Integration of Indigenous Research Methodologies, Traditional Ecological Knowledge and molecular scatology in an assessment of mesocarnivore presence, diet and habitat use on Yurok Ancestral Lands
Abstract
Partnerships between Tribes and researchers in wildlife monitoring and application of Traditional Ecological Knowledge (TEK) have taken a variety of forms, and some scholars have noted a need for culturally sensitive approaches. Guided by Indigenous Research Methodologies, this research is coupled with Yurok TEK, or hlkelonah 'ue-megetohl (‘to take care of the earth’), enabling an applied, culturally sensitive approach in partnership with the Yurok Tribe. We present results from a molecular scatology study of wildlife within the ancestral territory of the Yurok Tribe. Scats were collected opportunistically on road transects. All samples (N = 132) were analysed via DNA barcoding and results matched to documented 'Oohl 'we-toh (Yurok language) names to determine the depositor species (N = 8). Though there were four focal mesocarnivore species in our study, only bobcat (Chmuuek; Lynx rufus) and gray fox (Wergers; Urocyon cinereoargenteus) were detected as depositor species. Post hoc analyses were conducted to explore distribution, habitat use and selection in a use-availability context, and food habits of these two species. We found almost complete separation of bobcat and gray fox use of transects, as well as indication of partitioning of vegetation cover types and food. We demonstrate an integrated framework of Western and Indigenous sciences that allows the Indigenous researcher to transcend structured academic disciplinary boundaries. Our approach can be modified for partnerships between Tribes, agencies, academics and students for wildlife monitoring in broader geographic regions in various research applications.
1 INTRODUCTION
The development and implementation of approaches to wildlife conservation efforts that are both ecologically meaningful and culturally appropriate can be important to Indigenous communities (Housty et al., 2014; Lyver et al., 2015; Ramos, 2018). Given the marginalization of Indigenous histories and present-day narratives in Western academic institutions and scientific disciplines (Johnson, 2016), Indigenous scholars have advocated that research conducted with Indigenous communities should respect their worldviews, forms of government, community and culture (Champagne, 2015). One example of a framework for integrating conventional scientific research with a culturally appropriate research agenda was developed with the Heiltsuk First Nation for grizzly bear (Neekwech; Ursus arctos horribilis) monitoring in Canada. The research methodology was guided by six principles of Heiltsuk customary law, and authors provided examples of each principle in action as well as contributions of appropriate scientific knowledge or tools. The methodology enabled results of applied molecular genetics of grizzly bears to be embedded in a socially and culturally appropriate context (Housty et al., 2014).
Additionally, there have been efforts by various researchers to integrate Traditional Ecological Knowledge (TEK) and conventional wildlife research and monitoring (Learn, 2020). Application of Indigenous Research Methodologies (IRM) to understand TEK through a community's cultural lens has been suggested as a step towards culturally sensitive wildlife research (Ramos, 2022). IRM were developed to focus on ethically and culturally appropriate ways to conduct research with Indigenous communities (Lavallee, 2009). IRM conform to Indigenous ethical protocols that shape the methods according to local cultural imperatives (Porsanger, 2004). Approaches to research that apply IRMs will actualize differently with each study location, in time and place, as well as with the intentions of the researcher (Chalmers, 2017).
Federally recognized Native American Tribes in the United States of America maintain sovereignty to manage wildlife within their jurisdictions and many have developed wildlife management programs (Czech, 1995; Ramos et al., 2016). For the Yurok, a federally recognized Tribe whose ancestral lands are located along the Lower Klamath River in northern California, the inherent relationship with the environment as part of TEK has been important since time immemorial (Ramos, 2022). The Klamath River Reservation, established in 1855, included lands within Yurok and Hoopa ancestral territory. Through a series of subsequent executive orders, the Yurok reservation was created in 1988 (Huntsinger & Diekmann, 2010). In 1993, the Yurok community created a formal government with a constitution (Yurok Tribe, 1993), and in 2008, a wildlife program (Yurok Tribe, 2022a, 2022b).
At its inception, the primary purpose of the wildlife program was to conduct a feasibility study to reintroduce the culturally significant California condor (Pregoneesh; Gymnogyps californianus) to Yurok ancestral lands (West et al., 2017; Yurok Tribe, 2022a, 2022b). Yurok TEK, conceptualized as hlkelonah 'ue-megetohl (‘to take care of the earth’), a system where Yurok people and wildlife collaboratively strive to create and maintain balance of the Earth via physical and spiritual management in tandem (Ramos, 2019: 86), underlies much of the Yurok Wildlife Department's activities. Over time, the program expanded to include a broad range of projects related to wildlife conservation and management (Yurok Tribe, 2022a, 2022b).
As indicators of ecosystem integrity, due to their wide-ranging habits and reliance on numerous prey populations, mesocarnivores are ideal survey species (Slauson et al., 2010). Four sympatric mesocarnivores on Yurok ancestral lands, the Humboldt marten (Wohpeyroks; Martes caurina humboldtensis; hereafter, ‘marten’), Pacific fisher (Le'goh; Pekania pennanti; hereafter, ‘fisher’), bobcat (Chmuuek; Lynx rufus; hereafter ‘bobcat’) and gray fox (Wergers; Urocyon cinereoargenteus; hereafter, ‘gray fox’), might compete for food and space. The marten is extirpated from >95% of its historical range in California, with one of the two extant populations occurring in north coastal California within areas under Yurok Tribe jurisdiction (Slauson et al., 2019a). The marten is a habitat specialist; the majority of contemporary sites where martens have been detected in California occur in old-growth forest with dense shrub cover (Slauson et al., 2019a).
The current range of the fisher in California is less than half of the historical range and consists of two isolated native populations, one in the northwestern portion of the state (Zielinski et al., 2004). The fisher, also a habitat-specialized species, historically occurred in mixed conifer forests in north coastal California (Zielinski et al., 2004). Both marten and fisher are culturally significant to Yurok, participating in ceremony as ko'lsonkehl (regalia; R. Clayburn, Yurok Tribe Heritage Preservation Officer, personal communication). The gray fox and bobcat are generalists (Slauson et al., 2010), adapted to a wide variety of forest structure and management regimes for cover, den sites and prey (Harris & Ogan, 1997).
Some degree of partitioning must occur in the realized niche of coexisting species, which can occur at the trophic and habitat selection levels (Soto & Palomares, 2015). Food is a primary niche dimension that competitors may use to partition life requisites (Molles, 2010; Neale & Sacks, 2001a). However, carnivore detection across the landscape to determine habitat use and other biological measures is challenging due to the wide-ranging, low-density and elusive nature of most carnivores. All carnivores leave sign (identifiable evidence) of their presence, such as faeces, which can be collected noninvasively (not requiring animals to be baited, captured or handled) and genetically analysed for diet (Long et al., 2008). Of the four focal species in our study, we did not detect marten or fisher as depositor species. Therefore, we addressed the following post hoc research questions with only bobcat and gray fox: (1) did species use vegetation cover types differently from each other? (2) which environmental variables among vegetation cover types and road density are important to species' habitat selection? (3) what is the frequency of occurrence of prey items in the diet and is there any diet overlap between depositor species?
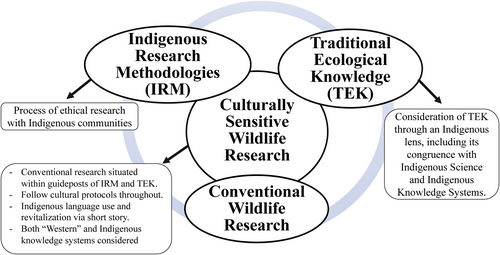
Our overarching objective was to conduct culturally sensitive wildlife research to examine mesocarnivore presence, diet and habitat use on Yurok ancestral lands by integrating IRM, TEK and molecular scatology via DNA barcoding. We situated a conventional wildlife research approach within a coupled IRM and Yurok TEK context (Figure 1) to investigate interspecific relationships between the focal species with respect to space use and food habits. Readers interested in hearing audio for pronunciation of Yurok words presented in this paper may visit Yurok language websites with archived recordings and additional resources (U.C. Berkeley Department of Linguistics, 2023; Yurok Tribe, 2022a, 2022b).
2 MATERIALS AND METHODS
2.1 Study area
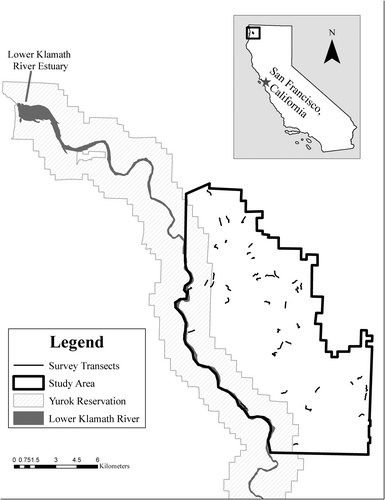
This study took place on Yurok ancestral lands in northwestern California, USA. Yurok ancestral lands are centred on the Klamath River where it meets the Pacific Ocean, covering more than 161,874 ha (Huntsinger & Diekmann, 2010) and include several contemporary jurisdictions, including the Yurok reservation, Redwood National and State Parks, Six Rivers National Forest, and privately owned timberlands under management by Green Diamond Resource Company (GDRC). Our study area (Figure 2) was approximately 770 km2 (123°47′9.5′′ W, 41°27′20.84′′ N), entirely within Yurok ancestral lands, and included portions of the Yurok reservation, acquisition lands in the process of purchase by the Yurok Tribe from GDRC, and lands under the management of GDRC. The climate is an inland expression of the maritime region with wet, cool winters (December–February) and mild, dry summers (June–August). Coastal fog occurs throughout the year (Starns et al., 2015). Average annual precipitation varied from approximately 1200 to 1800 mm and occurred mostly as rain, with 90% of precipitation falling between October and April (Kolbe & Weckerly, 2015).
Structural composition of coastal forests in the region has changed dramatically during the last century due to many factors, timber harvest being one of the most substantial (Huntsinger & Diekmann, 2010). More than 90% of northern California coastal forests have been harvested, and most of the logging of forests on private lands has been through clear-cutting. On industrial timberlands in north coastal California, road construction has also contributed to forests with highly altered structures and compositions (Thornburg et al., 2000). Though Yurok people have been divested of access to natural resources, land, traditional customs, and spiritual practices (Lara-Cooper & Lara, 2019), many have worked to maintain TEK via cultural survival and their relationship with wildlife for food and ceremony (Ramos, 2022).
2.2 Research design
We applied seven tenets of IRM: (1) follow tribal protocols; (2) intentionally build community capacity through the research agenda; (3) facilitate community ownership of data; (4) build long-term relationships with the community; (5) consider potential impacts of historical events due to settler colonialism and how the research empowers the community; (6) give back to the community (i.e. bringing back new knowledge or taking the needs of the people into account when formulating the research agenda); and (7) distribute research results and outcomes in an appropriate and meaningful way (i.e. more than just sending a copy of the final written product, Table 1). These tenets are a subset of IRM themes from published literature (Kovach, 2016; Lambert, 2014).
| IRM tenet number | IRM tenet description | Implementation |
|---|---|---|
| 1 | Follow Tribal protocols | In addition to obtaining collaborative land access permits, we held meetings with the Yurok Tribal Council, Yurok Culture Committee and the Yurok Natural Resources Committee throughout the research process to provide updates and receive guidance in our approach. We entered field sites with reverence and culturally appropriate behaviour, as directed by the Yurok Culture Committee |
| 2 | Research agenda should intentionally build community capacity | We collaborated with the Yurok Tribe Environmental Program to hire a Yurok person to assist with fieldwork |
| 3 | Take care that the community owns data | We provided species identifications and GIS data to the Yurok Tribe |
| 4 | Build a relationship with the community that is long-term |
The first author attended public and cultural events for years before consideration of conducting this research. Throughout the research process, she engaged with the Tribal Committees listed under implementation of IRM tenet 1. She continued involvement with the community many years beyond project completion There likely exist different opportunities for allies to build relationships with Indigenous communities than Indigenous peoples. Though not a replacement for personal investment with a given community, gaining understanding of the IRM and TEK literature prior to developing research plans might be helpful in building relationships |
| 5 | Consider impacts of settler colonialism and how research empowers the community | We considered the history of Yurok people and TEK through the Yurok cultural lens (Ramos, 2022) in the development of our research questions and approach. By bringing forward cultural context and TEK, we empower the community |
| 6 | Give back to the community | The results of this research were used to inspire a children's Yurok immersion story, developed in collaboration with the Yurok language community. Copies of the original book were delivered to schools across the region, the Yurok Language Program and the cultural library with the Indian Tribal & Educational Personnel Program at a local higher education institution. The story was revised with higher-level grammar for this publication (Figure 7). This method of implementation for tenet 6 could also be appropriate for tenets 4 and 7 |
| 7 | Distribute research results and outcomes in an appropriate and meaningful way | A presentation of this research was delivered to the Yurok Tribal Council and community members prior to publication of this paper |
- Abbreviation: TEK, Traditional Ecological Knowledge.
We conducted our study in conjunction with another research project to understand Yurok TEK through the Yurok cultural lens (Ramos, 2022), where we intended for TEK research to serve as a contextual foundation in which to situate ‘conventional’ wildlife research. The first author, who is Yurok and Karuk, met with the Yurok Tribal Council in 2009, upon her initial consideration to pursue research in natural resources with the Tribe to ask if such a pursuit would be supported. Upon support of the Tribal Council, she developed and refined her proposal in 2010, consulting with the Yurok Tribal Council, Yurok Culture Committee, Yurok Natural Resources Committee and the Yurok Tribe Environmental Program (YTEP) throughout the research process to provide updates and receive guidance in our approach. Additionally, the first author regularly met with non-tribal agency partners throughout the research process.
Field sites were accessed through collaborative permits between the Yurok Tribe and GDRC. We entered field sites with culturally appropriate behaviour, as directed by the Yurok Culture Committee. Following tribal protocols aligns with IRM tenet 1 (Table 1). The first author collaborated with YTEP to hire a Yurok person to assist with fieldwork (IRM tenet 2). The first author met with Yurok Tribal Council on 26 October 2022, to discuss various data archival options. Yurok Council expressed certain data should be considered sensitive, such as Global Positioning System (GPS) locations. Therefore, in support of Indigenous data sovereignty and governance (Carroll et al., 2019), limited data were uploaded to Dryad. The full dataset was given to the Yurok Tribe (IRM tenet 3).
In Yurok TEK and worldview, the creation story begins with animals and humans in spirit form with the ability to communicate with each other. Therefore, in a spiritual sense, animals are viewed as people (Ramos, 2022). However, relationships between Yurok people and wildlife have changed, in part, because of the historical impacts of early European contact and subsequent changes to the landscape and wildlife populations. Based on this context, we intentionally developed research questions where it was not necessary to trap or handle animals. Considering impacts of settler colonialism and how the research empowers the community aligns with IRM tenet 5.
We utilized molecular scatology as a noninvasive, and culturally sensitive, method to detect wildlife species. Prior to genetic scat identification methods, researchers relied on morphological characteristics, such as size of scats, to identify depositor species. However, where similar-sized carnivores co-occur, such as coyote (Segep; Canis latrans; hereafter, ‘coyote’), bobcat and gray fox, identification of the correct depositor species is unreliable and may be a substantial impediment to predator identification and subsequent dietary analyses (Farrell et al., 2000). The benefits of scat surveys: include multispecies objectives can be easily incorporated, there is minimal field expense, species detection is unbiased with lure or bait, and DNA analyses can be performed (Long et al., 2008).
2.3 Scat collection
We selected random point locations across the study area and delineated transects on the nearest roads. Transects included contemporary drivable roads as well as decommissioned logging roads. Our study design consisted of 48 approximately 500 m transects (Neale & Sacks, 2001a), with adjustment in the field due to terrain (range 300–832 m; mean = 559 m). We recorded the GPS location and road width (m) of each of the transects. Each transect was surveyed once, with two passes each (one from the start to the end; one in the opposite direction), during daylight hours.
We opportunistically collected scats detected on each transect during the summer of 2013 (21 June–20 September), under a protocol approved by The University of Arizona Institutional Animal Care and Use Committee. For each scat, we recorded the GPS location of the collection site. We followed Naidu et al. (2011) to collect and store samples prior to analyses. Apart from black bear (Chyer'ery; Ursus americanus; hereafter, ‘bear’), we did not attempt in-field identification of scat via morphology. We did not collect scats with the characteristics of fruit scat deposited by adult bears, as these were abundant and bear was not a focal species for our study. Although the appearance of bear scat varies depending on diet, bear fruit scats have distinguishable characteristics in size and appearance (Elbroch, 2003). We recorded the GPS location of each bear fruit scat and included these data with our depositor species data.
2.4 Depositor species identification
We performed scat-DNA extractions in a laboratory dedicated to processing ancient or otherwise degraded tissue specimens (Culver Conservation Genetics Laboratory, The University of Arizona, Tucson, USA). The DNA laboratory was located in a separate building from where contemporary tissue DNA extractions are performed. We washed each sample in a petri dish with approximately 500 μL ATL buffer (Qiagen, Inc., Valencia, CA, USA), or a volume such that the buffer appeared brown upon swirling the sample, making contact with only the outside surface of the scat. We ceased washing at approximately 30 s or upon observation of a change in colour of the solution from clear to brown, indicating the potential presence of epithelial cells from the predator's intestinal tract. We then used a DNeasy Blood & Tissue Kit (Qiagen, Inc.), following a modified protocol from the manufacturer for DNA extraction from animal tissue, to extract DNA from 300 μL of the epithelial cell solution, with a final elution volume of 200 μL.
For species identification, we used mammalian polymerase chain reaction (PCR) primers mcb398 and mcb869 (Verma & Singh, 2003) to amplify a 472 bp segment of the mitochondrial cytochrome b gene. Each PCR amplification was performed in a 20 μL reaction volume with the following final concentration: 1 × PCR Buffer (Qiagen, Inc.), 1 mM MgCl2 (Qiagen, Inc.), 0.2 mM dNTPs (Qiagen, Inc.), 0.05% BSA (Sigma-Aldrich, St. Louis, MO, USA), 0.5 U of Taq DNA Polymerase (Qiagen, Inc.), 0.5 μM each of forward and reverse primers, and 6 μL of template DNA. PCR conditions consisted of initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 45 s, annealing at 51°C for 1 min, extension at 72°C for 2 min, and a final extension step at 72°C for 10 min. Post-amplification, PCR products were cleaned with ExoSAP-IT (USB Corporation, Cleveland, OH, USA) and sequenced on a 3730 Automated DNA Analyser (Applied Biosystems, Foster City, CA, USA) at the University of Arizona Genetics Core (Tucson, AZ, USA). We used Sequencher v5.2 (Gene Codes Corporation, Ann Arbor, MI, USA) to assemble, align and edit forward and reverse DNA sequences. Due to scat samples having low-quality or degraded DNA, we trimmed, edited and differentiated sequences manually.
We used the online Basic Local Alignment Search Tool (BLAST), optimized for similar sequences (blastn) and run remotely from the National Center for Biotechnology Information, to query DNA sequences and identify species of origin based on best matches obtained with reference sequences in GenBank. We identified species by selecting the first hit for each query sequence meeting the criteria of a maximum identity ≥95% and an e-value of 0.0. If a sequence exhibited high background peaks, no discernible peaks for a stretch of more than 1–2 bp, or many Ns, representing ambiguous base calls, and did not result in a match in GenBank, the chromatogram and unidentified bases were examined to determine if further improvements could be made to the base calls. The sequence was then queried again. We deemed the species identity to be inconclusive if: the sample failed to yield PCR products, the BLAST search yielded a result of ‘no significant match’, or the BLAST percent identity or e-value did not meet our criteria. As language is a component of Yurok TEK (Ramos, 2022), we searched for the Yurok names for species on the Yurok Language Project website (http://linguistics.berkeley.edu/~yurok/), operated at the University of California, Berkeley (Berkeley, CA, USA).
2.5 Habitat use and selection
Indigenous peoples have used the location of scat to identify the presence and location of animals, and ecologists have also used the distribution of scat as an indicator of space use at various scales, recognizing that the microhabitat placement of scat (e.g. on top of a rock or on a road) is under different selection pressures (Neale & Sacks, 2001a, 2001b; Telfer et al., 2006). In this study, we analysed the relationships between scat locations and the surrounding environment from data in a GIS, assuming the scat location revealed information about general habitat use. All undermentioned statistical analyses were conducted in Rstudio (v. 1.4.1106).
We used GIS-based vegetation coverage of the study area from the Yurok Tribe Geospatial Information Technology Program to evaluate habitat use patterns. The vegetation cover layer consisted of polygons of various vegetation cover types that included six categories: barren, herbaceous, shrub, conifer, hardwood and mixed hardwood–conifer. We excluded the herbaceous cover type from analyses because no scats deposited by our focal species were detected in herbaceous sites. We considered the vegetation cover type of each scat detection location on transects as the sampling unit; we used the spatial join tool in ArcMap to extract the cover type of each sample. We pooled samples from the conifer, hardwood, and mixed hardwood–conifer into a category, ‘forest’, resulting in three final vegetation cover types: barren, shrub, and forest.
To determine whether bobcat and gray fox used vegetation cover types equally across categories, we conducted a chi-square test of homogeneity. We assessed population-level habitat selection of both species across the entire study area based on used and available transects. We used detections of bobcat and gray fox scats to identify variables to include in a used-available resource selection function (RSF; Rstudio, v. 1.4.1106) for each mesocarnivore. We removed two gray fox samples that had been deposited on top or bottom of another gray fox scat and one bobcat sample that had been deposited on top or bottom of another bobcat scat. We used a random point generator to identify ‘available’ habitat locations; we selected random points (n = 69) within transects that were not used by either bobcat or gray fox. We included vegetation cover type (barren, shrub, forest) and road density (km2) as predictor variables. We converted a road's shapefile to a 30-m raster and then used density tools in spatial analyst with the nearest neighbour option (ArcMap v. 10.5.1) to create map of road density. We then extracted road density values for each scat and available location. We utilized a generalized linear model to compare characteristics of all predictors (vegetation cover types and road density) associated with species scat detection locations to characteristics of available locations, as well as a null intercept-only model.
2.6 Food habits
To assess overall representation of prey in bobcat and gray fox diet, scat samples identified as deposited by bobcat or gray fox were subsequently dissected for bones. We randomly selected one bone from each scat for analyses. We used a SPEX SamplePrep Freezer/Mill 6770 cryogenic grinder (Spex CertiPrep, Metuchen, NJ, USA) to pulverize each bone sample into powder. We then decalcified up to 50 mg of powder from each sample and used the DNeasy Blood & Tissue Kit (Qiagen, Inc.) to extract DNA from the bone powder, and used a final elution volume of 200 μL. We then followed the procedures described above for PCR for species identification, DNA sequencing and BLAST to analyse sequences to the lowest possible taxonomic category.
For bobcat diet items, we calculated relative frequency of occurrence as the total number of occurrences of each prey species divided by the total number of occurrences of all prey species expressed as a percentage. To assess niche breadth and overlap for bobcat and gray fox diet, we pooled prey into six categories: small mammal, medium mammal, large mammal, reptile, bird and plant/insect. We calculated Levin's index (Serafini & Lovari, 1993): to determine diet breadth for bobcat and gray fox. To assess diet overlap between bobcat and gray fox, we used Pianka's index (Pianka, 1973; Thornton et al., 2004): , where pi is the proportion of food item i in the diet of predator p, and qi is the proportion of food item i in the diet of predator q. A value of 0 resulting from Pianka's index indicates complete dissimilarity, and a value of 1 indicates complete similarity.
3 RESULTS
3.1 Depositor species identification
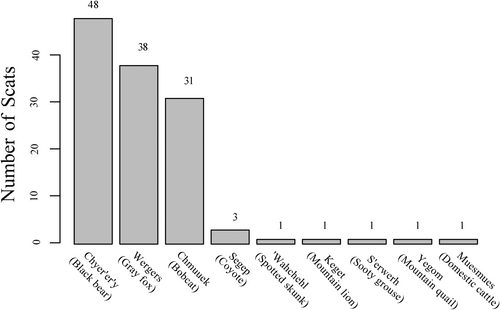
We observed a total of 167 scats across 41 transects and collected 131 of these (scats field-identified as deposited from bear were not collected). We obtained DNA sequence data meeting our criteria and determined the depositor species of 89 scats, totalling eight species including bobcat (n = 31) and gray fox (n = 38). Other species detected via molecular analysis include bear (n = 12), coyote (n = 3), mountain lion (Keget; Puma concolor; n = 1), sooty grouse (S'erwerh; Dendragapus fuliginosus; n = 1), mountain quail (Yegom; Oreortyx pictus; n = 1), domestic cattle (Muesmues; Bos taurus; n = 1) and Western spotted skunk ('Wahchehl; Spilogale gracilis; n = 1; Figure 3). The depositor species of the remaining 42 scats were inconclusive. We did not detect marten or fisher as scat depositors. Fruit scats deposited by bear were identified in the field (n = 36).
We found all species names in the online Yurok dictionary, with the exception of the marten. During our study, a Yurok language instructor found the Yurok name for marten in an audio recording that was stored with the Yurok Language Program but had not yet been included in texts such as the online dictionary (James Gensaw, Yurok Language Instructor, personal communication).
3.2 Habitat use and selection
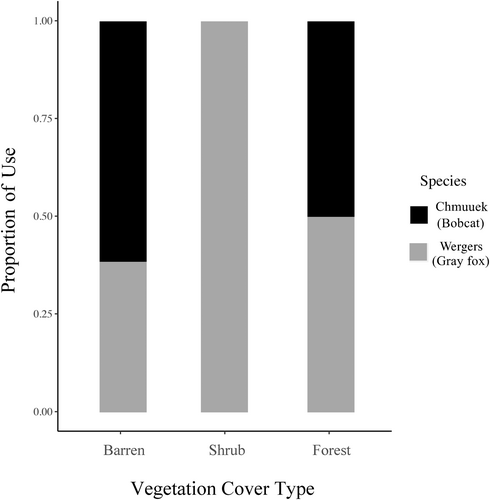
Of the 41 transects surveyed, 25 were used by bobcat and gray fox (bobcat only = 7 [28%]; gray fox only = 17 [68%]; both bobcat and gray fox = 1 [4%]). The most bobcat samples collected from one transect was 17 (54.8%), while the most gray fox collected from one transect was six (15.8%). There was statistical evidence that bobcat and gray fox did not use vegetation cover types equally (χ2 = 10.086, df = 2, p < .01; Figure 4): bobcat used barren areas more than gray fox, while gray fox used the shrub cover type more than bobcat.
The RSF model for bobcat with all predictors explained 9.8% of deviance (AICc = 117.56). The null intercept-only model was not competitive (AICc = 123.49). Compared to the barren cover type, there was statistical evidence that bobcat used the forest cover type less than expected given its availability (p < .01). Road density was not shown to be a strong predictor of bobcat scat deposition (p = .49). The RSF model for gray fox explained 19.95% of deviance (AICc = 116.08). The null intercept-only model was not competitive (AICc = 137.05). Like bobcats, gray foxes also used the forest cover type (p < .01) less than expected given its availability. Road density was a statistically significant predictor of gray fox scat location (p < .001); the likelihood of selection decreased in the model as road density increased.
3.3 Food habits
We used DNA barcoding to identify prey items for all three coyote scats, as coyote is considered a mesocarnivore (Buskirk & Zielinski, 2003) and is sympatric with bobcat and gray fox (Fedriani et al., 2000); we detected woodrat (Tergers; Neotoma fuscipes; hereafter, woodrat; n = 2) and domestic cattle (n = 1). We analysed 38 gray fox scats for diet. Of the gray fox scats, only three contained bones. The remaining gray fox scats (n = 35) were composed primarily of soft mast (e.g. seeds) and a few contained insect parts. We used DNA barcoding to identify one of the bone samples as kingsnake (Chergercheryerh or Kwegey; Lampropeltis getula) and one as gray fox. Researchers have found cannibalism to occur among gray foxes (Fedriani et al., 2000). However, the result could also have been due to contamination (e.g. depositor DNA on the bone sample); therefore, we removed the sample from our dataset. The third bone sample from the gray fox diet was inconclusive.
For bobcat, we analysed 22 scats for diet and successfully identified 20 items of prey remains via DNA barcoding. Two bobcat scats did not contain bones but did contain unidentifiable plant and insect parts. Two of our bobcat diet samples were matched as red squirrel (Tamiasciurus hudsonicus) via BLAST. T. douglassii (hereafter, ‘Douglas squirrel’) is the only species of Tamiasciurus that has been shown to occur in our study area (Arbogast et al., 2001; Steele, 1998, 1999). In addition, Yurok have a long-standing name for the Douglas squirrel (Hegoyekw), but no name for T. hudsonicus. Therefore, we determined the samples to be Douglas squirrel.
We detected six prey species in the bobcat scats; the most common was woodrat, consisting of 45.5% of the identified items. Chipmunk (Smechken; Tamias spp.) was the second most common prey species in the bobcat diet and accounted for 22.7% of identified items. Other species identified in the bobcat diet included deer mouse (Negeneech; Peromyscus maniculatus), Pacific band-tailed pigeon (He‘mee’; Patagioenas fasciata monilis) and marten (Figure 5).
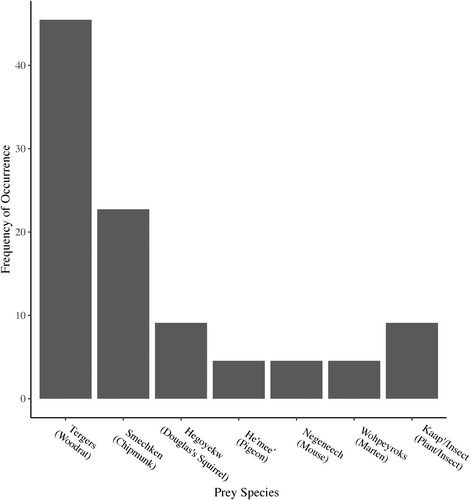
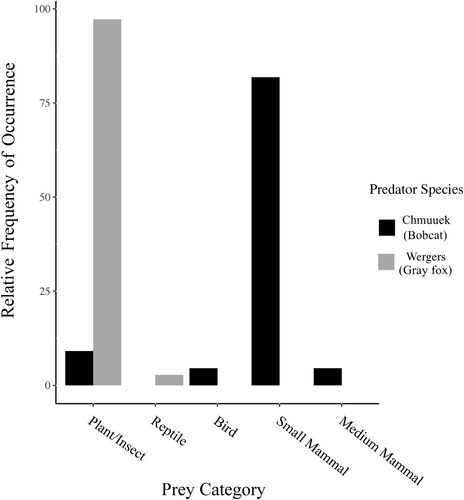
We analysed total number of diet items across categories from bobcat (n = 22) and gray fox (n = 36) scats (Figure 6) in assessments of diet breadth and diet overlap. Diet breadth (Levin's index) was slightly broader for bobcat (1.441) than for gray fox (1.057). Pianka's index of overlap, based on total counts of all diet categories, showed an overlap value of 0.104, due to slight similarity between bobcats and gray foxes in use of plants and insects.
4 DISCUSSION
Our interdisciplinary framework of utilizing molecular scatology methods, IRM and TEK allowed for a culturally sensitive approach to wildlife research with the Yurok Tribe. The first author, with support of the second author, was able to align action with seven IRM tenets, such as building a long-term relationship with the community (IRM tenet 4) that has continued from proposal stage to data collection, initial write-up and beyond. During the final stages of preparation of this paper, the first author delivered a presentation of the research to the Yurok Tribe Council and community members (IRM tenet 7) and co-authored a companion story in the Yurok language that was inspired by the research (IRM tenet 6; Figure 7). While one might not envision an Indigenous language immersion story as material for a scientific journal, language is an important component of Yurok TEK and Indigenous Science (Ramos, 2022). The 2024 special issue, ‘Indigenous Contributions to Molecular Ecology’, in Molecular Ecology Resources, in which this paper is included, provides an opportunity to honour IRM by being more inclusive of various types of works that can be utilized by Indigenous communities.

With the addition of a companion story in the Yurok language, the authors of this paper and the companion story recognize the United Nations International Decade of Indigenous Languages, 2022–2032. Yurok was historically an oral, not a written, language. In the 1950s, fewer than 20 people had conversational speaking ability beyond words and phrases (Robins, 1958). Since then, there have been initiatives to document and revitalize the language (Garrett, 2014; Trull, 2003). To this day, there continue to be different ways of spelling used among the Yurok Tribe, community members and programs such as the Yurok Language Project at the University of California, Berkeley. We used a combination of the Yurok Tribe's New Alphabet and the spelling system of the U.C. Berkeley Yurok Language Project (Garrett, 2014). At the heart of efforts to create materials, such as this story, is the common goal of community language use and continued revitalization of TEK.
By considering historical and cultural contexts (Ramos, 2022) in the design of this research and in publishing this work in an Indigenous-focused special issue of a journal steeped in Western science, we empower (IRM tenet 5) not only the Yurok people but also others who strive to conduct research inclusive of Indigenous science and community needs. We reverse the approach of seeing TEK as a dataset to be extracted but rather as a way of life, thereby broadening the space of inclusive science. While we believe our research framework creates more space for Indigeneity in [Western] science, we caution that completing projects in this space require extensive time investment with a given community. We encourage researchers to consider building relationships with the community and gain understanding of the IRM literature before formulating research plans.
We also acknowledge that conducting research with Indigenous communities will often present different processes for Indigenous peoples as compared to allies. In our research, the second author served as a mentor in wildlife genetics, as well as an ally who facilitated institutional processes for the research and supported the first author in career advancement to a postdoctoral position, and subsequently to an assistant professor position in STEM. While these latter actions extend beyond the research, they serve here to illustrate how allies can empower Indigenous peoples in STEM.
We used Yurok language in our paper as one aspect of Indigenous science and Yurok TEK (Ramos, 2022), where the use of language is of importance to Yurok people in cultural revitalization and relationships with wildlife. Although we were able to match the majority of species identifications from our genetic analyses with the Yurok name, there were instances where there were discrepancies; such an instance occurred with the chipmunk. Four bones in the bobcat diet matched with high percent identity (96%–99%) to both T. senex and T. siskyou in GenBank. We determined these samples to be T. siskyou, as our study area overlapped the species' range near the Klamath River, and not the range of T. senex (Reid, 2006). Though both species occur within Yurok ancestral territory, we found there is only one Yurok word for chipmunk (smechken). Similarly, we found only one word for woodrat (tergers), though both N. fuscipes and N. cinerea occur in the study area (Reid, 2006).
Our deliberate use of roads meant that transect placement was not random with respect to microhabitat; it is conceivable that the results were biased because roads are not randomly placed on the landscape. Nevertheless, our study site was large enough to encompass approximately 12–296 bobcat home ranges (Conner et al., 2001) and 220–770 gray fox home ranges (Deuel et al., 2017), and 13 fisher home ranges (Zielinski et al., 2004). Further, a population of marten that persists within the study area was monitored during the same time period as our study (US Fish and Wildlife Service & USDI Forest Service, 2019). At the landscape scale, our results indicate that bobcat and gray fox use roads more than fisher or marten, as we detected scats from the former two species but not the latter. These results align with previous research: in the North-coast redwood region of California, gray foxes and bobcats favour roads and have high detection probabilities (Cooperrider et al., 2000). In a study of mesocarnivores, including the focal species, in our study area, both marten and fisher were found to occur but used forest interiors significantly more than roads. Conversely, bobcat and gray fox used roads more frequently than forest interiors (Slauson et al., 2010).
The distribution of mesocarnivores is largely determined by the interaction of a number of factors including habitat structure, community interactions and prey availability (Slauson et al., 2010). Coexistence of species may be privileged when species with different degrees of habitat and/or trophic specialization are present (Soto & Palomares, 2015). We observed niche separation between bobcats and gray foxes across spatial deposition of scats, habitat use and selection, and food habits. Niche separation is particularly obvious in heterogeneous landscapes, where coexistence is facilitated between similar species, with the selection of different habitats as one of the main processes promoting sympatry (Soto & Palomares, 2015). Our study area was heavily managed prior to transfer to the Yurok Tribe and contained areas with various vegetation cover types, including regenerating clear-cut forest and older second-growth forest stands. Across transects used by bobcat and gray fox (n = 25), only one had scats deposited by both species. Further, the two species were selected for different vegetation types when compared to each other, with gray fox using more shrub cover types than bobcat, and bobcat using more barren cover types than gray fox.
The RSF models for both species explained less than 10% of deviance, though fit better than the null models. Both species avoided forest relative to the barren cover type. Road density at the landscape level was not a significant predictor for bobcat scat but was for gray fox scat. Both of these mesocarnivores are generalists who use roads; the gray fox scats in our study were distributed more broadly across the landscape. One study of kit foxes (Vulpes macrotis) in Utah suggested that scat deposition transects on roads may be more useful for monitoring kit fox abundance than determining resource selection, as roads in the study area were placed in lower elevations while movement of kit foxes determined by telemetry locations extended into higher elevations (Dempsey et al., 2015). In our study area, road drivability varied due to the inclusion of decommissioned logging roads to establish our transects. Our transects also spanned the elevational range of the study area. For future research in our study area, random placement of scat deposition transects may increase the utility of RSF studies. However, probabilities of detecting (or finding) scat of gray fox and bobcat scats in native vegetation would likely decline and the efficacy of random transects would need to be considered.
We found evidence of diet partitioning between gray foxes and bobcats. Bobcat showed slightly broader breadth of prey items than gray fox, and almost no overlap in diet items. Predators might consume a prey item in one area and then defecate in another; location of scat does not necessarily translate to occupancy by the animal. However, it does indicate that the animal used the area in the recent past (Long et al., 2008). Although timber harvesting in northern California has contributed to habitat loss for marten and fisher (Zielinski et al., 1997), the resulting early successional vegetation has led to increases in populations of woodrat (Hamm & Diller, 2009), a prey item for all four species of interest (Golightly et al., 2006; Harris & Ogan, 1997; Maser et al., 1981; Nussbaum & Maser, 1975). We found woodrat in the bobcat diet of nearly half of our samples where we were able to conclusively determine species identification. Lagomorphs and rodents are the most common prey items of bobcat in the Western United States (Buskirk & Zielinski, 2003). Although we obtained inconclusive (e.g. percent identity was just below our criteria) matches to lagomorphs (Herkwerh; Sylvilagus spp.) in four bobcat diet samples, woodrat occurred more frequently. As the study area had been actively managed by timber harvest, woodrat is common in young (5–20 years) forest stands that result after harvest (Hamm & Diller, 2009) and bobcats used barren areas (which could have resulted from clear-cutting), bobcats may utilize recently harvested areas to find prey.
We detected woodrat in the diet of coyote, as well, consistent with findings from other researchers (Neale & Sacks, 2001b). Although gray fox have been found to prey on woodrat (Harris & Ogan, 1997), we did not detect woodrat in the gray fox diet in our study. In a study in Arizona, the primary food of gray fox in February through November was soft mast (fruit and berries), but rodents, insects and rabbits were also common (Cunningham et al., 2006). In another study in California, coyote and gray fox relied on small mammals year-round, though they also ate significant amounts of fruit (Fedriani et al., 2000). The gray fox scats in our study almost exclusively contained soft mast and no bones.
Predation rates by habitat generalist carnivores in intensively managed landscapes, such as our study area, likely represent a limiting factor for marten populations (Slauson et al., 2019b). Bobcat have been known to prey on marten in April–November in northeastern Oregon (Bull & Heater, 2001), coinciding with the months of our study in June–September. Previous researchers have detected mortality of marten by bobcat based on necropsies and circumstantial evidence at kill sites in Oregon (Bull & Heater, 2001) and in our study area (US Fish and Wildlife Service & USDI Forest Service, 2019). Our results provide genetic evidence supporting not only bobcat predation on marten but also consumption. Although coyote have been found to prey on marten (Bull & Heater, 2001), we did not detect marten in the coyote diet in our study area. However, we only detected three coyote scats.
Noninvasive survey methods are increasingly being used to determine animal presence and monitor populations. Although we used a noninvasive survey design in our aim to be culturally sensitive, we acknowledge that there may be cases where the trapping and handling of animals would be advantageous to species recovery efforts. Communication with Tribes in culturally sensitive studies such as ours is important, as Tribes may support projects where animals are handled, especially in cases where it is for the sake of at-risk species. However, it is important to remember that resources, capacity, values and needs vary between Tribes and each collaborative endeavour with Tribes might pose unique opportunities or restrictions. Future surveys in our study area could include repeated visits to the established transects, as well as, geospatial analyses to investigate species distribution in relation to various forest age classes and other environmental variables. Further, our study exemplifies how wildlife monitoring can be designed in a framework that includes molecular methods, IRMs and TEK, and our approach may be utilized in other geographic areas, such as lands under Tribal jurisdiction and public lands of cultural significance to Tribes.
AUTHOR CONTRIBUTIONS
Conceived and designed the study: SCR. Collected the samples: SCR. Analysed the data: SCR. Contributed reagents/materials/lab space/equipment: SCR, MC. SCR wrote the initial version of the manuscript, with subsequent review by MC.
ACKNOWLEDGEMENTS
We thank the Yurok Tribe, Green Diamond Resource Company, National Park Service, U.S. Fish and Wildlife Service and Wildlife Conservation Society for their support in fieldwork logistics and in-kind contributions. This research was supported by The University of Arizona/Sloan Indigenous Graduate Partnership Program (Award 2010-3-03), as well as a collaborative agreement between the National Park Service and the Wildlife Conservation Society. We thank the Yurok Tribe Environmental Program, especially K. Sloan, for in-kind support with fieldwork. We are grateful to L. Alvarado (Yurok) for assistance with fieldwork and A. Rosenberg and K. Vargas for assistance with lab work. We thank T. Shenk, W. Zielinski, K. Slauson, J. Koprowski, B. Colombi and R. Trosper for valuable guidance in the initial stages of this research. A. Ochoa, R. Fitak, A. Naidu, S. Rinkevich, E. Vaughn and C. Voirin provided support in laboratory methods. We are grateful to the associate editor, three anonymous reviewers, M. Johnson and K. Hamm for thoughtful reviews that improved the manuscript. The Yurok Tribe Geospatial Information Technology Program kindly provided GIS vegetation coverage. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the US Government. The material in this publication is based in part on work supported by the National Science Foundation Postdoctoral Research Fellowship in Biology (Grant 1906338). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
BENEFIT-SHARING STATEMENT
We consulted with the Yurok Tribe and utilized Indigenous Research Methodologies, which are described throughout this research paper. The results of the research have been shared with the Yurok Tribe and broader scientific community (see “Data Availability Statement”). We also include a storybook in the Yurok language, ‘The Little Bobcat and His Mother’, as a companion piece to this research paper.
Open Research
DATA AVAILABILITY STATEMENT
DNA sequences used to determine depositor species and diet items are available through the Dryad repository (https://doi.org/10.5061/dryad.bzkh189dx).




