carcinoma, and treat bronchial mucosal lesions (Fig 3a).
- PDT first irradiation: Intravenous injection of photosensitizer (Porfimer sodium), after 40 hours (when the difference in drug concentration between tumor tissue and surrounding normal tissue is optimal), point spectroscopy can be used to detect blood concentration level, or directly perform optical fiber irradiation. Intermittent irradiation (irradiation 3–5 minutes, interval 1–3 minutes) is significantly better than continuous irradiation, because intermittent irradiation is conducive to the recovery of tissue oxygen concentration, so it can improve the efficacy (Fig 3b).
- The second exposure of PDT: Before irradiation on the third day of administration, the necrosis on the surface of the treatment site should first be removed. Care should be taken with excessive cleaning in order to avoid bleeding. If the amount of bleeding is great, the cleaning scope is far beyond the depth of photodynamic treatment and should be stopped immediately. The energy of the second irradiation is subject to effective tumor treatment and should not exceed the energy density of the first irradiation. If the radiation is repeated within 72–96 hours after the injection of the drug, there is no need to inject photosensitizer. In fact, most patients receive secondary radiation during treatment (Fig 3c).
- Choice of anesthesia method: For superficial tumors without obvious luminal stenosis, a flexible bronchoscope can be used to direct irradiate under local anesthesia or diazepam. For large tumors in the trachea and main bronchi with lumen obstruction, general anesthesia is recommended (Fig 3d).
- Choice of treatment: For patients with an obvious obstruction in the tracheobronchial bronchus, insertion of a rigid bronchoscope through the mouth under general anesthesia is recommended, and a flexible bronchoscope should be placed through a hard lens. Techniques such as hard shovel cutting, carbon dioxide freezing, laser/argon knife cauterization and ablation, electric loop ligature and other techniques should be carried out to remove the tumors. Photodynamic therapy should then be used to treat tumor stumps, which have been shown to be more efficacious.26
- Cleaning of necrosis: During treatment, the necrosis should be removed before each irradiation, and respiratory symptoms treated at any time (Fig 3e). The necrosis on the surface of the lesion should be cleaned again after one week of photodynamic irradiation to avoid lumen obstruction (Fig 3f).
Operational skills
The cylindrical optical fiber is placed into the lesion area to be irradiated during bronchoscopy. When the tumor is relatively flat, the fiber can be placed on one side of the tumor. For a large and intraluminal type of tumor, the fiber can be inserted into the tumor. A cylindrical optical fiber is usually used in patients with central airway obstruction. In general, different length of fiber is selected according to the length of the tumor to be treated, so that the fiber exceeds 0.5 cm at both ends of the lesion, and it is necessary to completely involve the tumor tissue and avoid excessive irradiation of nontumor tissue, when the lesion range is wide, segmental irradiation should be carried out, and care taken to avoid excessive repeated irradiation of the tumor tissue. Light penetration at a wavelength of 630 nm is about 5–10 mm in lung and tumor tissues, depending on power density and irradiation time.
The currently used light source is a semiconductor laser. The laser it emits is a nonthermal laser that does not cause burning in the airway. The photoactivation of the hematoporphyrin derivative is primarily controlled by the total irradiation dosage. In the treatment of bronchial tumors, after setting the power of the laser therapeutic device, the irradiation time is calculated according to the formula, and the corresponding irradiation performed. The irradiation area (cm2) of cylindrical optical fiber = 2πrh (h is the length of the emitting portion of cylindrical optical fiber, and r is the distance from the light-emitting portion to the lesion)
Points for attention
Irradiation problem
After 40–48 hours of infusion of photosensitizers (hematoporphyrin derivatives), patients in these studies27-29 were irradiated twice with a power density of 100 mW/cm2 and energy density of 100–150 J/cm2 on each occasion.
- If the tumor lumen obstruction in the area I–IV of the central airway is blocked by more than <50%; cytoreductive surgery (CRS) under bronchoscope should be carried out first. When the lumen stenosis was less than 50%, photodynamic therapy was performed. Tracheal stents can be placed immediately after PDT if necessary, but they need to be removed before the second irradiation.30
- Lumen obstruction caused by tumor in the central airway I–IV area ≥50%; perform cytoreductive surgery (CRS) under bronchoscopy, if the luminal stenosis <50% after treatment, then perform photodynamic therapy, if necessary, place the tracheal stent immediately after PDT, and this needs to be taken out before the second irradiation.
-
Photodynamic therapy can be performed directly on patients with bronchial (central airway V–VIII area) tumor-induced luminal stenosis,30 regardless of the degree of stenosis.
• If the bronchial luminal stenosis <50%, then perform photodynamic therapy
• If the bronchial luminal stenosis is >50%, it is feasible to reduce the stenosis by <50% after cytoreductive surgery is performed, and then carry out photodynamic therapy.
• If the bronchial luminal stenosis is >50%, the optical fiber can be directly inserted into the tumor, and interstitial photodynamic therapy performed, or combined with surface photodynamic therapy, and intraoperative bleeding reduced by cytoreductive surgery.
• If the bronchial luminal stenosis is >50%, the bronchial stent can be placed immediately after photodynamic therapy. Remove the stent before the second exposure of irradiation.
- Patients with squamous cell carcinoma have early necrosis formation after irradiation, while those with adenoid cystic carcinoma have a relatively late formation of necrosis after irradiation, which may occur after one week of irradiation. After photodynamic therapy, it is necessary to pay close attention to respiratory symptoms. Once dyspnea occurs, timely bronchoscopy should be performed to remove any necrosis under bronchoscopy. It may be necessary to repeat this procedure several times a day.
Educating patients to avoid light
At present, the domestic marketed photosensitizers are only the first generation of HiPorfin, and it is necessary to focus on the education of protecting patients from light27, 31: to inform them of the time and extent of protection from light.
During the first week of administration, the patient's skin and eyes are very sensitive to light. In this case, it is necessary to avoid direct sunlight and direct exposure to sunlight. Patients are advised to stay in a dark room. This room can be lit using a table lamp with a yellow light bulb below 60 W, and patients can also watch TV with a safety distance of at least 2 m, and wear sunglasses. Using a computer or mobile phone are not recommended.
Patients still need to continue to wear sunglasses in the second week because the photosensitive drug is in the process of metabolism. The brightness of the indoor light should be gradually increased until the normal indoor light is restored. Also, patients still need to avoid using their mobile phone or computer and maintain a safe distance when watching TV.
In the first 3–4 weeks, the patient's skin is still sensitive to light. Direct sunlight and indoor glare should be avoided. Patients should travel at night and on cloudy days, wear sunglasses (<4% transmittance), gloves, wide-brimmed hats, long-sleeved shirts, trousers, and socks during the day. Direct sunlight and bright light such as a reading light should be avoided during this period.
After 30 days, patients are advised to perform a light sensitivity test, placing their hands in a paper bag with a 2 cm diameter hole with subsequent exposure to sunlight for 10 minutes; if swelling, redness, or blisters occur within 24 hours, the patient is advised to keep away from the light for two weeks and then retest; if no reaction occurs within 24 hours, the patient can gradually return to contact with sunlight. It is advised to try exposure to the light for 15 minutes on the first day, and if no response occurs, then the exposure time to light can be gradually increased. Initially, it is recommended to avoid the strongest sunshine period (10:00–14:00) and not to sunbathe, use sun lights or sunbeds for at least three months. Also, ophthalmic lighting inspection equipment should be avoided.
Efficacy evaluation
Regular evaluation should be conducted before and after treatment. Each evaluation requires plain and contrast-enhanced chest CT scan, bronchoscopy, and tissue biopsy as an objective evaluation basis. We used the evaluation criteria for photodynamic therapy of respiratory tumors (2019 edition). Based on a detailed study of the efficacy evaluation standards for photodynamic therapy at home and abroad, and the Response Evaluation Criteria in Solid Tumors (RECIST) standards and WHO standards, we reached the following consensus.
- Complete response (CR): Signifies that the tracheobronchial carcinogenesis was completely eliminated, and no tumor cells were found in the mucosal biopsy.
- Partial response (PR): Signifies that the product of the length of the tracheobronchial × thickness is reduced by ≥30% compared with before treatment, and there are still tumor cells in the mucosal biopsy.
- Stable disease (SD): Signifies that there has been no significant decrease or increase in the size of tracheobronchial carcinogenesis, and tumor cells can still be seen in mucosal biopsy.
- Progressive disease (PD): Signifies that there has been an increase of the range of tracheobronchial carcinogenesis more than that of the original lesion, and the mucosal biopsy shows tumor cells present.
- Total survival (OS): The time from the start of treatment to death for any reason;
- Progression-free survival (PFS): The time from enrollment to tumor progression or death.
- Duration of controlling disease: The period between the onset of treatment and the progression of disease.
Complications and preventative measures
Common complications
- Photosensitive reactions32-34: The incidence rate of a photosensitive reaction34-36 is reported to be 5%–28%. The clinical manifestations are mainly sunburn-like changes such as congestion, redness, hot pain, a small number of rashes, mostly erythema, papules, with itching or burning pain, and there may be peeling and blisters in severe cases. Pigmentation may occur in the later stages. Educating patients to avoid direct sunlight forms part of the overall treatment and it is important to offer advice on the use of protective clothing and precautions. If a reaction does occur, and the skin first starts to tingle or become erythematous, patients should immediately use cold water to wet pack red swelling spots, and avoid direct sunlight for two weeks. For those who develop a rash, anti-allergic drugs can be taken orally or applied topically with a hormone-containing ointment. For patients with obvious swelling and blisters indicating that a serious phototoxic reaction has occurred, it is necessary to intravenously inject hormonal drugs, and for anti-allergic drugs to be taken orally, and avoid exposure to sunlight.
- Cough35, 36: The reported incidence rate of cough is 15%–34%, and is mainly of an irritating nature, often accompanied by difficulty coughing, and the production of a small amount of white sticky phlegm. Oral antitussive drugs can be administered conventionally after irradiation.
- Dyspnea: The incidence rate of dyspnea is reported to be 18%–32%. It is characterized by chest pain and shortness of breath. It is caused by necrosis blocking the lumen after irradiation. If acute total atelectasis occurs, it may be accompanied by chest pain. Bronchoscopy is routinely performed on the first 1–2 days after photodynamic therapy to remove necrosis in the airway. In addition, it is necessary to perform a bronchoscopy timely in the case of dyspnea to clear up necrosis, and a temporary tracheal stent could be placed to maintain the lumen unobstructed.
- Fever: The body temperature of patients is between 37°C to 38°C, which can be caused by the absorption of heat of tumor necrosis or an obstructive pneumonia triggered by the formation of necrosis that blocks the lumen after tumor treatment. Therefore, an antipyretic, anti-infective treatment can be used, if necessary, and bronchoscopy should be undertaken to remove necrosis.
- Hemoptysis: The main clinical manifestation is bloody sputum which may be due to damage to the normal tissue when the necrotic material is cleaned, or due to the exfoliation of the tissue necrosis after irradiation of the loosely-structured tumor tissue, resulting in large wound and oozing blood. Hemostatic drugs should be administered or a bronchoscopy performed to stop the bleeding.
- Acute mucosal edema: The release of inflammatory factors after irradiation causes vasoconstriction, blood cell retention and agglutination, and stasis resulting in tissue edema. Clinically, edema may be characterized by sudden dyspnea, cyanotic lips, and laryngeal stridor, profuse sweating, inability to maintain a supine position, progressive decline in blood oxygen saturation, increased heart rate, and elevated blood pressure. Death from asyphyxia may occur in severe cases. Acute mucosal edema occurs mostly in the lesion located in the central airway I area adjacent to the glottis, caused by glottic edema after irradiation. In such patients, methylprednisolone 40 mg intravenously once a day for three days can be administered after surgery. In the case of dyspnea and a progressive decline in blood oxygen saturation, endotracheal intubation under bronchoscopic guidance is required. Thus, a tracheotomy kit should be readily available to ensure an immediate tracheotomy can be performed if intubation proves difficult.
- Perforation: In the case of tracheoesophageal penetrating lesions, perforation is more likely to occur after perform PDT due to necrosis of tumor tissues and the exfoliation of necrosis, such as tracheoesophageal/bronchial fistula, tracheobronchial mediastinum fistula and so on, which usually manifests as a cough, sudden aggravation of a cough, and the amount of blood in the sputum increases significantly. If patients have an irritable cough after having a meal or drinking water, clinicians should be alert to the possibility of a tracheoesophageal fistula. Chest CT, gastrointestinal imaging and bronchoscopy should be undertaken to confirm this as soon as possible. According to the location of the fistula, a stent should be selected which is the appropriate shape (metal or silicone stent) in order to block the fistula. No food should be given orally before the fistula has been successfully blocked, and placement of an enteral nutrition tube or a jejunumostomy is recommended in order to develop enteral nutrition support.
- Cicatricial stenosis: Tumor necrosis usually occurs after treatment, local mucosal fibrosis forms cicatrix, and cicatrix contraction leads to stenosis of the lumen. The patient can be asymptomatic in the early stages, with the aggravation of stenosis in the later stages, and symptoms of cough, dys-expectoration, and shortness of breath will gradually develop. Bronchoscopic examination showed that the tumor tissue disappears after PDT treatment, local mucosa formes scar, and the lumen becames narrow. Balloon dilatation and endotracheal stent placement can be used to maintain an unobstructed lumen.
- Fatal hemoptysis: The tumor often involves adjacent large blood vessels, and tumor cells become necrotic and exfoliates after treatment, forming a bronchial-arterial fistula, and causing fatal hemoptysis. Tracheal intubation should be performed immediately. For details, see the consensus on prevention and treatment of massive hemorrhage associated with bronchoscopy diagnosis and treatment.37
Photodynamic therapy
PDT combined with bronchoscopy interventional cytoreductive surgery26
For a large tumor in the central airway which is blocking the lumen, the combination of rigid lens cutting, snaring and ligation of electric snare, electric needle cutting, APC, laser ablation, carbon dioxide freezing and other interventional techniques can be used to quickly remove the lesions in the tracheobronchial cavity, and the stump of the lesion is treated with PDT, which can achieve a good therapeutic effect.
PDT combined with radiotherapy15, 38, 39
Radiotherapy combined with porphyrin photosensitizer-PDT showed both additive and synergistic effects, and PDT combined with radiotherapy was safe and effective. It is generally recommended for PDT to be performed before radiotherapy; however, if radiotherapy has been used first on a patient, then PDT is feasible after the acute inflammatory reaction period of radiotherapy one month later.40
PDT combined with chemotherapy41, 42
PDT combined with chemotherapy is effective and safe. The two methods can be used for synchronous or sequential therapy to achieve a downstaging, and if necessary, surgical resection is feasible.
PDT combined with molecular targeted drugs43
Current studies have shown that erlotinib combined with PDT can enhance the efficacy of PDT, while PDT can improve the resistance of TKI drugs, or improve the prognosis of such patients.
PDT combined immunotherapy44-46
Photodynamic immunotherapy (PDIT) is gradually attracting more attention. PDIT is a combination of photodynamic therapy and immunotherapy applied to the treatment of diseases, and the two therapies work synergistically. However, these studies are currently at the laboratory stage, and there is currently no evidence for large-scale clinical application.
Disclosure
The authors declare no conflicts of interest.




