An Optimised Method to Identify Reintroduced Swift Foxes (Vulpes velox) Through SNP Genotyping of Non-Invasively Collected Scat Samples Using In-Solution Hybridisation Capture
Funding: The Volgenau Foundation provided financial support for K.R.T. John and Adrienne Mars provided financial support for H.S., D.L.N., L.D.P., and research expenses. Bureau of Indian Affairs provided financial support for contracting a trapping specialist in 2020. Regina Bauer Frankenberg Foundation provided financial support for D.L.N. stipend, L.D.P.'s contract, and genomics lab work. US Tribal Wildlife Grants (13159519) provided financial support for genomics lab work. NSF 18-546 Tribal Colleges and Universities Program (2054877) provided financial support for research supplies. American Prairie grant provided financial support for student stipends and research supplies.
ABSTRACT
Genomic methods have become increasingly common in wildlife population studies over the past two decades. While noninvasive genetic sampling has been prevalent since the 1990s, the field has lagged in the adoption of high-throughput sequencing methods. In-solution hybridisation capture offers an efficient way to enrich and sequence degraded, low-quantity DNA with a large percentage of exogenous content (e.g., scat samples) for specific targets of interest. Despite their frequent use in ancient and historical DNA applications, hybridisation capture techniques have not been widely adopted for noninvasive genetic samples. Previous studies demonstrated that capture enrichment of single-nucleotide polymorphism (SNP) loci enables genotyping of field-collected kit fox and coyote scats to effectively identify species, individuals and sex. Here, we expanded on this work by (1) investigating whether probes designed for kit foxes can generate multi-locus SNP genotypes and identify sex in closely related swift foxes (Vulpes velox), (2) assessing the capability of the resulting genotypes to differentiate among swift foxes by calculating identity-by-state values between samples from the same individual, (3) exploring the impact of replicate index polymerase chain reactions (PCRs) on genotyping success, and (4) exploring the performance of the marker set for inference of population genetic structure. We applied these methods to samples from swift foxes reintroduced to the Fort Belknap Indian Reservation, Montana, and showed a success rate of 85%. Our developed methodology can be applied to monitor the success of swift fox reintroduction efforts by estimating dispersal, survival, and reproduction. We also showed that probes designed and optimised for one species can produce informative genotypes from closely related species, highlighting their versatility for broader applications in wildlife population studies.
1 Introduction
Over the past two decades, the adoption of population genomic tools enabled by high-throughput sequencing has revolutionised the study of wildlife populations and informed conservation management policies (Hohenlohe et al. 2021). Genomic data have been invaluable for estimating and monitoring population size and connectivity (e.g., Jensen et al. 2018; Hotaling et al. 2018), defining evolutionarily significant units (Funk et al. 2012), detecting hybridisation (Sinding et al. 2018), and evaluating adaptive differentiation (Funk et al. 2016). Non-invasive genetic sampling, or collection of material left behind by an animal, has enabled the study of rare, difficult-to-trap, or imperilled populations since the 1990s (Paxinos et al. 1997; Taberlet et al. 1997). Historically, most non-invasive genetic studies focused on sequencing mitochondrial genes for species identification (e.g., Paxinos et al. 1997; Dalén et al. 2004), and genotyping microsatellite (or short tandem repeat) loci to identify individuals (e.g., Smith et al. 2006; Schwartz et al. 2006).
Despite more than three decades of non-invasive genetic research, the field has lagged in the adoption of genomic techniques, primarily due to the challenges presented by non-invasively sampled DNA. Ancient DNA (aDNA) studies face similar challenges, including DNA degradation, high and variable percentages of exogenous (non-host) DNA, and the presence of polymerase chain reaction (PCR) inhibitors (Waits and Paetkau 2005). However, non-invasive studies have largely overlooked common aDNA methods such as modified, single-tube library preparation (e.g., Carøe et al. 2018; Kapp et al. 2021) and in-solution DNA hybridisation capture or “capture” methods (e.g., Cruz-Dávalos et al. 2017; Gnirke et al. 2009) which are designed to enrich targets of interest, usually endogenous DNA.
Several studies have shown the efficacy of capture methods to enrich primate DNA from scat (e.g., Hernandez-Rodriguez et al. 2018; Perry et al. 2010; White et al. 2019); however, these methods have rarely been applied to other taxa. Instead, most non-primate non-invasive studies that utilise single-nucleotide polymorphism (SNP) genotyping have relied on multiplex PCR reactions followed by fluorescence measurement (e.g., with the Fluidigm platform, von Thaden et al. 2017) or direct amplicon sequencing (e.g., Natesh et al. 2019; Forbes et al. 2025). These approaches present their own set of challenges, including the need for specialised equipment (e.g., microfluidic PCR technology, von Thaden et al. 2017), the need for species-specific genomic references to design primers, the complex optimisation of multiplex PCR reactions (von Thaden et al. 2020), and the potential for variable and/or high error rates when applied to scat samples (e.g., Arpin et al. 2024). Capture methods offer a more flexible approach, allowing enrichment at scales up to whole genomes (Ozga et al. 2021), whereas multiplex PCR panels are usually limited to fewer than 500 loci (Meek and Larson 2019; Solari et al. 2025).
Here, we apply non-invasive genetic techniques to swift fox samples with the goal of facilitating future monitoring of reintroduction outcomes. Swift foxes were once abundant in North America's short- and mixed-grass prairies (Egoscue 1979), but faced population declines due to habitat loss and incidental take from predator removal campaigns, which led to their extirpation from southern Canada and the northern United States in the early 1900s (Allardyce and Sovada 2003). The species has since re-colonised ca. 44% of its historic range (Moehrenschlager and Sovada 2016). Although the International Union for Conservation of Nature (IUCN) classifies swift foxes as Least Concern (Moehrenschlager and Sovada 2016), they remain regionally threatened in Canada and the United States (Kunkel et al. 2003).
Several translocations have been undertaken to restore swift foxes to their northern range (Ausband and Foresman 2007; Moehrenschlager and Macdonald 2003; Sasmal et al. 2016). However, a 350 km gap remains between fragmented northern and contiguous southern populations (Butler et al. 2021). To restore range-wide connectivity, the Fort Belknap Indian Reservation in Montana began a 5-year reintroduction programme in 2020, with wild swift foxes sourced from existing populations in Colorado and Wyoming. This study seeks to evaluate the applicability of the FAECES* method (Parker et al. 2022) for monitoring swift fox populations, specifically focusing on the Fort Belknap population. By analysing field-collected scat samples, this research seeks to determine if this method can effectively identify translocated individuals and provide reliable estimates of population density, survival rates and reproductive success. The findings could have significant conservation implications for swift fox populations in Montana and other regions.
In a recent study by Parker et al. (2022), capture methods were used to enrich nuclear SNPs, enabling the production of multi-locus genotypes from multiple canid species utilising field-collected scat samples, even those that were not freshly deposited. The authors successfully used the SNP genotypes to simultaneously identify species, putative recaptures, and sex of both coyotes (Canis latrans) and kit foxes (Vulpes macrotis). However, they relied on pairwise kinship estimation to infer recaptures due to the lack of multiple samples from known individuals. Here, we significantly expand upon the Fast and Accurate Enrichment of Canid Excrement for Species* (FAECES*) method presented in Parker et al. (2022) and improve its utility by (1) investigating the method's effectiveness in producing genotypes using scat samples from swift foxes (Vulpes velox), a close relative of the kit fox (Mercure et al. 1993), (2) determining whether the genotypes can differentiate among swift foxes by calculating pairwise identity-by-state (IBS) between samples from the same individual (blood and multiple scat samples), (3) exploring the impact of replicate index polymerase chain reactions (PCRs) on genotyping success, and (4) exploring the performance of the marker set for population genetic inferences. Finally, we compare the cost of this approach with that of non-invasive genotyping without enrichment. We demonstrate and expand the economic feasibility of enrichment probes by increasing the number of applications for each designed and tested set. To our knowledge, this study represents the first attempt to use paired scat and blood samples from the same individual to validate non-invasive recapture identification using SNP enrichment methods in a non-primate taxon. Insights from this research will provide critical information for future conservation studies that utilise non-invasive methods.
2 Materials and Methods
2.1 Study Locations
We sourced swift foxes from existing populations in Colorado and Wyoming (Figure 1) per previous reintroduction efforts (Moehrenschlager and Macdonald 2003; Sasmal et al. 2013, 2015). The habitat of all source populations is characterised as short-grass prairie within the Northern Great Plains region of North America. In Colorado, we trapped founders in the Comanche National Grassland (38.06430° N, 103.67704° W) in August of 2021. In Wyoming, we trapped founders in and around Shirley Basin (42.3225° N, 106.3089° W), north of Medicine Bow, in September of 2021. The two source areas were separated by ca. 525 km.

We conducted translocations on the Fort Belknap Indian Reservation (Figure 1) in north-central Montana (48.2000° N, 108.5340° W). Fort Belknap is and has been home to the A'aniiih and Nakoda Tribes since the Reservation was established in 1888 by the US government (Montana Office of Public Instruction 2009). The Reservation is bounded by three major land features: the Milk River to the north, and the Little Rocky Mountains as well as the Missouri River to the south. The landscape consists of short- and mixed-grass prairie (Charboneau et al. 2013). Prior to their reintroduction in 2021, swift foxes were absent from Fort Belknap for over half a century (Sovada et al. 2009; Paraskevopoulou et al. 2022).
2.2 Sample Collection and Storage
We handled all animals according to the guidelines of the American Society of Mammalogists for use of wild mammals in research (Sikes and the Animal Care and Use Committee of the American Society of Mammalogists 2016) and in compliance with the Smithsonian Institution Animal Care and Use Committee (NZP-IACUC 20-09). Additionally, we used standard and humane holding and release protocols based on other swift fox projects (Sasmal et al. 2015; Moehrenschlager et al. 2003). We captured swift foxes using Tomahawk traps (Tomahawk Live Trap, Hazelhurst, WI, USA) baited with meat scraps, sardines and/or skunk lure (Canine Call, Russ Carman Lures, New Milford, PA, USA). We set traps between 1800 and 2000 h and closed them between 0600 and 0800 h.
Captured foxes underwent a health check by state wildlife veterinarians at each source site, which included, when possible, collecting 3–5 mL of blood from the jugular or cephalic vein. All blood samples were stored in ethylenediaminetetraacetic acid (EDTA) tubes (BD Vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Moreover, each translocated fox was fitted with a cellular GPS collar (ES-400, Cellular Tracking Technologies, Rio Grande, NJ, Figure 2) combined with a unique numeric code (e.g., collar ID 48005) to identify individuals. Healthy foxes were then held in quarantine for up to 5 days prior to transport to Fort Belknap.

Once the foxes arrived at Fort Belknap, they were held in soft-release pens (Figure S1) for up to five additional days to promote site acclimatisation (Sasmal et al. 2015). We collected faeces from the live traps (within 18 h of deposition), during veterinary examinations (immediately after deposition) and throughout quarantine prior to transport (within 3 days of deposition, Table S3). We continued to opportunistically collect faecal samples every other day during soft-release pen husbandry visits at Fort Belknap. Individual collar IDs were documented for each blood and scat sample upon collection. We stored samples individually in sealable plastic bags with silica desiccant (Dry & Dry, Brea, CA, USA) (n = 47 blood; n = 223 faecal) and kept them frozen at −20°C until shipping them on dry ice to Smithsonian's National Zoo and Conservation Biology Institute (NZCBI) in Front Royal, Virginia, USA.
2.2.1 Laboratory Methods: Scat Subsampling and DNA Extraction
At NZCBI, we stored scat and blood samples frozen at −80°C until subsampling. Colorado samples were lyophilised (freeze-dried) prior to subsampling (Appendix S1). For all faecal samples, approximately 5 mL was taken from the ends of whole scats. Subsamples were then stored in −20°C freezers at the Smithsonian's Center for Conservation Genomics (CCG) in Washington DC, until DNA extraction and analysis.
In total, we trapped 48 swift foxes (Table S1) in Comanche National Grassland (n = 30 foxes; August 16–31, 2021) and Shirley Basin (n = 18 foxes; September 16–19, 2021). Of these, we selected a subset of 16 individuals from which we had at least one scat sample for molecular analyses (range 1–3, average 1.6 scats per individual, Table S2). We extracted DNA from 80 μL of whole blood with a Qiagen DNeasy blood and tissue kit, following the manufacturer's protocol for blood except we performed an extended overnight digestion at 56°C (Qiagen, Germantown, MD, USA). For the scat samples (n = 26, Table 1), we extracted DNA using a Qiagen DNeasy PowerSoil Pro QIAcube HT kit and a QIAcube HT robot (Qiagen, Germantown, MD, USA). For samples that did not pass through the columns using the robot vacuum, we re-extracted using the manual Qiagen DNeasy PowerSoil Pro kit. We followed the manufacturer's protocol for scat extractions (Qiagen, Germantown, MD, USA), except we incubated samples in CD1 solution overnight in a VorTemp 56 shaking incubator (Labnet, Edison, NJ, USA) at 35 rpm and 56°C, and did not perform bead beating.
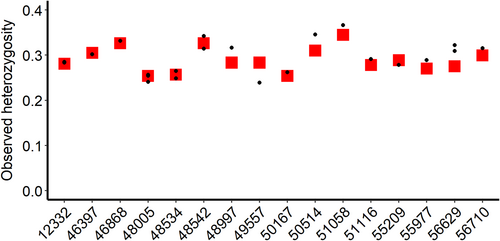
| Source location | Number of individuals | Total number of scats | Average number of scats per individual | Average observed heterozygosity | Average number of SNPs |
|---|---|---|---|---|---|
| Colorado | 10 | 17 | 1.7 ± 0.67 | 0.29 ± 0.02 | 288 ± 125 |
| Wyoming | 6 | 9 | 1.5 ± 0.84 | 0.29 ± 0.03 | 362 ± 30 |
2.2.2 Library Preparation and Capture Enrichment
We quantified DNA extracts using a Qubit fluorometer with a 1× dsDNA HS assay (Thermo Fisher Scientific, Waltham, MA, USA) and then sheared with a Bioruptor Pico sonication device (Diagenode, Liege, Belgium) to an average of ca. 250 bp using as many cycles as necessary of 30 s on, 30 s off (15–90 cycles). We assessed shearing by visualising the extracts with a TapeStation 4200 system (Agilent Technologies, Santa Clara, CA, USA) using High Sensitivity D1000 reagents after the first 15 cycles and every 30 cycles after that until samples were sufficiently sheared. For both blood and scat samples, we then prepared dual-indexed genomic DNA libraries using a single-stranded method designed for ancient DNA (i.e., the Santa Cruz Reaction, Kapp et al. 2021). For blood samples, we built libraries with an average of 117 ± 91 ng of DNA and for scat samples, 180 ± 229 ng. We determined the number of index PCR cycles by performing qPCR and adding one cycle to the Cq value for each library (Kapp et al. 2021).
We performed three replicate index PCR reactions on each scat library with unique index pairs for all three replicates so that sequences from each PCR reaction could be identified later. Index PCR replicates were combined for each scat sample prior to capture. For blood samples, we performed a single index PCR because these extracts are expected to have little exogenous DNA and therefore fewer nanograms of DNA are required for successful capture enrichment. We quantified index PCRs using the HS Qubit assay. We then enriched libraries for 835 autosomal SNP loci (ca. 364 of which are polymorphic in kit foxes) and the sex-linked ZFX/ZFY sex genes using FAECES* probes (Parker et al. 2022). We followed the myBaits Manual version 4.1 standard protocol with a hybridisation temperature of 62°C. We pooled two blood libraries per enrichment, with equal amounts of DNA from each sample, and enriched scat libraries in single-plex reactions. The hybridisation reaction proceeded for 36 h. We quantified the enriched and amplified libraries using Qubit and visualised them on the TapeStation. We pooled samples equimolarly and sequenced them with paired-end 150 bp reads on a shared run of an Illumina NovaSeq 6000 at the Clinical Genomics Centre (Oklahoma Medical Research Foundation, Oklahoma City, OK). We estimated 7 million reads for each scat sample and 3 million for each blood sample because we anticipated a lower mapping percentage of reads from scat samples than blood samples. Detailed bench protocols for sample processing are presented in detail in Parker et al. (2025).
2.3 Bioinformatic Sequence Processing
We evaluated demultiplexed sequence reads for quality and adapter content using FastQC version 0.11.8 (Andrews 2010, https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). We removed adapter sequences using TrimGalore version 0.6.4 (Krueger 2019, https://github.com/FelixKrueger/TrimGalore) and confirmed successful removal by running FastQC again. We concatenated raw sequence reads from the three index PCRs prior to bioinformatic analysis (Figure S2). We then mapped trimmed reads to the domestic dog (Canis familaris) reference genome (CanFam3.1, Hoeppner et al. 2014) using the ‘mem’ algorithm in BWA version 0.7.17 (Li 2013). The FAECES* probe set was designed by mapping kit fox and coyote reads to the CanFam3.1 reference, so although there is ca. 9–10 million years of divergence between dogs (tribe Canini) and foxes (tribe Vulpini: Lindblad-Toh et al. 2005), the impact of cross-species reference bias is limited. Here, we analyse SNPs for which mappability and genotype reliability are not expected to be affected by chromosomal rearrangements that may occur between dog and fox genomes.
We processed alignments with SAMtools version 1.3.1 (Li et al. 2009; Danecek et al. 2021) to sort and convert SAM to BAM format. We marked duplicates with Picard Tools version 2.20.6 (Picard Toolkit, 2019, https://github.com/broadinstitute/picard) and left-aligned reads around indels (insertions and deletions) using the GenomeAnalysisToolkit (GATK version 4.1.3.0, McKenna et al. 2010). These processes, from alignment to the reference genome through realignment around indels, are automated in a Nextflow pipeline (Prado et al. 2023; https://github.com/campanam/Elephants), which is also explained in detail in Parker et al. (2025). We identified sequence variants using BCFtools version 1.9 ‘mpileup’ (–C50 option, Danecek et al. 2021) and ‘call’ commands. Finally, we ran mapDamage version 2.2.2 (Jónsson et al. 2013) on final alignment files to calculate the length of mapped reads.
Next, we filtered the raw all-sample VCF file using VCFtools version 0.1.15 (Danecek et al. 2011) to remove indels and genotypes with quality less than 30 (minGQ), and to select the genomic regions targeted by the baits. We then filtered the remaining sample genotypes for a minimum depth of four reads covering a site per sample. We filtered sites with more than 25% missing data and a minor allele count less than two, and then thinned loci to include only one variant per baited region. Finally, we discarded samples with fewer than 100 SNPs (> 73% missing data).
To distinguish between the effects of additional sequence reads and multiple index PCRs on the number of variants recovered from each scat sample, sequence duplication and depth of sequence coverage, we randomly subsampled 2 million reads from each individual index PCR as well as the concatenated read files (n = 4 files per scat sample) and repeated the variant calling pipeline. We used the 'sample' command from seqtk version 1.3 (Li 2018, https://github.com/lh3/seqtk) to randomly sample 2 million reads. We followed the aforementioned pipeline for read trimming, mapping, variant calling and filtering, with the exception that the final, multi-sample VCF file was not filtered for missing data. Retaining loci with missing data allowed us to determine the number of SNPs recovered from each sample. We used VCFtools to calculate missing data (the proportion of loci with missing genotype data per sample), heterozygosity (number of heterozygous sites divided by the total number of genotyped sites), and mean depth of sequence coverage across loci per sample and SAMtools version 1.3.1 (Li et al. 2009) to calculate sequence duplication rates.
2.4 Population Genetics Analysis
For sex identification, we mapped trimmed sequence reads to the sex-linked zinc finger X and zinc finger Y (ZFX and ZFY) kit fox reference sequences (Ortega et al. 2004, GenBank AY310919 and AY310920, respectively). The targeted gene region is 401 bp and contains 26 diagnostic sites that differ between ZFX and ZFY. We followed the steps outlined above for alignment processing and removed unmapped reads from the BAM files with SAMtools ‘view’. We imported the BAM files into Geneious Prime version 2020.1.2 and generated consensus sequences with options ‘Assign Quality Total’, calling ‘Ns’ if coverage < 2 reads, and calling heterozygotes at > 30%. We aligned ZFX and ZFY consensus sequences to the reference sequences with the MAFFT version 7.490 (Katoh 2005). We determined sex visually; samples were considered male if the consensus sequences had at least 10 bases matching the ZFY reference sequence.
We used the SNPRelate version 1.8.0 package (Zheng et al. 2012) in R version 4.2.0 (R Core Team 2022, applies to all uses of R) to calculate pairwise identity-by-state (IBS) and identity-by-descent (IBD, or kinship) values to determine the range of values derived from pairs of samples from the same individual. For the IBD analysis, we used maximum likelihood estimation (R script available at https://github.com/LillyParker/VCF_to_PCA_IBS). We used VCFtools to calculate observed and expected heterozygosity. To estimate the power of the SNP data to differentiate between individuals, we calculated the probability of identity assuming siblings are present in the data (pIDsibs, Waits et al. 2001) using GenAlEx version 6.5.03 (Peakall and Smouse 2012) and the pID, the probability that two randomly sampled individuals would have identical genotypes (Armstrong et al. 2025).
To determine if there is significant population structure differentiating founder swift foxes from Colorado and Wyoming, we ran STRUCTURE version 2.3.4 (Pritchard et al. 2000) on a dataset containing blood samples from individuals who are not closely related (kinship < 0.2, following Manichaikul et al. 2010). We used a burn-in of 250,000 steps followed by 1 million recorded steps. We ran the programme twice: once with location priors and once without. For both runs we used the admixture model and assumed correlated allele frequencies (Falush et al. 2003). We performed simulations with K = 1–6 and 10 replicates for each value of K. We identified meaningful K values using the ΔK method (Evanno et al. 2005) implemented in STRUCTURE HARVESTER version 0.6.94 (Earl and von Holdt 2012).
3 Results
3.1 Performance of the Probe Set
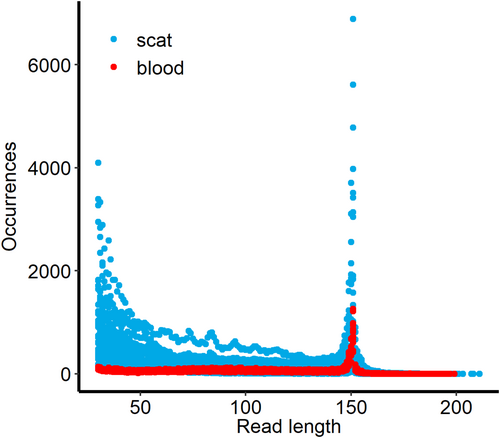
Extractions from blood yielded a mean of 1171 ± 916 ng of DNA. The DNA yield from scat samples was highly variable, with a mean of 1791 ± 1995 ng (range 44–7410 ng), including both exogenous and endogenous content. For the 16 blood samples, we sequenced a mean of ca. 4.5 million (±819,352) reads per sample, and for the 26 scat samples, we sequenced a mean of 2.8 million (±1.2 million) reads per index PCR replicate (SRA BioProject PRJNA1108678). A mean of 99% ± 0.06% of reads from blood samples and 79% ± 16% of reads from scat samples mapped to the domestic dog reference. Blood-derived sequences had a mean duplication rate of 32% ± 3.3%, while scat-derived sequences had a mean duplication rate of 52% ± 17%. The mean sequence coverage across loci was 119 ± 32 for blood samples and 50 ± 52 for scat samples. The lengths of reads mapped to the dog genome were shorter and more variable for scat samples compared to blood samples (Figure 3).
3.2 Identifying Recaptures
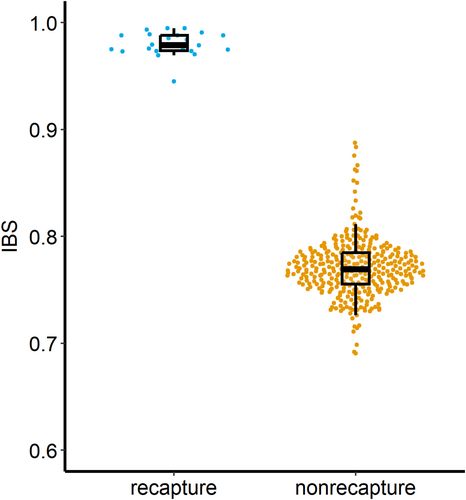
After filtering, the all-sample VCF file with concatenated PCR replicates contained genotype data for 374 loci (Table S2); of the 26 scat samples, 22 were successful, yielding > 100 SNPs (Table S2). The pIdsibs was 2.4 × 10−46 and the pID was 1.13 × 10−65. Pairwise IBS values between known recaptures (between blood and scats and multiple scats from the same individual) ranged from 0.945 to 0.995 (Figure 4). IBD (kinship) values between pairs of putative relatives (IBD ≥ 0.2, Manichaikul et al. 2010) ranged between 0.888 and 0.833 (Figure 4). Average observed heterozygosity calculated by VCFtools among swift fox blood samples was 0.29 ± 0.027 (Table 1). Observed heterozygosity between pairs of blood and scat samples from the same individual differed by a mean of 0.016 (range 0–0.047) (Figure 5). Pairs of blood and scat samples which yielded the same number of SNPs differed less (mean, 0.0075; range 0–0.016) than pairs of samples with different numbers of genotyped SNPs (mean, 0.023; range, 0.028–0.047).
Using the read data from all three index PCRs combined, an average of 7158 ± 7919 reads mapped to the ZFX reference. The sex estimated by ZFX/ZFY sequences matched the known sex for all scat samples that produced more than 100 SNPs (22/26 samples). Allelic dropout leading to misidentification of sex was not a concern due to sufficient sequencing depth.
3.3 Performance of Replicate PCRs
The results of the random subsampling analysis showed that at a standard sequencing effort of 2 million reads, the concatenated files that combined reads from three replicate PCRs had (1) significantly fewer PCR duplicates than single PCRs (mean 25% and 43% duplicates respectively, Wilcoxon test, p < 0.001), (2) recovered more SNP loci (average 220 and 171 SNPs, respectively), and (3) yielded higher average sequence depth of recovered SNPs than single PCRs (mean 10.6 and 7.2 reads per locus, respectively). The success rate of individual PCRs subsampled at 2 million reads was 49% (41/81 produced > 100 SNPs), and the success rate of the concatenated PCRs subsampled at 2 million reads was 65% (17/26 produced > 100 SNPs) (Table 2).
| PCR status | Mean number of SNPs | Mean sequence depth across SNPs | Percent duplication | Sample success |
|---|---|---|---|---|
| Concatenated | 220 ± 169 | 10.6 ± 12.6 | 25.3 ± 14.3* | 65.0% |
| Individual | 171 ± 163 | 7.2 ± 9.4 | 43.3 ± 17.6* | 49.4% |
- * Statistical significance (p < 0.001) in the difference between the two groups.
3.4 Preliminary Population Genetics
Kinship analysis revealed four individuals from Colorado that are first-order relatives (parent/offspring and/or siblings). After removing three of these individuals with high kinship (> 0.2) from the dataset, the results of the STRUCTURE analysis without and with location priors showed no evidence of significant genetic differentiation between foxes from Colorado and Wyoming (Table S4a,b). The highest mean log-likelihood value was for K = 1 and the ΔK analysis showed little difference with increasing K values (ΔK < 6, Table S4a,b).
4 Discussion
The FAECES* method (Parker et al. 2022) was designed to differentiate between coyote and kit fox scats and to identify individuals and their sex for both species. Here, we showed that this method is also effective for identifying SNP genotypes from swift fox scat samples, enabling the identification of individual swift foxes and their sex. By genotyping blood and multiple scat samples from known individuals, we validated the method's ability to correctly identify recaptured individuals and established the range of pairwise identity-by-state (IBS) values between pairs of samples from the same individual. Specifically, we showed that the set of 374 SNPs, with an average heterozygosity of 29%, yielded pairwise IBS values between 0.995 and 0.945 for multiple samples from a single individual. These values did not overlap with kinship values between samples from different, related individuals, the highest value being 0.888. The identified sex of each individual fox was consistent across its samples (Table S2).
4.1 Recommendations and Improvements for Future Studies
For future non-invasive studies, we recommend performing at least three index PCRs per library prior to enrichment. Our results demonstrate that performing index PCRs in triplicate prior to pooling for enrichment significantly increases library complexity, improves SNP recovery and decreases sample failure (Table 2). The index PCR reactions can be performed concurrently and do not significantly increase sample processing time, and because of the reduction in PCR replicates sequenced, combining multiple PCRs makes sequencing more efficient and cost-effective.
We demonstrate higher variability and shorter lengths of mapped reads derived from scat than those derived from blood, including a proportion of scat reads < 100 bp (Figure 3). This suggests post-defecation DNA degradation, which could make it difficult to apply multiplex PCR-based methods to genotyping of scat samples. Finally, we show that the SCR method, a single-stranded library preparation method designed for ancient DNA samples (Kapp et al. 2021) can be successfully applied to both blood and scat-derived DNA of variable DNA length and quantity.
4.2 Comparison With Other Methods
It is challenging to compare genotyping success rates across non-invasive genetics studies because field collection protocols, exposure to environmental conditions, and species' diets vary and significantly affect scat sample performance. Scat age (i.e., time since deposition), sampling month, and dietary contents have all been shown to impact genotyping success (Skrbinšek 2020; Vynne et al. 2012). However, our 85% sample success rate—without conducting any pre-screening for sample quality—is comparable to or higher than other non-invasive genotyping studies that utilised multiplex PCR methods, showing that our method can be a viable alternative depending on the specific application. For example, utilising field-collected polar bear scats, Hayward et al. (2022) reported a 30% success rate considering samples without pre-screening, and a 63% success rate considering only samples that successfully amplified in a qPCR targeting a nuclear gene. Using American pika scats, Arpin et al. (2024) achieved an 80% sample success rate; however, their final dataset included only 77 neutrally evolving SNPs with a relatively high error rate (28%), precluding confident determination of recaptures. The authors suggested that the high variance between technical replicates was due to differential allele amplification caused by low starting concentrations of target DNA and/or the presence of PCR inhibitors (Arpin et al. 2024). Similarly, Solari et al. (2025) reported a 77% success rate with samples previously identified as snow leopard by a PCR pre-screening step targeting the mitochondrial 12S rRNA gene.
Although we documented evidence of DNA degradation compared to blood samples (i.e., shorter, more variable DNA lengths, Figure 3), we collected relatively fresh scat samples: scats were collected from live traps or soft-release pens during wellness visits, and the maximum scat age is 3 days post defecation (Table S3). In future studies in which scats are of unknown age, we expect that the sample success rate may be lower. For example, Parker et al. (2022) reported a 44% success rate with the FAECES* method using samples that were of unknown age but potentially as much as 1 month old and collected in the desert. These lower-quality samples were also combined into pools of three samples per capture reaction, which may decrease the sample success rate. Environmental conditions can also significantly affect DNA preservation. For example, increases in moisture (Regnaut et al. 2006), temperature and UV exposure (Vynne et al. 2012) increase DNA degradation. Host diet may also change seasonally, and some dietary species, such as those high in chitin, cause inhibition of enzymatic reactions and negatively impact sample performance (Murphy et al. 2003; Panasci et al. 2011).
In addition to hybridisation capture and multiplex PCR enrichment strategies, some studies have used methylation-based enrichment to separate bacterial from animal DNA, followed by reduced-representation sequencing like RADseq (Chiou and Bergey 2018). However, other studies have suggested that even minimally degraded DNA from tissue samples (e.g., after 96 h of environmental exposure) produces poor-quality data and is generally not suitable for RADseq methods (Graham et al. 2015). Other studies have attempted “shotgun sequencing” of DNA extracted directly from scat without enrichment; as expected, this strategy revealed that only a small percentage of resulting sequence reads are endogenous, or from the host organism (e.g., 0.01%–10.8%, de Flamingh et al. 2023).
Despite declines in sequencing prices, it is still typically prohibitively expensive to perform population-scale genomic analyses on faecal samples with shotgun sequencing methods. For example, to perform low-coverage genomic analysis targeting 3× genomic coverage, and assuming 5% endogenous content and a 2.4 billion-bp genome, one would need 480 million 300-bp reads. Assuming 1.50 USD per million reads on an Illumina NovaSeq X plus platform, that equates to roughly 720 USD per sample or more if the endogenous content is lower. Moreover, non-invasive DNA is often fragmented into short pieces (< 300 bp), which will also increase the needed sequencing effort. Parker et al. (2022) estimated a per-sample cost of 38 USD for enrichment reagents for the FAECES* method if pooling three samples per reaction, and 114 USD if capturing each sample separately. Assuming 1.50 USD per million reads in this case, sequencing 7 million reads per sample is 10.5 USD. Excluding the methods common to each strategy (i.e., DNA extraction, quality control and library preparation), but taking into account triplicate index PCR reactions for capture, the reagent and sequencing price difference per sample between shotgun sequencing and enrichment when performing capture on one sample per capture reaction is 456 USD (789 USD and 332 USD for shotgun versus capture, respectively). Shotgun sequencing may be desirable if information about host gut microbiomes, diet and/or parasites is of interest in addition to host population genomics (e.g., Srivathsan et al. 2016). Otherwise, there are additional costs involved in the storage and processing of large amounts of data that are not directly utilised.
It is difficult to directly compare the cost of multiplex PCR (e.g., GT-seq, Campbell et al. 2015) with that of capture because the two workflows are different, and much of the cost of multiplex PCR is in the design of markers and the optimisation of multiplex PCR reactions. For example, Meek and Larson (2019) estimated the cost of SNP panel development for multiplex PCR to be ca. 12,000 USD (cost of primer pairs and reagents) and the development time to be 4 months. Once a panel is designed and optimised, the per-sample price depends on the scale of the project (i.e., the number of samples). Assuming 960 samples, for example, Meek and Larson (2019) estimated the per-sample price, including sample preparation and sequencing, to be $6 (Meek and Larson 2019). Similarly, Campbell et al. (2015) estimated the per-sample price to be $3.98 for ca. 2000 samples. For these reasons, multiplex PCR methods are better suited for applications where thousands of samples will be processed using the same panel, while capture methods may be more cost-efficient when there are fewer samples and flexibility is more important.
Flexibility is another consideration. Multiplex PCR typically includes around 500 loci or fewer in each panel (Meek and Larson 2019), whereas capture methods can include any number of markers, up to an entire genome (Ozga et al. 2021). Additionally, capture enrichment by hybridisation tolerates differences between bait sequences and target DNA, allowing the use of the same marker set designed to genotype coyotes and kit foxes on swift fox samples. By lowering the hybridisation temperature, stringency is reduced, allowing hybridisation with sample-bait divergences up to 25% (Arbor Biosciences myBaits manual version 5.03). By contrast, multiplex PCR primers need to be designed for each species, requiring population-scale data. Furthermore, a significant number of sequences below 100 bp (e.g., Figure 3) increases the likelihood of multiplex PCR failure or locus/allelic dropout (Pompanon et al. 2005).
4.3 Kinship Analysis and Implications for Conservation
Kinship analysis suggested that four individuals trapped in Colorado are close relatives, likely siblings or parent-offspring pairs (kinship > 0.2, Manichaikul et al. 2010). All four individuals were captured on the same trapline, with traplines having an average length of 3.5 km. Although our small sample size may introduce bias into the estimation of kinship (Wang 2017), this finding aligns with the typical litter size of swift foxes (3–7 pups, Asa and Valdespino 2003) and corroborates the team's efforts to translocate family units. Maintaining familial and other social bonds during translocation can improve welfare by reducing stress as well as enhancing post-release fitness (Creel et al. 2013; Shier 2006). This strategy is pertinent to kit and swift fox conservation, as both species are known to develop pair bonds and stable trios (Kitchen et al. 2006; Koopman et al. 2000; Ralls et al. 2007).
Interactions between individuals from different sources, specifically mate choice among translocated foxes, are key drivers of population dynamics informed by kinship analysis. Reintroduction practitioners rely on assessments of population dynamics, including survival rates and abundance estimates, to measure progress towards the establishment of a self-sustaining and persistent population (Griffith et al. 1989; Fischer and Lindenmayer 2000). The ability to identify recaptures using pairwise identity estimates (IBS), as shown in this study, suggests that FAECES* protocols can be applied to generate individual encounter histories from systematically collected scat samples. These encounter histories can then be used in mark-recapture models of population size and apparent survival (e.g., Forbes et al. 2025). In addition, the FAECES* method enables the estimation of population sex ratios by using sex identification data. Including sex ratio as a covariate in population models applied at a landscape scale will allow researchers to evaluate early establishment success in the Fort Belknap reintroduction and could be adopted by other conservation practitioners hoping to assess swift fox population dynamics.
5 Conclusion
We showed that the FAECES* method can be an efficient non-invasive tool for monitoring population trends in swift foxes, in addition to its previously established utility for coyotes and kit foxes. In the future, this method will be used to estimate demographic determinants of reintroduction success at Fort Belknap without the need to repeatedly trap individuals, thereby reducing unnecessary stress. Our study also expands the utility of the FAECES* canid probes and offers best practices for ensuring the success of non-invasive capture enrichment projects. For example, we recommend performing at least three replicate index PCRs prior to enrichment to increase library complexity, reduce missing data, and enhance the reliability of the genotyping results. We also demonstrated that the single-stranded library preparation methods commonly used in ancient DNA applications can be applied to non-invasive, field-collected DNA samples of unknown age. In this study, we used the SCR method (Kapp et al. 2021), but several commercial kits are now available that can achieve similar results including SRSLY kits by Claret Biosciences (Santa Cruz, CA, USA) and the xGen ssDNA & Low-Input DNA Library Prep Kit by IDT (Coralville, IA, USA). Finally, we showed that probes designed for one species can produce informative genotypes using samples from a different, closely related species. This increases the economic feasibility of enrichment probes by expanding the number of applications for a designed and tested set of probes. Ultimately, we hope that by implementing these improvements to the FAECES* protocols, researchers can bolster the efficacy of non-invasive genetic monitoring, thereby leading to better management and policy decisions for species of conservation concern.
Author Contributions
L.D.P., D.L.N., H.S., and J.E.M. conceived the project; L.D.P., K.R.T., and J.E.M. designed the laboratory experiments. D.L.N., N.S., W.J.M., M.S., T.M., J.E.M., and H.S. secured funding. K.R.T., D.L.N., T.M., and H.S. conducted field work and collected samples. L.D.P. and K.R.T. conducted laboratory work, and J.E.M. supervised the work. M.G.C. advised on bioinformatics analysis and FAECES* design. L.D.P. analysed and archived the data and wrote the manuscript with input from all authors. D.L.N. made figures and maps.
Acknowledgements
We would like to thank the following people and organisations: Nancy Rotzel McInerney and Robert Fleischer of Smithsonian's National Zoo and Conservation Biology Institute provided assistance with lab work and logistical support, respectively; Fort Belknap Fish & Wildlife assisted with soft-release pen construction and husbandry; A'aniiih Nakoda College assisted with pen construction; Wyoming Game and Fish Department and Colorado Parks and Wildlife contributed foxes for the reintroduction as well as veterinary and logistical support at trapping locations; the Volgenau Foundation provided financial support for K.R.T.; John and Adrienne Mars provided financial support for H.S., D.L.N., L.D.P., and research expenses; Bureau of Indian Affairs provided financial support for contracting a trapping specialist in 2020; Regina Bauer Frankenberg Foundation provided financial support for D.L.N. stipend, L.D.P.'s contract, and genomics lab work; US Tribal Wildlife Grants (13159519) provided financial support for genomics lab work; NSF 18-546 Tribal Colleges and Universities Program (2054877) provided financial support for research supplies; American Prairie grant provided financial support for student stipends and research supplies; Defenders of Wildlife provided in-kind support staff time during trapping; Montana Fish and Wildlife Department provided traps. Finally, we would like to thank three anonymous reviewers and Kristen Hayward for constructive comments that improved this manuscript.
Disclosure
Permitting Information: Translocation of foxes from Colorado and Wyoming was approved by the Wildlife Commissions in each state. Trapping was conducted under WGFD Permit 13-1305, and transport through Wyoming took place under a WGFD Chapter 10 permit granted to Colorado Parks and Wildlife. All foxes received certificates of veterinary inspection completed by respective state wildlife veterinarians, and import permits were obtained from the Montana State Veterinarian. Smithsonian IACUC SI-20-09 and SI-23039.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All DNA sequences generated as part of this study are available on SRA under BioProject PRJNA1108678. A Nextflow pipeline to automate the alignment of sequencing reads to the reference genome and process alignments with SAMtools can be found at https://github.com/campanam/Elephants. R scripts for pairwise identity-by-state analysis can be found at https://github.com/LillyParker/VCF_to_PCA_IBS.




