Gefitinib for asymptomatic brain metastasis from advanced non-small cell lung cancer: Report of a favourable outcome
Abstract
Brain metastasis (BM) is common in patients with non-small cell lung cancer (NSCLC). Although epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have now been included as standard treatment options for NSCLC harboring EGFR-activating mutations, only a few prospective reports demonstrate the efficacy of these agents in a BM setting. We report a case of a patient with advanced NSCLC, in which oral gefitinib documented a significant antitumor effect on parallel progression of extracranial lesion and BM occurred during chemotherapy.
Introduction
As an oral epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI), gefitinib was demonstrated to have significant antitumor activity in patients with advanced non-small cell lung cancer (NSCLC).1, 2 Further investigations and several randomized trials eventually led to the approval of both erlotinib and gefitinib as first-line therapies in advanced patients with activating EGFR mutations.1, 3-5
In patients with NSCLC, 10–20% have brain metastasis (BM) at diagnosis, while another 20% will develop BM during the course of the disease.6, 7 BM usually leads to significant symptoms and mortality and therapeutic options are still limited. Recently, emerging data from retrospective studies and prospective phase II series suggest that EGFR-TKI treatment for NSCLC patients with BM, especially for those harboring EGFR mutations, can obtain a response.8-10
In this paper, we report a case of a patient with advanced NSCLC who had a marked response to gefitinib for both extracranial lesions and BM, which progressed during chemotherapy.
Case report
A 59-year-old male patient with a cough and sputum lasting two months was referred to our hospital. On admission, the patient was confined to a wheelchair because of unbearable pain in his pelvis. A positron emission tomography-computed tomography (PET-CT) scan showed a right superior lobe mass (4.2*4.2 cm, Fig 1a) with multiple metastases in the bilateral supraclavicular and mediastinal nodal stations, bilateral lung (Fig 1c) and bone (Fig 1d), and a maximum standardized uptake value (SUVmax) of 13.4. Bronchoscopy found luminal narrowing of the upper bronchus of the right upper lobe (Fig 1b), and a biopsy showed adenocarcinoma (Fig 1e) expressing thyroid transcription factor-1 (Fig 1f) and P63. Analyses of biopsy specimens revealed adenocarcinoma with an EGFR mutation of an exon 19 deletion without a Kirsten rat sarcoma viral oncogene homolog mutation. Fluorescence in situ hybridization showed no rearrangement of the anaplastic lymphoma kinase gene or amplification of the C-Met gene. The patient was finally diagnosed with clinical stage IV (cT2aN3M1b) lung adenocarcinoma and began first line combination chemotherapy (500 mg/m2 pemetrexed [IV] on day [D] 1 plus 75 mg/m2 cisplatin [IV] on D2 of each 21 day cycle). In the meantime, intravenous bisphosphonate was administrated for bone metastasis. 20 Gy in five fractions of intensity-modulated radiation therapy using 6 MV-X was delivered to the painful metastasis lesion in the left iliac bone and acetabulum. The serious pain (pain score 9; scale: 0–10, 0 being no pain and 10 being the worst pain) was completely resolved seven days after the end of radiation (pain score 0).
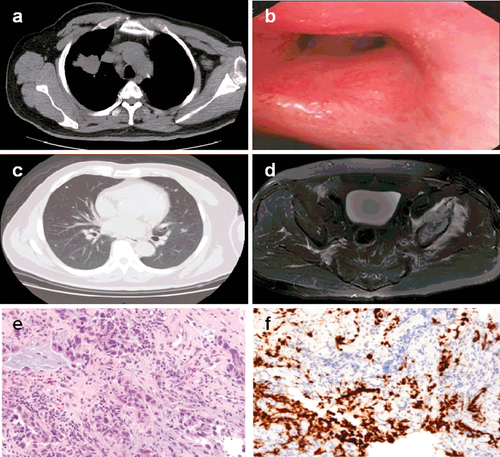
Examination results on admission. (a) Positron emission tomography-computed tomography (PET)-CT scan showing a right superior lobe mass and metastases in mediastinal nodal stations. (b) Bronchoscopy demonstrating luminal narrowing of the upper bronchus of the right upper lobe. (c) PET-CT scan showing multiple metastases in the bilateral lung. (d) Magnetic resonance imaging demonstrating metastases in the pelvic bone. (e) Bronchoscopic biopsy specimen showing adenocarcinoma. (f) Immunohistochemistry revealed that the bronchoscopic biopsy specimen expressed thyroid transcription factor-1.
The patient initially achieved a stable disease response through two cycles of chemotherapy, with grade 2 neutropenia and grade 1 gastrointestinal side effects. However, the disease subsequently progressed after the third cycle of chemotherapy. The progression included multiple brain metastases detected by brain magnetic resonance imaging (MRI) (Fig 2a,b), which led to transient head discomfort, as well as enlarged primary tumor and lymph nodes, observed by thoracic CT scan (Fig 2c,d).
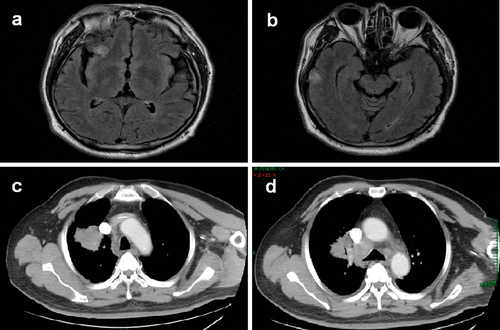
Examination results after chemotherapy. (a) Metastasis detected by brain magnetic resonance imaging (MRI) in the frontal lobe. (b) Metastasis detected by brain MRI in the temporal lobe. (c) Thoracic computed tomography (CT) showing an enlarged primary tumor. (d) Enlarged mediastinal lymph nodes detected by thoracic CT.
The patient was treated with 250 mg of gefitinib daily, which led to significant tumor regression, documented on thoracic CT scan one month later, with no main adverse events observed. Three months after the start of gefitinib treatment, the thoracic CT and brain MRI both demonstrated continuous tumor regression (Fig 3a–d).
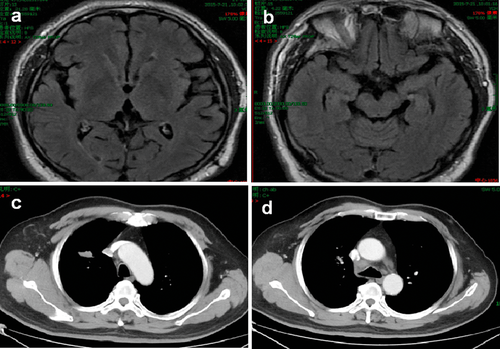
Examination results three months after the start of gefitinib treatment. (a) Regression of the metastasis in the frontal lobe on magnetic resonance imaging (MRI). (b) Regression of the metastasis in the temporal lobe on MRI. (c) Thoracic computed tomography (CT) reveals the primary tumor has shrunk (d) Thoracic (CT) reveals the mediastinal lymph nodes have shrunk.
Discussion
This report documents an NSCLC patient with activating EGFR responding to gefitinib treatment for both progressed extracranial lesions and brain metastases. This case highlights some valuable points related to gefitinib therapy for advanced NSCLC.
At present, EGFR-TKIs are the optimal systemic treatment in EGFR-mutated patients with advanced NSCLC as the first or further lines of treatment. In a meta-analysis, EGFR-TKIs were associated with a lower risk of progression-free survival (PFS) in the front-line setting (n = 13 trials, hazard ratio [HR] = 0.43; 95% confidence interval [CI] = 0.38–0.49; P < 0.001) and in second-line or subsequent treatment (n = 7 trials, HR = 0.34; 95% CI = 0.20–0.60; P < 0.001) in this subset of patients.11 Recently, another meta-analysis involving 1774 such patients confirmed the superiority of EGFR-TKIs as first-line treatment compared with chemotherapy in terms of PFS.12 Our case precisely belonged to this subset and gefitinib was the first choice after the patient's disease progressed during chemotherapy.
Furthermore, it is reasonable to administer gefitinib to NSCLC patients with asymptomatic BM, particularly for the subgroup with activating EGFR mutations. In early years, many case reports and small sample studies suggested that NSCLC-related BM could be treated successfully with gefitinib, with cerebral overall response rates of 10–60%.7, 13, 14 An association between BM response, BM gefitinib, and EGFR mutations in the primary tumor was later reported.8 Recently, in several retrospective or phase II trials, the application of EGFR-TKIs led to favorable outcomes, with an intracranial response rate of 70∼88%, and median PFS and overall survival time of 7∼15 and 12∼21 months, respectively.9, 10, 15, 16 Although some retrospective analysis found comparable intracranial tumor durable control and survival of whole brain radiation therapy (WBRT) in comparison with EGFR-TKIs, the toxicity profile of these TKIs at least suggest that WBRT could be delayed or even omitted in a certain number of patients.17
In a certain subgroup of advanced NSCLC patients with bone metastasis, local radiotherapy is the cornerstone of palliative treatment, based on the efficacy of pain relief demonstrated in several randomized trials.18, 19 Balancing efficacy and convenience, we conducted mild multiple fractionated radiation therapy for this potentially curable patient, in which persistent pain relief made the subsequent systematic treatment more feasible.20
This case represents a demonstration of gefitinib treatment producing a favourable clinical response for both extracranial progression and BM occurring during chemotherapy. Further trials are needed to better evaluate the role of this drug in these patients.
Acknowledgment
We thank the Pfizer Corporation for funding support.
Disclosure
No authors report any conflict of interest.




