UNBS5162 inhibits the proliferation of human A549 non-small-cell lung cancer cells by promoting apoptosis
Abstract
Background
Lung cancer is one of the most frequently diagnosed malignancies in the world, thus developing novel anticancer reagents for lung cancer treatment is critical.
Methods
We performed cell counting kit-8 and cell colony formation assays to investigate the role of UNBS5162 in the proliferation of A549 cells. Invasion and migration assays were applied to study the inhibitory effect of UNBS5162 on non-small cell lung cancer cells. To detect the effect of UNBS5162 on A549 cell apoptosis, Annexin-V fluorescein isothiocyanate and propidium iodide staining methods were used. Protein expression was analyzed using Western blot assay.
Results
UNBS5162 not only inhibited proliferation but also decreased invasion and migration in A549 cells. Most cells were intact (96.93%) under control conditions, but the number of intact cells decreased (84.8%) after 24 hours of treatment with UNBS5162, and the number of early and late apoptotic cells significantly increased (P < 0.05). Anti-apoptotic protein Bcl-2 expression in the UNBS5162 group was significantly decreased (P < 0.05), and expression of proapoptotic proteins Bim, Bax, and active caspase-3 were significantly increased (P < 0.05) compared to the control. In the PI3K signaling pathway, phospo-AKT and phospo-mTOR levels were significantly decreased (P < 0.05), while S6K and Cyclin D1 protein levels were significantly decreased in UNBS5162 treated A549 cells (P < 0.05).
Conclusion
These findings suggest that UNBS5162 could inhibit A549 cell proliferation and metastasis by inhibiting PI3K pathway mediated apoptosis.
Introduction
As one of the most frequently diagnosed malignancies in the world, lung cancer has become the number one cancer-related cause of death worldwide.1, 2 Approximately 85–90% of all lung cancer deaths are caused by non-small cell lung cancer (NSCLC), the molecular basis of which is complex and heterogeneous.3 Currently, surgical intervention, chemotherapy, and γ-irradiation are the major therapeutic modalities in clinical medicine. Chemotherapy and γ-irradiation can inhibit cancer cell proliferation, but can also kill normal cells and cause many side effects, such as loss of immunity. Studies have shown that the five-year survival rate in patients with lung cancer is less than 15% as a result of chemotherapy resistance;4, 5 therefore, many researchers have attempted to develop novel anticancer agents.6-8
Naphthalimides are DNA intercalating agents that have shown high preclinical anticancer activity against a variety of human tumor cells.9 Amonafide, the first naphthalimide evaluated in clinical trials as a potential anticancer agent failed to enter phase III trials because of dose-limiting bone marrow toxicity. This toxicity is linked to metabolism through N-acetyl transferase 2, a polymorphic enzyme, to a toxic metabolite, N-acetyl-amonafide.10, 11 Researchers have subsequently improved the therapeutic properties of naphthalimides by designing bis-intercalating agents.12, 13 UNBS3157 is a novel naphthalimide derivative that could avoid the specific activating metabolism that provokes the clinical hematotoxicity of amonafide.14 UNBS3157 is rapidly and almost completely hydrolyzed in physiological saline into one single product, UNBS5162, which accounts for its anti-cancer activity without generating amonafide.15 UNBS5162 diminishes proangiogenic CXCL expression when administered in a metronomic approach in vitro and significantly increased the survival rate in an orthotopic human PC-3 prostate cancer xenograft mouse model at almost the same magnitude as paclitaxel.15 Furthermore, there is a synergistic therapeutic benefit when UNBS5162 is administered in combination with paclitaxel.15 However, whether UNBS5162 has anti-cancer effect in human lung cancer is not yet clear.
To investigate the roles and underlying mechanism of UNBS5162 in NSCLC, we tested the proliferation and metastasis of human A549 NSCLC cells treated with UNBS5162 and found that UNBS5162 could inhibit A549 cell proliferation and growth by inhibiting the PI3K pathway to mediated apoptosis.
Methods
Cell culture
Human A549 NSCLC cell lines were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). The cells were grown in RPMI-1640 (Hyclone Laboratories Inc., Logan, UT, USA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and antibiotics (100 U/mL penicillin, 0.1 mg/mL streptomycin) (Sigma-Aldrich, St. Louis, MO, USA). The medium was changed every three days and human A549 NSCLC cells were maintained at 37°C in a 5% CO2 incubator. To evaluation protein expression, cells were seeded onto six-well culture plates.
Cell proliferation assay
Cells were cultured in 96-well plates. When the cells reached about 80% confluence, the medium (MedChem Express, Princeton, NJ, USA) in the UNBS5162 group was replaced with complete medium containing UNBS5162 (10 μM) for 24 hours and 0.1‰ dimethyl sulfoxide (DMSO) (AMRESCO, Solon, OH, USA) was employed as a vehicle control. A 10 μL cell counting kit-8 (CCK8) (Dojindo, Kumamoto, Japan) reagent was then applied to the medium, which was incubated for another 1.5 hours. The resulting formazan precipitates were dissolved in DMSO and the optical density at 450 nm was read using a microplate reader.
Cell colony formation
A total of 500 cells were cultured in Petri dishes (60 mm) containing 5 mL medium and 10 μM UNBS5162 or 1‰ DMSO, which was maintained for 14 days at 37°C in a humidified incubator with 5% CO2. The cells were then fixed with 4% paraformaldehyde for 30 minutes, after which the fixative was removed and the cells were dyed with crystal violet for 30 minutes using cells slowly washed with running water. The number of colonies was counted in each dish. The assay was repeated three times with duplicate samples.
Cell invasion and migration assay
Cancer cell invasion assays were performed using a 24-well transwell plate. Cells were suspended by 100 μL Matrigel matrix (serum-free medium diluted by 1:6) and seeded on the top chamber of the 24-well inserts (8 μm pore size) (Corning, Tewksbury, MA, USA). Serum-free medium was added to the lower compartments and the cells were incubated for 30 minutes to hydrate the basilar membrane. Cell suspension (1 × 105 cells/100 μL serum-free minimum essential media) was added to the upper compartments and complete culture solution (500 μL) was added to the lower compartments after incubation for 24 hours. The cells that did not migrate through the pores were removed by scraping the membrane with a cotton swab. The cells were fixed for 30 minutes in 4% paraformaldehyde after being passed through the filter and then stained for 20 minutes with 0.1% crystal violet. Five random fields (100×) were captured under the microscope. Finally, we counted the number of invasive cells and calculated the average values. Each experiment was conducted in triplicate.
The migration experiment was similar to the invasion assay procedure, but the transwell chamber was not required to be paved with Matrigel matrix and the number of cells seeded to the upper chambers was 5000.
Flow cytometric analysis
After the cells were treated with UNBS5162 for 24 hours, the medium was removed and serum-free starved for 24 hours. Evaluation of apoptosis was performed using an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit (Beijing 4A Biotech Co., Ltd), as previously described.16 The results were evaluated by a flow cytometer (BD Biosciences, San Jose, CA, USA) after the cells were labeled with Annexin-V and PI.
Western blotting
The cells were treated with UNBS5162 for 24 hours and then collected and lysed in radioimmunoprecipitation assay (RIPA) lysis buffer (Beijing ComWin Biotech Co. Ltd. [CWBIO], Beijing, China). The protein concentration was detected using a BCA kit (CWBIO). Subsequently, the equal proteins (20 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA, USA) and then transferred onto a polyvinylidene difluoride membrane.17 After blocking with 5% non-fat dry milk for one hour, the proteins were incubated with various antibodies overnight at 4°C, including Bcl-2, Bax, Bim, active-caspase3, AKT, p-AKT, mTOR, p-mTOR, and GAPDH (Proteintech Group Inc., Rosemont, IL, USA). Secondary anti-mouse or anti-goat horseradish peroxidase antibodies (1/2000 dilution) were added for one hour, and then the bands were visualized using electrochemiluminescence reagents and developed using Hyperfilm-ECL (GE Healthcare, Piscataway, NJ, USA).
Statistical analysis
All data were analyzed using SPSS version 18.0 (SPSS, Chicago, IL, USA). Data were expressed as means ± standard deviations. Statistical significance of the difference between two groups was determined by Student's t-test. P < 0.05 was regarded statistically significant.
Results
UNBS5162 inhibited the proliferation and growth of human A549 non-small cell lung cancer (NSCLC) cells
To investigate the role of UNBS5162 in the proliferation of human A549 NSCLC cells, we performed CCK-8 and cell colony formation assays. As shown in Figure 1, compared to the control, UNBS5162 significantly decreased the optical density value of A549 cells 72 hours after treatment (P < 0.05). The number of colonies formed in UNBS5162 treated human NSCLC cells decreased significantly (Fig 1). These results suggested that UNBS5162 inhibited the proliferation and growth of human A549 NSCLC cells.
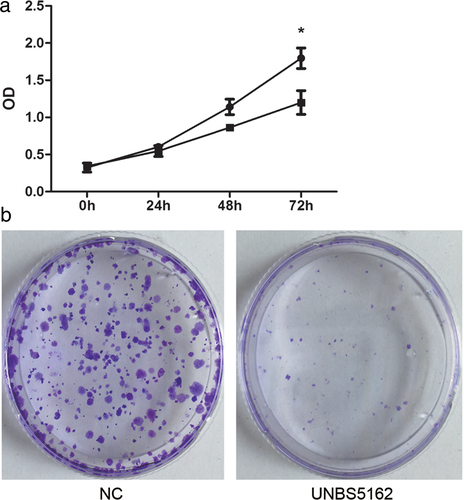
 ) NC and (
) NC and ( ) UNBS5162.
) UNBS5162.UNBS5162 suppressed human A549 NSCLC cell invasion and migration
We then observed the effect of UNBS5162 on human A549 NSCLC cell invasion and migration. In invasion assay, the number of positive crystal violet staining cells significantly decreased after treatment with UNBS5162 (P < 0.05) (Fig 2). Similar findings were observed regarding migration: the number of positive cells significantly decreased in the UNBS5162 treated group (P < 0.05) (Fig 2).
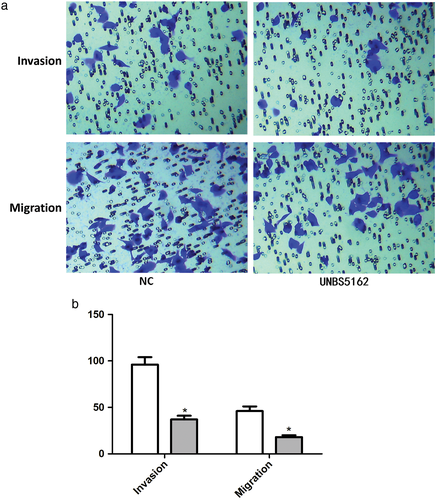
 ) NC and (
) NC and ( ) UNBS5162.
) UNBS5162.UNBS5162 induced apoptosis in human A549 NSCLC cells
To detect the effect of UNBS5162 on human A549 NSCLC cell apoptosis, Annexin-V-FITC/PI staining methods were used. Representative dot plots of Annexin-V-FITC/PI staining are shown in Figure 3a. Under control conditions, most cells remained intact (quadrant [Q] 4, AV-negative/PI-negative 96.93%). After 24 hours of treatment with UNBS5162, the populations of intact cells decreased (84.8%), while the number of early (Q3, AV-positive/PI-negative) and late (Q2, AV- positive /PI- positive) (19.8% > 5.8%) apoptotic cells significantly increased (P < 0.05).
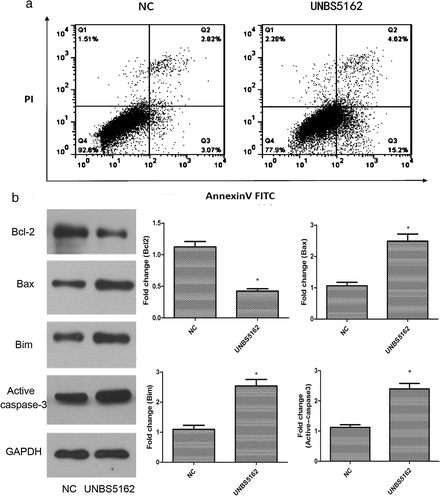
In addition, compared to the control group, anti-apoptotic protein Bcl-2 expression in the UNBS5162 group was significantly decreased (P < 0.05), and proapoptotic protein Bim, Bax, and Active caspase-3 expression was significantly increased (P < 0.05) (Fig 3b).
All of these results suggested that UNBS5162 could induce apoptosis in human A549 NSCLC cells.
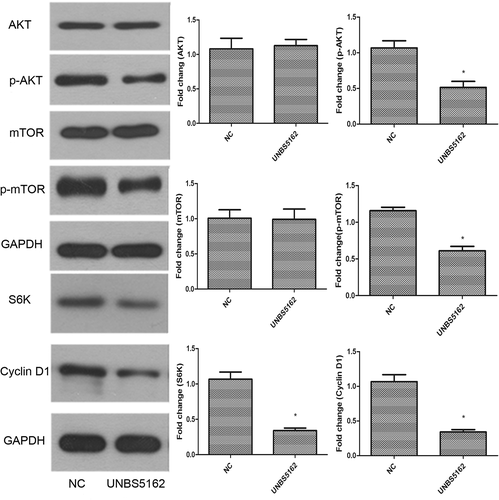
UNBS5162 inhibited the PI3K signaling pathway in human A549 NSCLC cells
It has been shown that the PI3K signaling pathway is closely related to tumor formation, therefore, the expression of related proteins was analyzed with Western blot assay.18-20 These results showed that phospo-AKT and phospo-mTOR levels were significantly decreased in UNBS5162 treated A549 cells (P < 0.05). S6K and Cyclin D1 protein expression was also significantly decreased in UNBS5162 treated A549 cells (P < 0.05). Collectively, these results suggested that UNBS5162 might inhibit the proliferation and metastasis of human A549 NSCLC cells by blocking the PI3K signaling pathway (Fig 4).
Discussion
Lung cancer is one of the most common causes of cancer-related death in the world. Despite advances in lung cancer treatment, the overall five-year survival rate has not substantially increased in the past few years.21 Elinafide, a naphthalimide, has revealed marked activity against tumors both in vitro and in vivo.9, 22 One early representative naphthalimide, amonafide, was evaluated in clinical trials as a potential anticancer agent; however, it failed to enter phase III as a result of dose-limiting bone marrow toxicity. Further novel amonafide analogues are still being evaluated in experimental cancer models.23-25 UNBS5162 is an improved naphthalimide; however, its effect on cancer has only been reported in regard to prostate cancer, advanced solid tumors, and lymphomas.15, 26 Although the UNBS5162 prodrug UNBS3157 has been associated with increased survival in NSCLC patients, the regulatory mechanism of UNBS3157 in NSCLC is not yet known.14 Therefore, we investigated the role of UNBS5162 in NSCLC in this study. The results showed that UNBS5162 significantly inhibited the proliferation of human A549 NSCLC cells and the number of colonies formed, suggesting that UNBS5162 inhibits the proliferation and growth of human A549 NSCLC cells. In addition, UNBS5162 reduced the invasion and migration of human A549 NSCLC cells. Collectively, UNBS5162 may be a novel drug for treating NSCLC in the future.
Apoptosis is a vital pathway regulating cell death and is also the most common cellular mechanism for naphthalimide inhibition in cancer cells.27 Insufficient apoptosis could result in uncontrolled cell proliferation, which has been shown to be involved in cancer.28-30 Ji et al. synthesized a triazolonaphthalimide, LSS-11, which exhibited strong cytotoxicity by inducing cell cycle arrest and apoptosis in selected human colon cancer cell lines, accompanied by DNA damage response.27 In our study, we found that after treatment with UNBS5162 for 24 hours, the populations of intact cells were decreased, while the numbers of early and late apoptotic cells were significantly increased. The expression of antiapoptotic protein Bcl-2 was significantly decreased, while the expression of proapoptotic proteins Bim, Bax, and active caspase-3 were significantly increased after treatment with UNBS5162 for 24 hours. Therefore, we hypothesize that UNBS5162 may inhibit A549 cell proliferation by promoting cell apoptosis.
The PI3K/AKT pathway is an important signaling pathway, which contributes to cellular growth. In the context of infection, activation of this pathway is associated with an increase in proliferation, cell survival, and cell migration, as well as enhanced protein synthesis via mTOR activation.31-33 Akt activation is known to regulate cell cycle progression, cell death, and cell growth. In our study, UNBS5162 exposure inhibited phosphorylation of Akt and its downstream protein, mTOR, in human A549 NSCLC cells. Moreover, S6K and Cyclin D1 protein expression in human A549 NSCLC cells were significantly decreased after treatment with UNBS5162 (Fig 4). S6K and Cyclin D1 are closely related to cell proliferation and the cell cycle.34 S6K is reported to be an analogous suppression of the phosphorylation of mTOR.35 Cyclin D1 activates Cdk4 and Cdk6, which are important in cell cycle progression through the G1 phase, and a reduction of Cyclin D1 expression impedes G1/S transition.36
In summary, these results provide a scientific basis for the application of UNBS5162 to NSCLC treatment in the future. UNBS5162 inhibited NSCLC cell proliferation and growth by suppressing the PI3K pathway to mediated apoptosis. In addition, UNBS5162 may mediate cell cycle progression through Cyclin D1 protein.
Acknowledgments
I would like to express my heartfelt thanks to Professor Jiaqiang Xing, who provides his expert guidance, direction and encouragement throughout my study. I am also sincerely grateful to Professor Yujun Gao, etc., who give me valued lessons and lectures during the period of my study.
Disclosure
No authors report any conflict of interest.




