Role of radiotherapy in treating patients with primary malignant mediastinal non-seminomatous germ cell tumor: A 21-year experience at a single institution
Abstract
Background
The aim of this study was to investigate the clinical characteristics and outcomes of patients with primary malignant mediastinal non-seminomatous germ cell tumor (MMNSGCT) by comparing the efficacies of different treatment modalities.
Methods
The charts of 62 consecutive patients with MMNSGCT between 1990 and 2010 were reviewed. Analyses included Kaplan-Meier survival and Cox multivariate regression.
Results
There was sufficient data of 61 patients for inclusion in the study. The median age was 25 years. At diagnosis, 35 patients had tumors located in the mediastinum, 26 had lung and/or distant metastases. At a median follow-up of 47.2 months, 32 patients had died and 43 had developed progressive disease. The one, three, and five-year overall survival (OS) and progression-free survival (PFS) rates were 72.1%, 50.8%, 49.2% and 47.5%, 32.8%, 32.8%, respectively. Patients who received radiotherapy in the primary treatment regimen showed improved five-year OS (68.2% vs. 38.5%, P = 0.043), PFS (45.5% vs. 20.5%, P = 0.023), and local recurrence-free survival (LRFS) (77.3% vs. 38.5%, P = 0.003) compared with those who did not receive radiotherapy. Multivariate analysis revealed that radiotherapy was an independent prognostic factor of five-year OS (hazard ratio [HR] 0.39, P = 0.037), PFS (HR 0.42, P = 0.017), and LRFS (HR 0.31, P = 0.019).
Conclusion
Radiotherapy in a chemotherapy-based treatment regimen could significantly reduce local recurrence and improve survival of MMNGCT patients.
Introduction
The mediastinum is the most common site of primary extragonadal germ cell tumors (GCTs), which represent approximately 10–15% of mediastinal tumors.1 It has been suggested that these tumors are derived from primitive germ cells that migrate aberrantly along the urogenital ridge during early embryogenesis.2 With the exception of prognosis, the primary malignant mediastinal non-seminomatous germ cell tumor (MMNSGCT) shares several features with its gonadal counterpart, such as similar histology, serologic expression of tumor markers, and characteristic genetic abnormalities.3 After the introduction of Cisplatin-based chemotherapy, survival of MMNSGCT patients has improved dramatically: the five-year survival rate ranges from 30% to 60%;4-8 however, this is still inferior to survival rates for tumors arising in the gonads,5, 8, 9 the retroperitoneum or the pineal body.10
Because primary mediastinal GCTs are rare, institutional publications studying large numbers of patients have been infrequent within the past 20 years.6, 11-13 There is no consensus as to whether systemic cisplatin-based chemotherapy combined with surgery or radiation therapy is effective against MMNSGCT. In clinical practice, radiation therapy has been used for unresectable or residual diseases. Despite the fact that modern techniques have advanced considerably in the past 20 years, no evaluation as to whether radiation could substantially benefit MMNSGCT patients has been undertaken.
To further advance knowledge and improve MMNSGCT treatment, we describe our experience with MMNSGCT management using radiotherapy, clinicopathological characteristics, and survival outcomes.
Materials and methods
Patients
We divided the GCTs of the mediastinum into two main categories:14 (i) teratomatous lesions, and (ii) non-teratomatous lesions (including seminomas and non-seminomatous GCTs). In this study, we focused on malignant non-seminomatous GCTs, including primary yolk sac tumors (YST), embryonal carcinomas (EC), choriocarcinomas (CC), non-teratomatous combined germ cell tumors (CGCTs), and teratomas with additional malignant components. The diagnosis of MMNSGCT was clinicopathologically defined in all patients when a bulky mediastinal mass was present in the absence of any clinically detectable testicular or ovarian masses during the course of the disease.15 Serum tumor markers (STM), especially alpha fetoprotein (AFP) and/or β-subunit human chorionic gonadotrophin (β-HCG), were measured preoperatively in 30 and 24 patients, respectively. A total of 62 cases with MMNSGCT were identified in our hospital over a 21-year period from 1990 to 2010. Sufficient data from 61 patients were obtained and included in this study.
The chemotherapy regimen used in each case was sequentially developed during the study period at our institute for GCT management. Patients with non-seminomatous GCT were mainly treated with a combination of cisplatin and Etoposide (PE) or a combination of Bleomycin, etoposide, and cisplatin (BEP).
Operative reports were reviewed, and the curability of resection (i.e. complete or incomplete resection) was recorded. Complete resection (R0) was defined as the absence of microscopic (R1) or macroscopic (R2) residual tumor, and incomplete resection was defined as the presence of macroscopic or microscopic residual tumor.
Radiotherapy was administered with a linear accelerator using 6–8 MV X-rays at 1.8–2 Gy per fraction (5 days per week) without concurrent chemotherapy, including techniques of conventional two-dimensional radiotherapy (2DRT) for 13 patients, three-dimensional conformal radiotherapy (3DCRT) for one patient, and intensity modulated radiation therapy (IMRT) for eight patients. For 2DRT, the irradiation fields covered the tumor bed, ipsilateral mediastinum, and ipsilateral hilum. The upper border is a suprasternal notch including ipsilateral supraclavicular fossa if the tumor is beyond the mediastinum, and the lower border is 5 cm below the distal margins of resection. 2DRT was performed with two parallel-opposed anterior–posterior fields to 40 Gy, followed by irradiation with two opposed oblique fields to the tumor bed in order to avoid the spinal cord, to boost the total dose to 50–60 Gy. For 3DCRT and IMRT, the clinical target volume (CTV) was defined as the gross/residual tumor volume visible on pretreatment images of chest computed tomography or the tumor bed plus a margin of 0.5 cm and 2 cm beyond the proximal and distal margins of tumor or resection, including ipsilateral supraclavicular fossa if the tumor was beyond the mediastinum. The CTV was expanded 0.5 cm to generate planning target volume (PTV). The treatment plan was 95% PTV 50–60 Gy. The main normal tissue limitation is the percentage of lung volume that received >20 Gy <25% and the mean lung dose <15Gy. Morbidity was assessed using Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.16
During the past 20 years, our institution has implemented surgery or radiotherapy as MMNSGCT treatments regardless of STM status; this practice was adopted because STM showed poor sensitivity and specificity for detecting viable malignancy in the residual mass after chemotherapy, and unsatisfactory results were achieved following second-line chemotherapy for MMNSGCT.17, 18
Based on previous reports,19, 20 the response criteria were defined as follows: complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). All evaluations were performed within three months of completion of the primary treatment plan.
End point of the study and statistics
The overall survival (OS), progression-free survival (PFS), local recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS) rates were calculated from the time of pathological diagnosis until the date of recurrence, death or last follow-up as the endpoint. By 30 April 2013, follow-up information was available for 55 (90.2%) patients. The follow-up time for the six patients that failed to be revisited was 45, 52.9, 62.6, 67.8, 75.2, and 95.9 months.
Statistical analyses were performed using the SPSS Package for Windows version 13.0 (SPSS Inc., Chicago, IL, USA). The continuous variables and frequencies between groups were compared by Mann-Whitney U test and chi-square or Fisher's exact test as appropriate. Survival time was calculated using the Kaplan–Meier method. The survival differences were compared with log-rank tests. Independent prognostic factors were identified using Cox stepwise regression analysis. All P-values were two-tailed, and confidence intervals (CIs) were calculated at the 95% level. A P-value of <0.05 was considered statistically significant.
Our Institutional Review Board approved this study.
Results
Clinical presentations
Table 1 summarizes the demographic distribution and treatment variables. Our patient population was overwhelmingly composed of men (52 men vs. 9 women) with a median age of 25 years (range 12–65 years). According to the clinical staging scheme by Moran and Suster,14 13 (21.3%) patients had Stage I tumors and 22 (36.1%) had Stage II; all were classified as limited disease (LD) in this study.21 Twenty-six (42.6%) patients had extensive disease (ED), 14 (23.0%) in Stage IIIa and 12 (19.7%) in Stage IIIb.
| Variables | No. of patients | % |
|---|---|---|
| Demographics | ||
| Male | 52 | 85.2 |
| Median age | 25.4 ± 11.3 | 12–65 |
| Histology | ||
| Yolk sac | 26 | 42.6 |
| Embryonal | 9 | 14.8 |
| Choriocarcinoma | 1 | 1.6 |
| CGCTs† | 12 | 19.6 |
| Teratomas with additional malignant components | 13 | 21.3 |
| Location of mediastinum | ||
| Anterior superior mediastinum | 57 | 93.5 |
| Middle mediastinum | 1 | 1.6 |
| Inferior mediastinum | 3 | 4.9 |
| Stage | ||
| Stage I | 13 | 21.3 |
| Stage II | 22 | 36.1 |
| Stage IIIa | 14 | 22.9 |
| Stage IIIb | 12 | 19.7 |
| Extension | ||
| Limited disease | 35 | 57.4 |
| Stage I | 13 | 21.3 |
| Stage II | 22 | 36.1 |
| Extensive disease | 26 | 42.6 |
| Metastasizing to lung and/or effusion (Stage IIIa) | 14 | 22.9 |
| Metastasizing to distant places (Stage IIIb) | 12 | 19.7 |
| Extramediastinal involved or metastasis | ||
| Intrathoracic lymph nodes | 3 | 4.9 |
| Extrathoracic lymph nodes | 2 | 3.3 |
| Chest wall | 5 | 8.2 |
| Lungs and/or effusion | 21 | 34.4 |
| Liver | 5 | 8.2 |
| Bone | 3 | 4.9 |
| Spleen | 1 | 1.6 |
| Symptoms | ||
| Chest pain | 22 | 36.1 |
| Dyspnea | 19 | 31.1 |
| Superior vena caval syndrome | 9 | 14.8 |
| Cough | 5 | 8.2 |
| Other | 6 | 9.8 |
| Median diameters of tumor | 12.8 ± 4.6 | 5–30 |
| STM | ||
| AFP | ||
| Tested | 30 | 49.2 |
| Elevated at diagnosis | 22 | 73.3‡ |
| β-HCG | ||
| Tested | 24 | 39.3 |
| Elevated at diagnosis | 10 | 41.7‡ |
| Primary treatment regimen | ||
| Chemotherapy | 48 | 78.7 |
| Cisplatin-based | 45 | 73.7 |
| Mean cycles | 4.1 | 1–8 |
| Surgery | 47 | 77 |
| R0 | 33 | 54.1 |
| R1 | 4 | 6.6 |
| R2 | 10 | 16.4 |
| Radiotherapy | 22 | 36.1 |
| Median dose | 52 | 30–66 |
| Response to treatments | ||
| CR | 23 | 37.7 |
| PR | 20 | 32.8 |
| SD | 2 | 3.3 |
| PD | 16 | 26.2 |
- †Non-teratomatous combined germ cell tumors; ‡Percentage in tested patients at diagnosis. AFP, alpha fetoprotein; β-HCG, β-subunit human chorionic gonadotrophin; CR, complete remission; MMNSGCT, malignant mediastinal non-seminomatous germ cell tumor; PD, progressive disease; PR, partial remission; SD, stable disease; STM, serum tumor markers.
Treatments and response
In the primary treatment regimens, triple-modality therapy (surgery, chemotherapy, and radiotherapy) was performed in 14 (23.0%) patients, dual-modality therapy (chemotherapy plus surgery (n = 21) or radiotherapy (n = 5) in 26 (42.6%) patients, and a single therapy of surgery (n = 10), chemotherapy (n = 8) or radiotherapy (n = 1) was performed in 19 (31.1%) patients; the remaining two patients received combined radiotherapy plus surgery.
First-line chemotherapy was given to 48 patients: regimens containing cisplatin in 73.7% (n = 45), Carboplatin/bleomycin (n = 1), Cyclophosphamide/Doxorubicin/Vincristine/other drugs (n = 1), and cyclophosphamide/Pirarubicin/vincristine (n = 1) (Table 1). Among these 48 patients, 34 patients received chemotherapy as initial treatment with 4.25 mean cycles; the remaining 14 patients were prescribed after surgery. Surgical resection was performed by median sternotomy in all 47 surgical patients; R0 resection was available for 33 patients. After initial chemotherapy, 44.1% (14/33) of these patients received salvage surgery (Table 1).
Twenty-two patients received adjuvant (n = 21) or definitive (n = 1) radiotherapy in the primary treatment regimen (Table 1), with a median dose of 52 Gy (rang 30 Gy-66 Gy). Of these patients, five died of distant metastasis, three died of local recurrence, and 14 enjoyed prolonged survival of 7 ± 6.5 years at which time two developed distant metastasis and two had local recurrence. Five patients received a radiation dose below 45Gy and had significantly poor outcomes, while two died of distant metastasis and two died of local recurrence. During the course of radiotherapy, four patients developed ≥ Grade II hematologic toxicity. Seven patients had Grade II (n = 4) and III (n = 3) acute esophagitis, and one patient had Grade III pneumonitis. Late complications were found in six patients with Grade I radiation pneumonitis. No other late morbidities presented during follow-up.
Prognosis
At a median follow-up of 47.2 months (0.7–260 months), the overall survival rate was 44% and 32 patients died, with only one achieving five-year survival. The overall median survival time (MST) and one, three and five-year OS and PFS were 35.1 ± 31.8 months, 72.1%, 50.8%, 49.2%, and 10.2 ± 3.6 months, 47.5%, 32.8%, 32.8%, respectively (Fig 1). All relapse events occurred within three years of treatment completion, and 43 patients suffered from disease progression, of whom 19 had local recurrence, 12 showed distant metastases, five had concurrent local recurrence and distant metastases, five showed uncontrolled disease, and recurrence details for two were unknown.
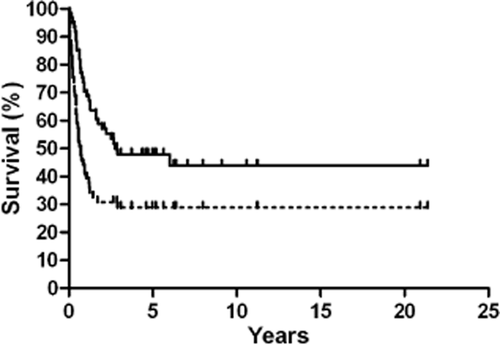
Kaplan-Meier overall survival and progression-free survival.  , OS;
, OS;  , PFS.
, PFS.
According to the Kaplan–Meier method stratified by variables, chemotherapy was significantly related with better OS, especially for LD patients. The five-year OS of 54.2% in patients who received chemotherapy was superior to that of the 30.8% in patients who did not (P = 0.008) (Table 2). Furthermore, cases that received cisplatin-based chemotherapy had remarkably higher five-year OS (57.8%, 26/45) and PFS (33.3%, 15/45) rates compared to patients who received non-cisplatin-based chemotherapy (0%, 0/3) (P < 0.001). There were no significant differences in five-year DMFS and LRFS between chemotherapy-treated and non-chemotherapy-treated cases. Including surgery in the primary treatment regimen did not appear to have an impact on survival outcome for any of the patients.
| Variables | Five-year OS | Five-year PFS | Five-year LRFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All patients | LD patients | ED patients | All patients | LD patients | ED patients | All patients | LD patients | ED patients | |
| Stage | P < 0.001 | P = 0.25 | P = 0.025 | P < 0.001 | P = 0.29 | P = 0.19 | P = 0.33 | P = 0.91 | P = 0.75 |
| Stage I | 76.9 | 76.9 | — | 53.8 | 53.8 | — | 53.9 | 53.9 | — |
| Stage II | 59.1 | 59.1 | — | 40.9 | 40.9 | — | 59.1 | 59.1 | — |
| Stage IIIa | 42.9 | — | 42.9 | 14.3 | — | 14.3 | 50.0 | — | 50.0 |
| Stage IIIb | 8.3 | — | 8.3 | 0 | — | 0 | 41.7 | — | 41.7 |
| Primary treatment regimen | |||||||||
| Chemotherapy | P = 0.008 | P = 0.88 | P < 0.001 | P = 0.11 | P = 0.87 | P = 0.001 | P = 0.20 | P = 0.74 | P = 0.30 |
| Yes | 54.2 | 65.5 | 36.8 | 31.3 | 44.8 | 10.5 | 54.2 | 58.6 | 47.4 |
| No | 30.2 | 66.7 | 0 | 23.1 | 50.0 | 0 | 46.2 | 50.0 | 42.9 |
| Surgery | P = 0.28 | P = 0.48 | P = 0.83 | P = 0.19 | P = 0.57 | P = 0.66 | P = 0.78 | P = 0.77 | P = 0.90 |
| Yes | 53.2 | 69.0 | 27.8 | 34.0 | 48.3 | 11.1 | 53.2 | 55.2 | 50.0 |
| No | 35.7 | 50.0 | 25.0 | 14.3 | 33.3 | 0 | 50.0 | 66.7 | 37.5 |
| Radiotherapy | P = 0.038 | P = 0.036 | P = 0.20 | P = 0.023 | P = 0.016 | P = 0.11 | P = 0.003 | P = 0.025 | P = 0.049 |
| Yes | 68.2 | 91.7 | 40.0 | 45.5 | 75.0 | 10.0 | 73.3 | 83.3 | 70.0 |
| No | 38.5 | 52.2 | 18.8 | 20.5 | 30.4 | 6.3 | 38.5 | 43.5 | 31.3 |
| Surgery | P = 0.37 | P = 0.75 | P = 0.55 | P = 0.16 | P = 0.60 | P = 0.45 | P = 0.22 | P = 0.39 | P = 0.62 |
| R0 | 57.6 | 71.4 | 33.3 | 39.4 | 52.4 | 16.7 | 60.6 | 61.9 | 58.3 |
| R1/2 | 42.9 | 62.5 | 16.7 | 21.4 | 37.5 | 0 | 35.7 | 37.5 | 33.3 |
| No | 35.7 | 50.0 | 25.0 | 14.3 | 33.3 | 0 | 50.0 | 66.7 | 37.5 |
| Response to treatments | P = 0.002 | P = 0.003 | P = 0.036 | P < 0.001 | P = 0.004 | P = 0.20 | P = 0.011 | P = 0.10 | P = 0.21 |
| CR | 60.9 | 66.7 | 37.5 | 47.8 | 60.0 | 25.0 | 65.2 | 66.7 | 62.5 |
| PR | 70.0 | 91.7 | 37.5 | 35.0 | 58.3 | 0 | 60.0 | 58.3 | 62.5 |
| SD | 50.0 | 100.0 | 0 | 0 | 0 | 0 | 50.0 | 100.0 | 0 |
| PD | 12.5 | 14.3 | 11.1 | 0 | 0 | 0 | 25.0 | 28.6 | 22.2 |
- ED, extensive disease; LD, limited disease; LRFS, local recurrence-free survival; OS, overall survival; PFS, progression-free survival.
Patients who received radiotherapy as a part of their primary treatment regimen had a better five-year OS compared to those who did not receive radiotherapy (68.2% vs. 38.5%, P = 0.038) (Fig 2), and this result was also observed in LD cases (91.7% vs. 52.2%, P = 0.036) (Table 2). Similar results were observed for the five-year PFS (Fig 3), and this result was also observed for LD cases (75.0% vs. 30.4%, P = 0.016). No differences in OS and PFS were observed between radiotherapy-treated and non-radiotherapy-treated ED cases. Analysis of five-year LRFS indicated that radiotherapy improved prognosis in all patients (77.3% vs. 38.5%, P = 0.003) (Fig 4), in LD patients (83.3% vs. 43.5%, P = 0.025) and in ED patients (70.0% vs. 31.3%, P = 0.049). However, no difference was observed in the five-year DMFS between patients with or without radiotherapy (68.2% vs. 87.2%, P = 0.98).
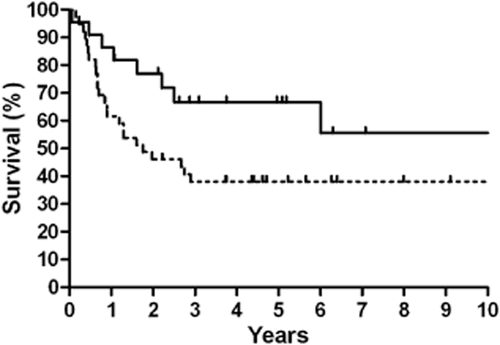
Kaplan-Meier 10-year overall survival stratified by radiotherapy.  , Radiotherapy;
, Radiotherapy;  , Without Radiotherapy.
, Without Radiotherapy.
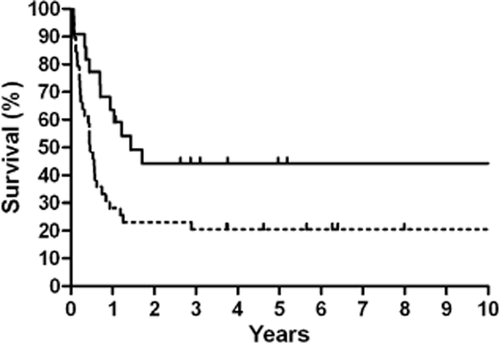
Kaplan-Meier 10-year progression-free survival stratified by radiotherapy.  , Radiotherapy;
, Radiotherapy; 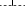 , Without Radiotherapy.
, Without Radiotherapy.
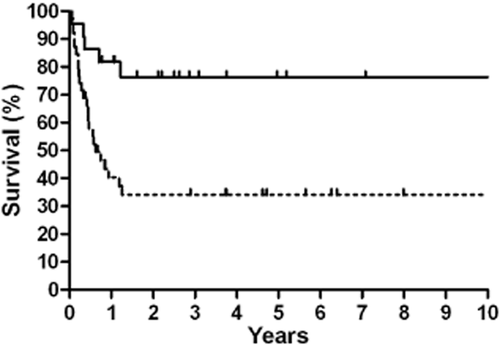
Kaplan-Meier 10-year local recurrence-free survival stratified by radiotherapy. 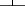 , Radiotherapy;
, Radiotherapy; 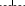 , Without Radiotherapy.
, Without Radiotherapy.
Multivariate analysis revealed that stage of disease (hazard ratio [HR] 2.20, 95%, P < 0.001), chemotherapy (HR 0.22, P = 0.001), radiotherapy (HR 0.39, P = 0.037), and response (HR 1.40, P = 0.037) in the primary treatment regimen were independent from the prognostic factor for OS. Stage of disease (HR 1.99, P < 0.001), chemotherapy (HR 0.38, P = 0.013), radiotherapy (HR 0.42, P = 0.017), and response (HR 1.36, P = 0.019) were independent predictors of PFS (Table 3). Radiotherapy (HR 0.31, P = 0.019) and response (HR 1.43, P = 0.022) were related to five-year LRFS in all patients.
| Factors | Five-year OS | Five-year PFS | Five-year LRFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | P | 95% CI | HR | P | 95% CI | HR | P | 95% CI | |
| Moran clinical stage | 2.20 | <0.001 | 1.48–3.27 | 1.99 | <0.001 | 1.44–2.74 | — | — | — |
| Chemotherapy | 0.22 | 0.001 | 0.09–0.55 | 0.38 | 0.013 | 0.18–0.82 | — | — | — |
| Radiotherapy | 0.39 | 0.037 | 0.16–0.94 | 0.42 | 0.017 | 0.20–0.85 | 0.31 | 0.019 | 0.11–0.82 |
| Response to treatments | 1.40 | 0.037 | 1.02–1.93 | 1.36 | 0.019 | 1.05–1.77 | 1.43 | 0.022 | 1.05–1.93 |
- CI, confidence interval; HR, hazard ratio; LRFS, local recurrence-free survival; OS, overall survival; PFS, progression-free survival.
Discussion
The clinical features and tumor behavior of our 61 patients were similar to those in previous reports.8, 22-25 For instance, the majority of the study cases were young men, the tumor mass was huge at diagnosis, patients exhibited mediastinal compression symptoms, serum tumor markers were elevated, and high rates of metastasis were observed. Furthermore, the five-year OS was 49.2% and PFS was 32.8%, in agreement with other studies.4-8, 22 As previously reported,4, 18, 26 patients with extra-mediastinal disease, particularly those with extra-thoracic disease at presentation, showed poor survival compared to patients who presented tumors confined to the mediastinum, supporting the staging system devised by Moran and Suster.14, 27
To our knowledge, this report represents the largest study of malignant mediastinal NSGCT treated with radiotherapy. Our results indicated that radiotherapy was an effective local treatment option and an independent prognostic factor of outcomes. Approximately two thirds of patients in this study did not receive radiotherapy and showed worse OS, PFS, and LRFS. The outcomes might have been improved if more patients received radiotherapy after first-line chemotherapy.
Given the presumed systemic nature of the disease, patients with MMNSGCTs are usually not considered for primary surgery, but are recommended for chemotherapy first. However, compared to other extragonadal or testicular tumors, MMNSGCTs do not respond as well to chemotherapy. For local treatment, all resections for GCTs were previously performed as “salvage surgery” which is intended to remove chemotherapy-resistant disease, and the histology of the excised specimen is used to assess the pathological response and to guide additional chemotherapy treatments. However, histological analysis may not detect malignant tissue within the large surgical specimen, and undetectable metastases may exist outside the surgical field.28 Moreover, the clinical significance and indications of salvage surgery in the treatment of mediastinal GCTs remain unclear. As in this study, the patients who received surgical resection or achieved R0 resection showed slightly better survival compared with those who did not, but no statistical significance was detected in Kaplan–Meier survival or multivariate analysis.
In contrast, oncologists at our institution chose radiotherapy or surgery for locally residual tumors following first-line chemotherapy. Under certain circumstances, radiotherapy treatment was used to shrink the tumor in order to make it resectable or to reduce the tumor burden following chemotherapy. Twenty-two patients underwent radiotherapy in the primary treatment regimen and showed remarkably improved OS and PFS, particularly those with limited disease; this treatment in LD or ED patients most likely led to 50% reduced local recurrence.
NSGCT are sensitive to radiation, but the role of radiation therapy in MMNSGCT has not been defined, and the optimal dose and fields of radiation therapy have, therefore, not been established. In addition to palliation, radiation therapy has been used to improve local control. Renata et al.29 reported administering radiotherapy (45 Gy) to one patient with a residual tumor that was later resected, with no viable malignant cells found in the specimen. Another patient, treated with chemotherapy followed by radiotherapy and surgical removal of the necrotic mass, has been alive and free of the disease for 14 years. An explanation for this outcome may come from the addition of radiotherapy to an aggressive chemotherapy regimen. However, depending on the amount of bone marrow treated, irradiation given before chemotherapy may adversely affect the patient's ability to tolerate full cytotoxic doses. Consistent with this possibility, Newlands et al.30 noted a decreased survival rate among patients receiving radiation therapy prior to chemotherapy compared to non-radiation-treated patients (56% vs. 82%, respectively).
Nowadays, objections to the use of radiation therapy are no longer warranted because modern techniques allow for more limited fields and a smaller volume of marrow irradiation. In our opinion, radiotherapy with modern techniques may provide effective alternatives to surgical resection for refractory tumors, as well as local relapse disease previously treated with first-line and sometimes second-line chemotherapy, given that surgery alone has obvious limitations. Complete R0 en-bloc excision should always be attempted. Patients should be carefully selected based on radiographic findings suggesting resectable disease and on a general health status of their ability to tolerate aggressive surgery.31 Aggressive forms of the disease may require a cardiopulmonary bypass or a major mediastinal vessel replacement; thus, the level of experience of the surgical oncology team is a crucial factor in the prognosis.28, 32 On the contrary, radiotherapy has lower general health requirements for patients and can target all residual lesions, even those close to vital mediastinal structures. If radiation therapy is contemplated, it should be administered following maximal response to chemotherapy, and high doses of 55–60 Gy or more appear to be necessary to achieve better local control.33 All vital mediastinal structures can tolerate radiotherapy doses in the 50–60 Gy range,34 which provides an opportunity to treat residual lesions with a higher dose.
Our single-center study has several limitations, including the retrospective design, limited number of patients, and selection of initial treatment. Moreover, we did not obtain all of the patients’ data of STM pre-chemotherapy and post-chemotherapy, which were reported to be better survival indicators in patients with primary mediastinal NSGCT.35 However, several studies indicated that the presence of pre-chemotherapy and preoperative STM elevation did not impact five-year OS.4, 26 In addition, STM status after the completion of platinum-based chemotherapy may not consistently reflect the presence of residual cancer.28 Thus, we believe it is possible that incomplete STM data did not compromise our results because the survival and relapse indicators were objective and patients with or without radiotherapy showed significantly different outcomes.
Conclusions
In conclusion, because of the rarity of the type of tumor and the undefined role of surgical resection in local treatment, our data provides substantial evidence that radiotherapy could be a wise option for the treatment of tumors refractory to platinum-based chemotherapy in order to reduce local recurrence and improve survival; moreover, doses of 55–60 Gy or more may be necessary to achieve local control.
Disclosure
No authors report any conflict of interest.




