Continuous infusion of high-dose ulinastatin during surgery does not improve early postoperative clinical outcomes in patients undergoing radical lung cancer surgery: A pilot study
Abstract
Background
Ulinastatin can prevent the perioperative increase in proinflammatory cytokines for lung resection surgery; however, its impact on early clinical outcomes remains unknown.
Methods
The study enrolled 108 non-small cell lung cancer (NSCLC) patients who were randomly allocated into two groups: ulinastatin (group U) and control (group C). Patients in group U ( n = 52) were continuously intravenously infused with ulinastatin at a rate of 20 000 U/kg/hour for the first hour after anesthesia induction, and then at a rate of 5000 U/kg/hour until the conclusion of surgery. Patients in group C ( n = 56) received an equivalent volume of normal saline. The primary outcome was to record the postoperative pulmonary complications that occurred during hospital stay. Other clinical courses, such as hospital mortality, blood loss, respiratory parameters, postoperative chest drainage, and duration of intensive care unit and postoperative hospital stay, were also observed and analyzed.
Results
There were no significant differences between the two groups in early postoperative pulmonary complications, hospital mortality, blood loss, or other perioperative laboratory values, except for the duration of postoperative chest drainage and serum creatinine level. The frequency of pulmonary complications was lower in patients treated with ulinastatin compared with the control (38.46% in group U vs. 48.21% in group C).
Conclusion
Administration of high-dose ulinastatin during surgery did not reduce postoperative pulmonary complications, hospital mortality, or hospital stay for patients undergoing lung radical thoracotomy. However, a protective trend of ulinastatin was observed.
Introduction
Lung cancer is one of the most common cancers and the leading cause of cancer related death worldwide.1-3 Surgery remains the best curative option for patients with lung cancer.4 However, postoperative pulmonary complications (PPCs) are observed at high frequencies and are the major cause of postoperative morbidity and mortality in patients undergoing radical lung surgery.5-7 Mechanical ventilation, surgical procedure, and anesthesia can all induce a severe inflammatory response; prolonged high levels of inflammatory cytokines and neutrophil elastase are related to the development of postoperative complications.8
Ulinastatin, a human protease inhibitor found in urine, has the capacity to inhibit both the activity of neutrophilic elastase and the activation of proinflammatory cytokines.9, 10 Previous studies have demonstrated that ulinastatin can improve clinical outcomes in patients with acute inflammation, such as acute pancreatitis, shock, cardiac surgery, and organ transplantation, and can alleviate acute lung injury as a result of severe burns, sepsis, esophagectomy surgery, and hemorrhagic shock.11-14
Although some trials have demonstrated that ulinastatin can prevent the intraoperative and postoperative increase in proinflammatory cytokines for lung resection surgery, its impact on postoperative complications and clinical outcomes remains unknown. Therefore, we designed this randomized prospective pilot study for further multi-center research to examine the effects of intraoperative high-dose ulinastatin treatment on early postoperative clinical outcomes in patients undergoing lung resection surgery.
Methods
Clinical data and grouping
This trial was performed in accordance with the principles of the Declaration of Helsinki. The Ethical Committee of West China Hospital, Sichuan, China approved the protocol of this study. All patients signed written informed consent before entering the study.
Non-small cell lung cancer (NSCLC) stage I–IV patients (aged 30–80 years, American Society of Anesthesiologists classification ≤ III) were enrolled into the study. All patients had undergone elective radical thoracotomy in the Lung Cancer Treatment Center, West China Hospital of Sichuan University, China, in 2014 or 2015. Patients: (i) with allergies to ulinastatin; (ii) who had secondary surgery for recurrent tumors; (iii) with other malignant tumors; (iv) who required preoperative mechanical ventilation (> 1 hour) within two weeks; and (v) with a body mass index (BMI) ≥ 35 kg/m2, were excluded.
The study was a randomized, prospective, placebo-controlled, double blind trial. The consecutively enrolled 108 patients were randomly assigned into ulinastatin group (group U) and control group (group C) according to the computer generated random numbers.
Ulinastatin therapy
Patients in group U were intravenously infused with ulinastatin (Techpool, Guangdong, China) at a rate of 20 000 U/kg/hour for the first hour after anesthesia induction and then at a rate of 5000 U/kg/hour (diluted into 20 mL using normal saline) until the conclusion of surgery. For group C, the ulinastatin solution was replaced with normal saline in the same volume and infused at the same rate. A nurse who was not participating in the study opened the randomized envelopes and prepared the study drug solution.
Anesthetic method and perioperative management
The same surgeon performed all surgeries. Five-lead electrocardiogram (ECG), pulse oximetry (SPO2), end-tidal carbon dioxide tension (EtCO2), bispectral index (BIS), invasive blood pressure (BP), and central venous pressure (CVP) were recorded throughout the surgery. Arterial blood samples were collected through the radial artery before surgery, 30 minutes after one lung ventilation (OLV), and 30 minutes after re-two lung ventilation (re-TLV). The respiratory parameters and vital signs were recorded at the same time.
Patients received volume-controlled mechanical ventilation with a tidal volume (VT) of 6–8 mL/kg of predicted body weight, a positive end-expiratory pressure (PEEP) of 6–8 cm of water and a recruitment maneuver (applying a continuous positive airway pressure of 30 cm of water for 30 seconds), repeated every 30 minutes for lung re-expansion before and after OLV to avoid atelectasis. In case of desaturation during OLV (defined as a peripheral oxygen saturation less than 90%), rescue therapy was proposed and consisted of increasing FiO2 to 100% and continuously uptaking oxygen in the non-ventilated lung for 3–5 L/minutes or discontinuing TLV until oxygen saturation had reached the target value (oxygen saturation ≥ 90%). The fluid infusion speed was adjusted according to bleeding quantity and CVP, and blood products were used according to blood transfusion guidelines. After surgery, all patients were transferred into the intensive care unit (ICU), with a tracheal tube.
The postoperative clinical course of each patient was carefully observed daily until discharge and pulmonary complications were evaluated. For patients discharged within 30 days after surgery, information was retrieved via telephone follow-up. Postoperative complications were observed and evaluated by a blinded assessor.
Ulinastatin-related adverse events were defined as shock, rash, itching, nausea, vomiting, diarrhea, allergy, or neutropenia.
Outcomes
The primary outcome of the study was to determine postoperative pulmonary complications (PPCs). PPCs were defined as the presence of pneumonia, bronchopleural fistula, massive atelectasis, adult respiratory distress syndrome (ARDS), acute lung injury (ALI), or respiratory failure without signs of pneumonia and/or atelectasis that required prolonged management by artificial ventilation.15 Pulmonary complications resulting from postoperative recurrent nerve palsy were excluded.16 PPC morbidities were attributed in a binary fashion by patient to ensure that a patient with more than one event was only counted once.17
The secondary outcomes were hospital mortality, chest drainage, cardiovascular complications (arrhythmia, acute cardiac failure), wound infection, and duration of ICU and postoperative hospital stays. Operative mortality was defined as patient death after surgery before discharge from the hospital or within 30 days of surgery.17
Other clinical outcomes evaluated included blood routine and biochemical tests in the first 24 hours after surgery.
The respiratory index (RI), dynamic lung compliance (Cdyn), and oxygenation index (OI) during surgery were calculated according to the following formulas: RI = P(A–aO2)/partial pressure of O2 in arterial blood (PaO2) = ([fraction of inspired oxygen {FiO2} × {760–47}– partial pressure of carbon dioxide in arterial blood {PaCO2}/0.8–PaO2]/PaO2); Cydn = (Cydn [mL/cmH2O]) = VT/(Pmax-PEEP); and OI = PaO2/FiO2.
Statistic analysis
Statistical analyses were carried out with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Normally distributed continuous data were expressed as mean ± standard deviation; non-normal distributed data were expressed as median (interquartile range). Enumeration data were expressed as count proportion. One-way analysis of variance was used for continuous variables. Chi-square or Fisher's exact tests were used to compare categorical data. P values < 0.05 were considered statistically significant.
Results
Baseline characteristics of enrolled patients
Between November 2014 and August 2015, of 338 patients screened, 117 patients were enrolled; 108 (92.31%) patients completed the pilot study, with 52 in the ulinastatin and 56 in the control group (Fig 1).
The baseline patient characteristics were balanced between the two groups (P > 0.05), except for base excess (BE), which was lower in group U (−0.28 ± 1.67) than in group C (0.49 ± 1.91; P = 0.046; Table 1).
| Characteristic | Ulinastatin (n = 52) |
Control (n = 56) |
P |
|---|---|---|---|
| Age (years) | 61 (53.00–66.00) | 60.0 (51.25–60.00) | 0.319 |
| Gender (male/female) | 37/15 | 41/15 | 0.832 |
| Height (cm) | 165.12 ± 6.153 | 164.38 ± 6.57 | 0.556 |
| Weight (kg) | 62.0 ± 9.16 | 61.40 ± 9.11 | 0.735 |
| Body-mass index (kg/m2) | 22.82 ± 2.81 | 22.67 ± 2.56 | 0.756 |
| Tumor status | |||
| I | 6 | 12 | 0.476 |
| II | 11 | 13 | |
| III | 29 | 27 | |
| IV | 6 | 4 | |
| ASA classification | |||
| I | 16 | 13 | 0.19 |
| II | 32 | 42 | |
| III | 4 | 1 | |
| ABG (room air) | |||
| PaO2 | 80.57 ± 9.63 | 79.57 ± 19.67 | 0.764 |
| PaCO2 | 36.49 ± 2.90 | 38.69 ± 7.25 | 0.065 |
| BE | −0.28 ± 1.67 | 0.49 ± 1.91 | 0.046* |
| Lung function | |||
| FVC (L) | 3.05 ± 0.8 | 3.13 ± 0.82 | 0.643 |
| FEV1/FVC (%) | 73.79 ± 9.76 | 71.06 ± 13.37 | 0.265 |
| Breath holding test (seconds) | 33.8 ± 10.21 | 35.18 ± 14.25 | 0.593 |
| Smoke index | 400 (0–600) | 530 (0–800) | 0.598 |
| Pre-existing comorbid condition | |||
| Hypertension | 5 | 6 | 0.553 |
| Diabetes mellitus | 8 | 9 | 0.567 |
| Heart disease | 3 | 4 | 0.542 |
| COPD | 14 | 13 | 0.412 |
| Type of surgery | |||
| Sublobectomy | 4 (7.69%) | 5 (8.93%) | 0.993 |
| Lobectomy | 33 (63.46%) | 36 (64.29%) | |
| Biolectomy | 13 (25.00%) | 13 (23.21%) | |
| Peneumonectomy | 2 (3.8%) | 2 (3.4%) | |
| Blood loss during surgery (mL) | 231.00 ± 120.33 | 257.22 ± 190.11 | 0.399 |
| Duration of OLV (minutes) | 95.46 ± 39.97 | 86.41 ± 40.05 | 0.264 |
| Duration of surgery (minutes) | 154.97 ± 57.227 | 147.11 ± 58.41 | 0.508 |
- * P < 0.05 compared with the control. ABG, arterial blood gases; ASA, American Society of Anesthesiologists; BE, base excess; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; OLV, one lung ventilation; PaO2, partial pressure of O2 in arterial blood; PaCO2, partial pressure of carbon dioxide in arterial blood.
Clinical outcomes
Primary clinical outcome
Primary clinical outcomes are presented in Table 2. Similar results were obtained between the two groups for primary clinical outcomes. PPCs were observed in 20 patients (38.46%) in group U and 27 (48.21%) in group C. Pulmonary complications occurred less frequently in group U; however, there was no significant difference (P = 0.307).
| Outcome | Ulinastatin (n = 52) |
Control (n = 56) |
P |
|---|---|---|---|
| Primary outcome | |||
| Pulmonary complications (n) | |||
| Pneumonia (n) | 14 (26.92%) | 16 (28.57%) | 0.808 |
| Bronchopleural fistula (n) | 3 (5.8%) | 2 (3.6%) | 0.465 |
| Respiratory failure (n) | 8 (15.38%) | 7 (12.50%) | 0.665 |
| ARDS/ALI (n) | 11 (21.15%) | 14 (25.00%) | 0.636 |
| Total | 20 (38.46%) | 27 (48.21%) | 0.307 |
| Secondary outcomes | |||
| Hospital mortality (n) | 2 (3.8%) | 2 (3.4%) | 0.649 |
| Chest drainage ≥ 5 days (n) | 8 (15.4%) | 18 (32.1%) | 0.039* |
| Chest drainage (mL) | 761.30 ± 541.49 | 836.45 ± 582.79 | 0.514 |
| Arrhythmia (n) | 7 (13.5%) | 15 (26.8%) | 0.069 |
| Heart failure (n) | 1 (1.9%) | 1 (1.8%) | 0.733 |
| Wound infection (n) | 2 (3.8%) | 3 (5.36%) | 0.709 |
| Length of stay, median (IQR) | |||
| ICU (days) | 3.49 ± 1.82 | 3.78 ± 2.34 | 0.492 |
| Postoperative hospital (days) | 12.71 ± 3.33 | 13.16 ± 4.09 | 0.574 |
- * P < 0.05 compared to control. ARDS, adult respiratory distress syndrome; ALI, acute lung injury; ICU, intensive care unit; IQR, interquartile range.
Secondary clinical outcomes
Secondary clinical outcomes are presented in Table 2. Except for chest drainage duration, no significant differences were observed between the groups (P > 0.05). Chest drainage duration in eight patients (15.4%) in group U and 18 (32.1%) in group C was longer than five days (P = 0.039). Hospital mortality did not differ between the groups (P = 0.649). Two patients in each group died within 30 days after surgery. There were no differences in the amount of chest drainage, arrhythmia, heart failure, or length of ICU or postoperative hospital stay between the two groups (P > 0.05).
The level of postoperative creatinine in the serum in group U (87.31 ± 33.16) was higher than in group C (80.53 ± 19.95; P = 0.013; Table 3). Other postoperative clinical courses between the two groups were similar (P > 0.05).
| Laboratory value | Ulinastatin (n = 52) |
Control (n = 56) |
P |
|---|---|---|---|
| Routine blood count (pod1) | |||
| WBC count | 14.81 ± 3.71 | 15.18 ± 3.89 | 0.627 |
| Neutrophil (%) | 87.04 ± 5.09 | 87.53 ± 3.40 | 0.566 |
| Lymphocyte (%) | 6.33 ± 3.85 | 6.20 ± 2.20 | 0.835 |
| Biochemical test | |||
| BUN | 6.43 ± 2.03 | 6.5519 ± 1.76 | 0.748 |
| Cr | 87.31 ± 33.16 | 80.81 ± 19.95 | 0.013* |
| LDH | 226.80 ± 41.25 | 237.59 ± 58.63 | 0.135 |
- * P < 0.05 compared to control. BUN, blood urea nitrogen; Cr, creatinine; LDH, lactate dehydrogenase; WBC, white blood cell.
Intraoperative respiratory index
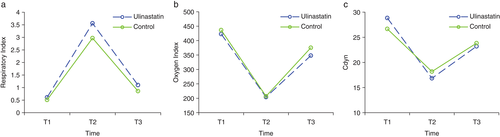
There were no significant differences observed in RI, OI, or Cdyn at all three time points (preoperative; OLV 30 minutes; re-TLV 30 minutes) between the two groups (P > 0.05; Fig 2).
Safety analysis
No adverse events related to ulinastatin administration were observed during the study.
Discussion
In this clinical trial, the observed rate of postoperative complications was slightly higher than previously reported (3.3–40%).18 Of the 108 patients enrolled, 38.46% patients in the U group and 48.21% in the control developed pulmonary complications. In part, this was a result of the inclusion of NSCLC stage III–IV patients (61.11%) who experienced more extensive surgery trauma, as well as patients with chronic obstructive pulmonary disease (varying from mild to severe), both of which are associated with increased morbidity rates.19, 20 Most previously reported studies only included early stage NSCLC patients with almost normal lung function.21
Continuous intraoperative infusion of high-dose ulinastatin was not observed to lead to significantly improved clinical outcomes after radical lung cancer resection in this trial. Ulinastatin has been shown in previous studies to alleviate inflammatory response and to improve the respiratory parameters in patients undergoing lung resection surgery.22, 23 Few studies, especially prospective, randomized, and controlled studies, however, have observed the effect of ulinastatin on clinical outcomes in patients undergoing radical lung cancer resection. Although some studies found that ulinastatin was effective in the treatment of ALI and ARDS, these findings were mainly from patients with sepsis, acute pancreatitis, and burn injuries, and the treatment always lasted for a long period.24-26 A systematic meta-analysis demonstrated that ulinastatin significantly decreased ICU mortality and the length of ICU stay in patients with ALI and ARDS, while 28-day mortality was not significantly affected.27 Another systematic meta-analysis revealed that intraoperative ulinastatin did not affect hospital mortality, postoperative complication rate, or length of ICU stay in patients undergoing cardiac surgery.13 Lung cancer surgery consists of complex trauma, including the surgical removal of part of lung, OLV, mechanical ventilation, and anesthesia, which can induce pulmonary and systemic injuries. In our study, over 60% of the patients were stage III–IV NSCLC patients, considered high-risk. Our results suggest that ulinastatin may have limited benefits for high-risk patients or those undergoing complicated surgery. However, injuries caused by lung surgery may last days or weeks, and ulinastatin was only used during surgery in our study. Further research is required to determine whether prolonged administration of ulinastatin after surgery is of benefit.
Pulmonary complications occurred less frequently in patients treated with ulinastatin (38.46% in group U vs. 48.21% in group C). We also observed that ulinastatin administration led to a shorter duration of postoperative chest drainage. These findings suggest, to some extent, that intraoperative ulinastatin might promote the recovery of lung injuries caused by surgery.25 As a protease inhibitor of neutrophil elastase, ulinastatin can alleviate pulmonary edema, hemorrhage, and inflammatory exudation in acute lung injuries through mitigation of the inflammatory reaction and inhibition of immunosuppression.28-31
We used ulinastatin at high doses until the conclusion of surgery. It has been demonstrated that ulinastatin works in a dose-dependent manner and was safe at a dose of 20 000 U/kg in a tolerance trial.26, 32-34 Thus, we designed this pilot study to treat patients with ulinastatin at 20 000 U/kg/hour for the first hour after anesthesia induction and then 5000 U/kg/hour until the conclusion of surgery in an attempt to achieve the maximal anti-inflammatory effect. This practice suggested that an increase in the dose of ulinastatin during surgery might be limited; however, an examination of the use of ulinastatin after surgery may be worth further trials.
Our study has some limitations. As only 108 patients were enrolled, the population might not be large enough to determine differences. Large-scale research is needed for verification of the possible protective effects of ulinastatin in thoracotomy patients. The patients enrolled underwent four types of surgeries: sublobectomy, lobectomy, biolectomy, and peneumonectomy. Although the distribution between U and C groups was well balanced, this might be a confounding factor and requires further stratified analysis. We only measured direct clinical indicators and did not measure inflammatory markers in this pilot study. As mentioned, ulinastatin reduces the injury caused by inflammatory response; therefore, the protective effect of ulinastatin may be reflected by changes in inflammatory markers. Finally, we did not observe the effect of ulinastatin on long-term outcomes. Ulinastatin is believed to not only protect tissues and organs from severe inflammatory response, but also inhibit tumor invasion and metastasis.9, 35 Further studies are necessary to confirm the inhibitory effect of ulinastatin on lung cancer invasion and metastasis.
In summary, the present study revealed no significant positive effects of ulinastatin on postoperative pulmonary complications and other outcomes for lung cancer patients undergoing radical thoracotomy. However, a protective trend of ulinastatin was observed. Further research is warranted.
Acknowledgments
This study was supported by the Natural Science Foundation of China (NSFC; 30571785; 30972862); the Sichuan Projects of China (2012FZ0121); and the Techpool Research Fund.
Disclosure
No authors report any conflict of interest.




