Long-lasting response to third-line crizotinib treatment in a patient with non-small cell lung cancer with brain metastases and poor performance status
Abstract
Identification of the anaplastic lymphoma kinase (ALK) gene has refined the classification of non-small cell lung cancer (NSCLC) and promoted research on molecularly targeted drugs such as crizotinib, an ALK inhibitor with good efficacy, in ALK-rearranged NSCLC. At present, few studies have reported the efficacy of crizotinib in patients with ALK-rearranged NSCLC with brain metastases. In a patient with NSCLC harboring ALK-rearrangement who had brain metastases and poor performance status (PS), we obtained a durable response with crizotinib administered following multi-line chemotherapy regimens.
Introduction
Anaplastic lymphoma kinase (ALK) rearrangement is identified in approximately 5% of patients with non-small cell lung cancer (NSCLC), and these patients have been found to respond well to crizotinib, an oral ATP-competitive inhibitor of ALK.1, 2 In the recent PROFILE clinical trials, a promising intracranial disease control rate (I-DCR) and median intracranial time to progression (MI-TTP) were reported with crizotinib in patients with ALK-rearranged NSCLC with brain metastases.3, 4 In this report, we document the response to third-line crizotinib therapy in a patient with ALK-rearranged NSCLC with brain metastases and poor performance status (PS) who had previously received multi-line chemotherapy.
Case report
A 44-year-old female patient with no history of smoking presented with right chest pain and a cough in June 2012. A chest computed tomography (CT) scan revealed a mass with a maximum diameter of 4.5 cm in the right upper lung and right pleural effusion, but an upper abdominal CT scan and bone emission CT revealed no abnormalities. Magnetic resonance imaging (MRI) revealed multiple nodules in the brain with a maximum diameter of 1 cm, but no peripheral edema. Biopsy of the pleura confirmed a poorly differentiated lung adenocarcinoma. An epidermal growth factor receptor (EGFR) gene mutation test was negative. The patient was diagnosed with NSCLC with brain metastases (cT4N0M1, stage IV). Her Eastern Cooperative Oncology Group PS was 0.
Initially, the patient received first-line chemotherapy with six cycles of pemetrexed and cisplatin (AP). A partial response (PR) was achieved in the lung (shrinkage of the pulmonary tumor and pleural effusion) and a complete response (CR) was achieved in the brain, with elimination of the metastases. The patient subsequently received maintenance treatment with pemetrexed and palliative radiotherapy for the right upper lung lesion. However, after six cycles of pemetrexed maintenance treatment, the disease progressed. A recurrent nodule was detected by brain MRI in the left frontal lobe (maximum diameter, 3 mm), and bilateral lung and liver metastases (multiple small bilateral lung lesions with maximum diameters of 5 mm and single liver nodules with maximum diameters of 3 cm) were detected by an 18F-labeled fluorodeoxyglucose-positron emission tomography-CT scan. As her PS was adequate (PS = 1), the patient received second-line chemotherapy with docetaxel and cisplatin (DP), which was administered for four cycles. In addition, she was treated with γ-knife radiotherapy for her recurrent brain lesion. Following two cycles of DP therapy, tumor assessments revealed stable disease (SD). However, CT and MRI scans after four cycles of DP showed enlargement of the bilateral lung tumors and brain lesions (maximum diameters, 4 and 6 mm, respectively; Fig 1).
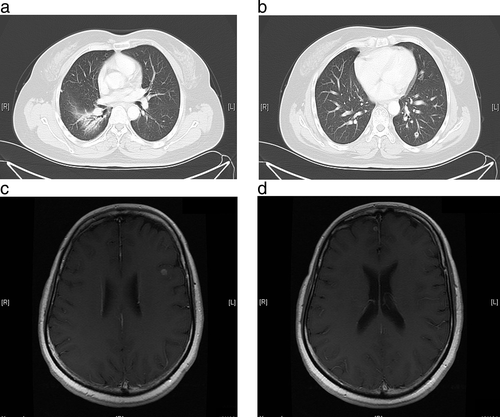
Around this time, the patient developed severe anemia, fatigue, and neurotoxicity as a result of the platinum-based chemotherapy, and her PS worsened (PS = 2). Because of the stressful nature of further biopsies of the small lesions, the original pleural sample was used for detection of ALK rearrangement by fluorescence in-situ hybridization testing. As the tumor was positive for ALK rearrangement, the patient was started on crizotinib 250 mg orally twice daily in June 2014. Two months after the initiation of crizotinib therapy, CT and MRI examinations revealed a decrease in the size of the bilateral lung metastases, and the brain and liver metastases had disappeared (Fig 2). After a year of crizotinib therapy, a brain MRI showed two new nodules in the left temporal lobe (maximum diameter, 3 mm) and the right frontal lobe (maximum diameter, 2.5 cm), but the pulmonary lesions remained stable. X-knife radiotherapy was then administered to control the two new brain metastatic lesions.
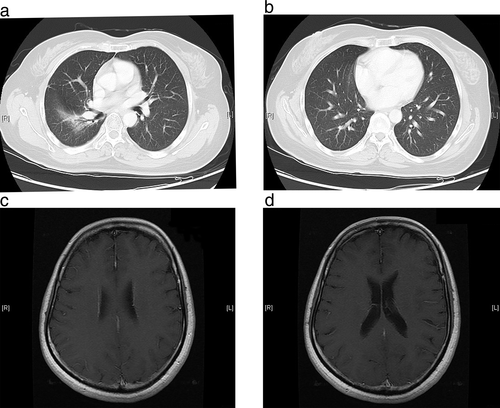
By June 2016, the patient had received crizotinib treatment for 24 months without any evidence of disease progression. Although she experienced a slight blurring of vision, fatigue, and nausea during the first month of crizotinib therapy, these symptoms gradually disappeared after a month.
Discussion
Approximately 10–20% of patients with NSCLC are found to have brain metastases at the time of initial diagnosis, and several retrospective studies have reported that 20–30% of patients with brain metastases have ALK-rearranged NSCLC.5-8 Because of the blood–brain barrier (BBB), antineoplastic drugs commonly exhibit poor penetration into the brain; therefore, the central nervous system (CNS) has been considered a sanctuary for many types of cancer. However, studies have shown that the BBB can be disrupted by brain metastases, and targeted therapies, such as EGFR and ALK tyrosine kinase inhibitors (TKIs), have shown great potential in treating brain metastases.3,4,9-12
Crizotinib is a first-generation ALK inhibitor approved by the United States Food and Drug Administration because of its effectiveness in the treatment of ALK-rearranged NSCLC.1 Recently, some studies have focused on the CNS activity of crizotinib in NSCLC patients with brain metastases.3, 4 A pooled retrospective study analyzed the clinical benefits of crizotinib in ALK-rearranged NSCLC with brain metastases after failure of at least one prior chemotherapy regimen in patients who had participated in the PROFILE 1005 and PROFILE 1007 clinical trials.3 The 12-week I-DCR with crizotinib in patients with previously untreated asymptomatic brain metastases was 56%, compared with 62% in patients with previously treated brain metastases, and the MI-TTP were 7 and 13.2 months, respectively.3 In a prospective study (PROFILE 1014) that compared first-line crizotinib with pemetrexed plus cisplatin or carboplatin chemotherapy in ALK-rearranged NSCLC, a significantly higher I-DCR at 12 weeks was achieved with crizotinib in patients with stable treated brain metastases (85% vs. 45%, respectively; P < 0.001), and the median progression-free survival (PFS) was also significantly longer with crizotinib (9 vs. 4 months, respectively; P < 0.001).4 In our patient, third-line therapy with crizotinib achieved intracranial progression-free survival (I-PFS) of 12 months, and an additional 12 months of brain lesion control was achieved when X-knife radiotherapy was performed, together with continued crizotinib therapy for the new lesions. As crizotinib has demonstrated good efficacy in the subset of ALK-rearranged NSCLC patients with brain metastases and is relatively well tolerated, patients with a poor PS score should be given the opportunity of treatment with crizotinib. Because the patient we treated showed sensitivity to the first-line therapy administered, it may be that the efficacy of crizotinib is better in chemotherapy-sensitive patients. This has been reported in EGFR-TKI therapy, and could be one of the reasons for the long PFS.13 Although isolated CNS progression occurred in our patient after 12 months of crizotinib treatment, no progression was found in her extracranial disease. Two new nodules were detected in the brain but the previously identified brain metastases were stable. The likely reason for this may be poor drug penetration in the non-metastatic brain area.14, 15 Isolated CNS relapse is a common disease progression pattern in patients with ALK-rearranged NSCLC who are treated with crizotinib.3 In a retrospective study, Takeda et al. analyzed the clinical impact of continuing crizotinib treatment after radiotherapy for isolated CNS progression in patients with ALK-rearranged NSCLC.16 In addition to controlling the extracranial lesions, the brain metastases were controlled for another 5.5 months.16 In our patient, isolated CNS progression occurred after one year of crizotinib treatment. However, continued crizotinib therapy combined with irradiation of the brain lesion achieved long-lasting survival.
Continuous EGFR-TKI treatment combined with local treatment has been proven an effective strategy for patients with locally advanced NSCLC.17, 18 Our findings indicate that similar benefits can be obtained with continuous ALK-inhibitor treatment for locally advanced disease.16
Acknowledgment
Editorial assistance was provided by Content Ed Net, Shanghai Co. Ltd.
Disclosure
No authors report any conflict of interest.




