Barcoding and traditional health practitioner perspectives are informative to monitor and conserve frogs and reptiles traded for traditional medicine in urban South Africa
Abstract
Previous literature suggests that Indigenous cultural practices, specifically traditional medicine, are commonplace among urban communities contrary to the general conception that such practices are restricted to rural societies. We reviewed previous literature for records of herptiles (frog and reptile species) sold by traditional health practitioners in urban South Africa, then used visual confirmation surveys, DNA barcoding and folk taxonomy to identify the herptile species that were on sale. Additionally, we interviewed 11 IsiZulu and SePedi speaking traditional health practitioners to document details of the collection and pricing of herptile specimens along with the practitioners' views of current conservation measures for traditional medicine markets. The 34 herptile species recorded in previous literature on traditional medicine markets included endangered and non-native species. Spectrophotometry measurements of the DNA we extracted from the tissue of herptiles used in traditional medicine were an unreliable predictor of whether those extractions would be suitable for further experimental work. From our initial set of 111 tissue samples, 81 sequencing reactions were successful and 55 of those sequences had species-level matches to COI reference sequences on the NCBI GenBank and/or BOLD databases. Molecular identification revealed that traditional health practitioners correctly labelled 77% of the samples that we successfully identified with DNA barcoding in this study. Our mixed methodology approach is useful for conservation planning as it updates knowledge of animal use in Indigenous remedies and can accurately identify species of high conservation priority. Furthermore, this study highlights the possibility of collaborative conservation planning with traditional health practitioners.
1 INTRODUCTION
Traditional medicine, or the Indigenous knowledge and practices that people of different cultures use for maintenance of physical and mental health, is prevalent across the world (World Health Organization, 2000, 2019). A World Health Organization report stated that 88% of 179 member states that responded to a global survey acknowledged use of traditional medicine by their citizens (World Health Organization, 2019). South Africa is among the developing countries where use of traditional medicine is common, and this practice also occurs in the country's most urbanized areas such as Johannesburg and Durban (Du Toit, 1980; Longmore, 1958; Ngwenya, 2001; Williams & Whiting, 2016). Traditional cultural practices have also been recorded in urban areas of other countries such as Bolivia (Macía et al., 2005), the United States of America (Balick et al., 2000) and Brazil (Alves & Rosa, 2007). South Africa's urban traditional medicine markets generally trade in illegally acquired wildlife, a majority of which are plant species (Williams & Whiting, 2016). Both lethal and non-lethal collection techniques are used to collect materials from plants and animals for traditional medicine practices. Non-lethal collection for traditional medicinal use, for example, only involves taking leaves of some plants. Conversely, lethal collection methods involve killing some plants to use their roots, removing fungi (with their mycelium) from their substrate or killing animals to utilize their tissue.
Although traditional medicine mostly relies on plants (Nascimento et al., 2016; Solovan et al., 2004), animal use in Indigenous remedies nonetheless remains important to society as demand for animal-based remedies can lead to overexploitation of animal species (Alves et al., 2013; Still, 2003). Investigating this use of animals in traditional medicine is important for updating knowledge of this Indigenous practice, informing collaborative conservation management of animals used in traditional medicine and exploration of the economic value of animal trade for medicinal purposes (Alves et al., 2013). Studies of traditional medicine use also provide an opportunity to understand how custodians of Indigenous practices perceive conservation planning thus further informing collaborative approaches, and this collaboration is especially important in South Africa where lax inclusivity in conservation practices is a concern (Leonard, 2013; Musavengane & Leonard, 2019). Research interest in the use of animals in traditional medicine has been low (Solovan et al., 2004). Among animals that are used in Indigenous remedies across the world, there are at least 331 herptile species (47 amphibian and 284 reptile species) and the number of herptile species known to be used for traditional medicine purposes could increase when comprehensive studies of traditional medicine use are conducted (Alves et al., 2013). Traditional health practitioners are sometimes reluctant to discuss their practices (Whiting et al., 2011), thus attempts to comprehensively study traditional medicine practices or collaborate with traditional health practitioners may be hampered. The term traditional health practitioner broadly refers to people that are deemed capable of incorporating plants, animals or minerals in healing practices that are based on Indigenous cultural practices (World Health Organization, 1978).
Research focused on traditional medicine markets generally has a problem with identification of specimens available at those markets (Veldman et al., 2020). Morphology-based identifications (or identification based on distinctive characters) of animal specimens from traditional medicine markets in urban South Africa showed that some specimens could only be identified to genus or higher taxonomic ranks depending on how well the morphological traits are preserved (Ngwenya, 2001; Simelane & Kerley, 1998; Whiting et al., 2011). Species-level identifications of traditional medicine market specimens with poorly preserved diagnostic traits can be obtained with DNA barcoding (Whiting et al., 2011). Furthermore, the Indigenous names that traditional health practitioners use for species of interest can also be compared to molecular taxonomy with DNA barcoding (Veldman et al., 2020). Introducing DNA barcoding to studies of herptiles in urban traditional medicine markets is thus beneficial for helping traditional health practitioners confirm that they are using the correct species (instead of a cryptic species with a different folk name) and assisting conservation practitioners to keep track of endangered species with traditional medicine value. DNA barcoding is an effective tool to identify both known and unknown species by comparing fragments of an individual's DNA with DNA sequences of individuals from several species (Hebert, Ratnasingham, & de Waard, 2003). Using DNA barcoding to confirm the identity of species in traditional medicine markets helps to increase our knowledge on the (number of) species that are being sold at those markets and the related conservation pressures (Veldman et al., 2020), and to also detect substitution of species in Indigenous remedies (Newmaster et al., 2013; Veldman et al., 2020). Substitution of plant species in Indigenous remedies poses a risk to human health if non-toxic plants are substituted with toxic species (Ouarghidi et al., 2012). DNA barcoding of traditional medicine specimens in this instance is vital to identifying human health risks in addition to confirming species' identification. Use of DNA barcoding to confirm the identity of Indigenous medicine specimens hence has promising prospects, but its use remains low (Mishra et al., 2016). DNA barcoding would be of even greater use in identifying species used in Indigenous remedies if an extensive DNA library of focal taxa is established.
Research focusing on urban areas in developing countries provides opportunity for innovative scientific approaches that can benefit urban sustainability (Nagendra et al., 2018). As growth of cities on the African continent continues to place pressure on their surrounding environment to meet the needs of the urbanized human populations (Grant, 2015), studies that investigate urban utilization of wildlife contribute to context-specific interventions to mitigate conservation threats posed by city-dwellers' use of wildlife. Updated understanding of threats to South Africa's anuran amphibians (i.e. the only group of amphibians that occur in the country collectively called frogs) and reptiles, this study's focal taxa, is important as 5% of reptile species and 12% of frog species described from the country are listed on the International Union for Conservation of Nature (IUCN) Red List of threatened species (IUCN, 2021). We aimed to increase understanding of the use of herptiles in traditional medicine, document traditional health practitioners' perceptions of conservation interventions aimed at traditional medicine markets and update records of herptile species targeted for South Africa's urban traditional medicine trade. To achieve these aims, we addressed the following questions: (1) Can DNA barcoding pinpoint which herptile species are sold for traditional medicine purposes in South African cities? (2) Can an adequate quantity/quality of DNA be retrieved from herptiles collected and preserved through Indigenous methods? (3) What is the correspondence between the Indigenous names for herptiles found in traditional medicine markets and names assigned through scientific taxonomy? (4) What are the perceptions of traditional health practitioners towards current conservation measures aimed at traditional medicine markets?
2 MATERIALS AND METHODS
To achieve the aims of this study, we reviewed existing literature on South Africa's urban traditional medicine markets for records of trading in frogs and reptiles. Additionally, we interviewed traditional health practitioners to increase current understanding of their practices, and used visual confirmation during visits to traditional medicine markets and DNA barcoding targeting a fragment of the cytochrome c oxidase subunit 1 gene (COI) to identify the herptile specimens that practitioners traded in.
2.1 Literature review
We searched for the keywords: animal + traditional medicine + “South Africa” on Google Scholar (https://scholar.google.com/) to obtain results of literature for which titles and abstracts were pre-screened for mentions of animal use in South African traditional medicine. Following this initial screening, we studied suitable articles to find records of herptiles sold specifically in South Africa's urban traditional medicine markets or shops. Subsequently, we checked the availability of reference DNA sequences for the herptile species matching the inclusion criteria of the literature review on the National Center for Biotechnology Information (NCBI) GenBank database (using the search query ((Species name) AND (CO1[Gene Name] OR COI[Gene Name])) and the Barcode of Life Data Systems (BOLD) database (using the search query “Species name”).
2.2 Fieldwork: Interviews, tissue sampling and visual observation
The first author conducted visual confirmation surveys of herptile specimens available for sale at traditional medicine markets/shops by visiting six urban locations in Durban, Johannesburg, Pietermaritzburg, Polokwane and Pretoria from August to December 2020 (Figure 1). Species identification through visual confirmation was based on their morphology using wildlife guides (books) as a reference (Alexander, 2007; Marais, 2008). North-West University's Biodiversity and Ecology Research Ethics Committee approved human participation in this study. This study which is part of a bilateral scientific cooperation between two universities received approval from institutional research committees of both universities that are signatory to the bilateral agreement: North-West University Animal Care, Health and Safety Research Ethics Committee (Ethics number: NWU-00185-18-S5) and Hasselt University Social-Societal Ethics Committee (Reference: REC/SMEC/VRAI/189/127). Furthermore, we conducted research in a manner that complies with the Nagoya Protocol on Access and Benefit-sharing (UID: ABSCH-IRCC-ZA-257320-1).
In accordance with North-West University Health Research Ethics Committee's guidelines, the first author sought participation of traditional health practitioners at the markets/shops after explaining the purpose of this study in SePedi (language spoken by people of Pedi culture) to practitioners from Limpopo and IsiZulu (language spoken by people of Zulu culture) for Gauteng and KwaZulu-Natal practitioners. SePedi was the preferred language for the Limpopo participants, IsiZulu was the most spoken language at the markets/shops in Gauteng and KwaZulu-Natal. Following explanation of this study, 11 traditional health practitioners consented to participate in this study (two in Limpopo, two in Gauteng and seven in KwaZulu-Natal). The first author used an informal conversational interview approach to collect data about herptiles of traditional medicine value; their Indigenous names, and the collection and preservation methods used for those herptiles were documented. This interview approach relies on continuous participant observation without predetermined questions (Gall et al., 2003). The approach was chosen due to traditional health practitioners expressing apprehension towards researchers based on what they explained as past unpleasant experiences with researchers and conservation practitioners. This interview was guided by the first author's conversation with participants and questions were introduced to the conversation when participants were forthcoming with details about their practices. The first author wrote answers to these questions in a field book as practitioners did not give consent for their participation to be recorded using electronic devices. The reasons for the practitioner's apprehension towards researchers were also noted.
Tissue samples from dry specimens sold by nine participants were collected by the first author at four of the six localities visually surveyed in Gauteng and KwaZulu-Natal markets/shops (Figure 1) as they gave consent for this collection while the two participants in Limpopo said they could not give consent as they did not own the traditional medicine shops. A total of 111 samples were collectively obtained from Gauteng and KwaZulu-Natal (Data set S1). Practitioners were asked the IsiZulu names for each sampled specimen and the first author recorded these names, while also noting any visible morphological features that could be used to supplement molecular identification. Recording of names and the observed morphological traits of specimens entailed writing them in a field book.
Many sampled specimens lacked distinctive morphological traits as sometimes all that remained were ventral scutum, or bones with flesh but no skin. We opted to collect tissue samples instead of taking entire specimens to minimize this study's environmental impact as removal of entire specimens may prompt traditional health practitioners to acquire replacement specimens to satisfy demand from customers or patients.
2.3 DNA extraction and absorbance measurements
We shaved/scraped off and discarded outside layers of tissue from the acquired samples as they were most likely exposed to contamination (through handling and contact with other specimens) before taking ~25 mg of tissue for DNA extraction. To extract this tissue's genomic DNA, we used the standard extraction protocols for animal tissue provided by the manufacturer for the NucleoSpin®Tissue Genomic DNA Tissue Kit (Macherey-Nagel).
We assessed DNA purity to evaluate whether DNA extracted from our traditional medicine samples could be used for further experimental work. To assess DNA purity, we used ultraviolet–visible spectroscopy (UV–visible spectrophotometry), where the peak absorbance of pure nucleic acid is 260 nm (Desjardins & Conklin, 2010; Koetsier & Cantor, 2019). We used the NanoDrop One Spectrophotometer (Thermo Scientific), a cost-effective preliminary DNA assessment (Ponti et al., 2018), according to the manufacturer's instructions, to measure absorbance. Blanking measurements were performed using 2 μL of the reference solution (elution buffer used during DNA extractions) to minimize this solution's contribution to the absorbance of the extracted DNA. To be able to make inferences about purity of the extracted DNA, we compared spectrophotometry results from this study's samples to typical absorbance of pure nucleic acid for DNA; a 260/280 nm (A260/280) absorbance ratio of ~1.8 (1.85–1.88) and a 260/230 nm (A260/230) absorbance ratio in the range of 1.8–2.3 (Desjardins & Conklin, 2010; Koetsier & Cantor, 2019). Samples with 260/280 nm and 260/230 nm absorbance ratios greater or equal to 1.8 are generally considered suitable for further experimental work (Koetsier & Cantor, 2019), but there are likely to be exceptions to these general guidelines for interpreting absorbance ratios.
Outliers or extreme values in the 260/280 nm absorbance ratio might be due to the presence of contaminants (protein, phenol or others) that have strong absorbance near 280 nm while minor outliers in the 260/230 nm absorbance ratio may be an indication that DNA extraction procedures require improvements (Desjardins & Conklin, 2010). Strong absorbance of contaminants such as phenol and guanidine lowers the 260/230 nm ratio of a sample by increasing absorbance below 240 nm (Page & Gomez-Curet, 2011). Negative absorbance ratios could be an indication that contaminants in the sample are emitting light. We used the interquartile rule to determine outliers, where the minor outliers in the absorbance ratios are lower than the first quartile value minus 1.5 times the interquartile range (i.e. Q1–1.5(IQR)), the major outliers are higher than the third quartile plus 1.5 times the interquartile range (i.e. Q3 + 1.5(IQR)) and interquartile range is calculated by subtracting the first quartile value from the third quartile value (i.e. IQR = Q3 – Q1).
After we measured absorbance, we used polymerase chain reaction (PCR) to amplify DNA barcode fragments for each of the 111 samples. In addition to PCR being a step towards obtaining DNA barcodes, we consider its outcomes as an indication of whether the success of this amplification (as further experimental work) conforms to absorbance ratio guidelines. We used primers RepCOI-F (5′-TNT TMT CAA CNA ACC ACA AAG A-3′) and RepCOI-R (5’-ACT TCT GGR TGK CCA AAR AAT CA-3′ (Nagy et al., 2012)) to amplify a target region of 664 bp of the COI gene. For DNA barcoding animals, the 5′ end of the mitochondrial COI gene is proposed as a universal barcode marker (Hebert, Ratnasingham, & de Waard, 2003). We performed PCR reactions in total volumes of 25 μL: 12.5 μL ready-to-use Thermo Scientific DreamTaq Green PCR Master Mix (X2) (with DreamTaq DNA Polymerase, 2X DreamTaq Green buffer, dNTPs, at 0.4 mM each and 4 mM MgCl2), 1.25 μL (10 μM) of each of the two RepCOI primers mentioned above, 3 μL of the template DNA elution and 7 μL Thermo Scientific nuclease-free water (PCR-grade). The reactions were carried out in the Applied Biosystems SimpliAmp Thermal Cycler (Thermo Fisher Scientific Inc) using the following PCR protocol: initial denaturation at 95°C for 3 min, 40 cycles of denaturation at 95°C for 30 s, annealing at 48.5°C for 30 s and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min, and subsequent storage of PCR products at 4°C. We then visualized PCR products on a 1% agarose gel under ultraviolet light on the E-BOX CX5 stand-alone gel imaging system (Vilber Lourmat Deutschland GmbH).
2.4 Sequencing protocol
We outsourced purification and sequencing of PCR products to a commercial sequencing company (Inqaba Biotechnical Industries (Pty) Ltd). They cleaned PCR products using the ExoSap Protocol: 10 μL amplified PCR product and 2.5 μL ExoSAP master mix (Exonuclease I 20 U/μL and Shrimp Alkaline Phosphatase 1 U/μL) mixed well and incubated at 37°C for 15 min then held at 80°C for 15 min. The Nimagen, BrilliantDye™ Terminator Cycle Sequencing Kit V3.1, BRD3-100/1000 was used to sequence fragments according to manufacturer's instructions. The sequencing company provided the following cycle sequencing protocol: 10 μL NEB OneTaq 2X MasterMix with standard buffer, 1 μL PCR product (10–30 ng/μL), 1 μL of forward and reverse primer each (10 μM) (using the same primers, RepCOI-F and –R, as in initial amplification), and 7 μL nuclease-free water. The sequencing PCR profile was 94°C for 5 min, 35 cycles of 94°C for 30 s, 50°C for 30 s and 68°C for 1 min, followed by 10 min at 68°C and subsequent storage at 4°C. Subsequently, they cleaned products with the ZR-96 DNA sequencing clean-up kit, ran the cleaned products on an Applied Biosystems ABI with a 50-cm array (using POP7) and used FinchTV software to analyse sequence chromatograms.
We trimmed the sequences obtained from the commercial sequencing company with the Decontamination Using Kmers (BBDuk) trimmer, paired, then assembled them using De Novo assembly on the Geneious Prime® 2022.0.2 (https://www.geneious.com/prime/) sequence analysis software (Biomatters New Zealand Ltd). Subsequently, we used BOLD identification system (IDS) for comparisons of this study's sequences with reference samples on the BOLD database (https://v3.boldsystems.org/index.php/IDS_IdentificationRequest) for verification of the sequence and species identity using neighbour-joining placement (Ratnasingham & Hebert, 2007). Using the Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1990), we carried out further comparison of this study's sequences with published sequences on the NCBI Nucleotide collection (nr/nt) database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine sequence and species identity using the MegaBlast (Zhang et al., 2000). We used a difference of 2% or less between DNA sequences as a limit for discriminating between species (Hebert, Cywinska, et al., 2003; Pereira et al., 2013). The sequences we obtained from this study were deposited in the NCBI GenBank database under the accession numbers [GenBank: OQ360757–OQ360811].
3 RESULTS
3.1 Literature review
We recorded a total of 34 herptile species (one anuran and 33 reptile species) from the previously published literature that we reviewed, with other herptiles only being identified to genus or higher taxonomic ranks from the South African urban traditional medicine markets/shops of Eastern Cape (Simelane & Kerley, 1998), Gauteng (Whiting et al., 2011) and KwaZulu-Natal (Ngwenya, 2001). Eight of those 34 herptile species reported in previous literature did not have COI reference samples available on either BOLD or NCBI GenBank databases at the drafting of this article in June 2022 (Table 1).
| Scientific name | Common name | COI reference availability | |
|---|---|---|---|
| BOLD | NCBI | ||
| Frogs | |||
| Schismaderma carens (Bufonidae)a | African Red Toad | YES | YES |
| Reptiles | |||
| Acanthocercus atricollis (Agamidae)a,b | Blue-headed Tree Agama | NO | NO |
| Acontias plumbeus (Scincidae)a | Giant Legless Skink | YES | YES |
| Bitis arietans (Viperidae)a,b,c | Puff Adder | YES | YES |
| Chamaeleo dilepis (Chamaeleonidae)a | Flapneck Chameleon | YES | YES |
| Chersina angulata (Testudinidae)a | Angulate Tortoise | YES | YES |
| Cordylus tropidosternumd (Cordylidae)a | Tropical Girdled Lizard | NO | NO |
| Cordylus vittifer (Cordylidae)a | Common Girdled Lizard | YES | NO |
| Crocodylus niloticus (Cordylidae)a,b,c | Nile Crocodile | YES | YES |
| Dendroaspis angusticeps (Elapidae)a | Green Mamba | YES | YES |
| Dendroaspis polylepis (Elapidae)a | Black Mamba | YES | YES |
| Dispholidus typus (Colubridae)a | Boomslang | NO | NO |
| Eretmochelys imbricata (Cheloniidae)a – CR | Hawksbill Sea Turtle | YES | YES |
| Gerrhosaurus flavigularis (Gerrhosauridae)a | Yellow-throated Plated Lizard | YES | NO |
| Gerrhosaurus major (Gerrhosauridae)a | Rough-scaled Plated Lizard | NO | NO |
| Hemachatus haemachatus (Elapidae)a,b | Rinkhals | YES | YES |
| Kinixys bellianad (Testudinidae)a | Bell's Hinge-back Tortoise | YES | YES |
| Kinixys natalensis (Testudinidae)b – VU | Natal Hinge-back Tortoise | YES | YES |
| Kinixys speckii (Testudinidae)a | Eastern Savanna Hinge-back Tortoise | YES | NO |
| Lamprophis aurora (Lamprophiidae)a | Aurora House Snake | NO | NO |
| Naja melanoleucad,e (Elapidae)b | Central African Forest Cobra | YES | YES |
| Naja annulifera (Elapidae)b | Snouted Cobra | YES | YES |
| Naja mossambica (Elapidae)a,b | Mozambique Spitting Cobra | YES | YES |
| Psammophis phillipsiid (Psammophiidae)a | Olive Grass Racer | YES | YES |
| Psammophylax rhombeatus (Psammophiidae)a | Rhombic Skaapsteker | YES | NO |
| Psammophylax tritaeniatus (Psammophiidae)a | Striped Skaapsteker | NO | NO |
| Pseudaspis cana (Pseudaspididae)a | Mole Snake | YES | YES |
| Python natalensis (Pythonidae)b,c | Southern African Python | YES | YES |
| Smaug giganteus (Cordylidae)a – VU | Giant Girdled Lizard | YES | YES |
| Smaug warreni (Cordylidae)a | Lebombo Girdled Lizard | YES | YES |
| Stigmochelys pardalis (Testudinidae)a,b | Leopard Tortoise | YES | NO |
| Thelotornis capensis (Colubridae)b | Vine Snake | NO | NO |
| Varanus albigularis (Varanidae)a,b,c | Rock Monitor | NO | NO |
| Varanus niloticus (Varanidae)a,b,c | Nile Monitor | YES | YES |
- Note: CR, Assessed to be Critically Endangered (IUCN, 2022). VU, Assessed to be Vulnerable (IUCN, 2022).
- a Records from Gauteng (Whiting et al., 2011).
- b Records from KwaZulu-Natal (Ngwenya, 2001).
- c Records from Eastern Cape (Simelane & Kerley, 1998).
- d Species not native to South Africa.
- e Some studies previously identified N. subfulva as N. melanoleuca using DNA barcoding (Mulcahy et al., 2022; Wüster et al., 2018), it is not possible to verify whether this was also the case with the cited literature relying solely on morphology-based identification.
From the species recorded in previous literature (Table 1), Kinixys natalensis Hewitt, 1935 and Smaug giganteus (Smith, 1844) have their conservation status as vulnerable, while Eretmochelys imbricata (Linnaeus, 1766) is critically endangered (IUCN, 2022). Furthermore, previous literature has records of four species from South Africa's urban traditional markets that are not native to the country: Cordylus tropidosternum (Cope, 1869) is endemic to Eastern African countries (Broadley & Branch, 2002), Kinixys belliana Gray, 1831 occurs in the north of the Southern African region and beyond (Turtle Taxonomy Working Group, 2021), Naja melanoleuca Hallowell, 1857 is from Central and West African countries (Wüster et al., 2018) and Psammophis phillipsii (Hallowell, 1844) occurs in West African countries (Leaché et al., 2006).
3.2 Fieldwork: Interviews, tissue sampling and visual observation
Through visual surveys of traditional medicine shops and open markets in the urban areas of three South African provinces (Gauteng, KwaZulu-Natal and Limpopo), we identified nine of the 34 species recorded in previous literature (Table 2). The traditional health practitioners we interviewed explained that their apprehension towards conservation practitioners resulted from conservation law enforcement previously confiscating specimens in their possession instead of seeking to collaborate with them to introduce measures that both adhered to environmental laws and respected Indigenous cultural practices. They further explained that such collaboration is something they were willing to consider.
| Scientific name | Common name | Identification method | |
|---|---|---|---|
| Visual | DNA | ||
| Reptiles | |||
| Acanthocercus atricollis (Agamidae)a | Blue-headed Tree Agama | Site B, D | - |
| Bitis arietans (Viperidae)a | Puff Adder | Site B, D | Site B, D |
| Chamaeleo dilepis (Chamaeleonidae)a | Flapneck Chameleon | - | Site D |
| Crocodylus niloticus (Cordylidae)a | Nile Crocodile | Site B–E | Site A |
| Dendroaspis angusticeps (Elapidae)a | Green Mamba | - | Site B, C, D |
| Hemachatus haemachatus (Elapidae)a | Rinkhals | Site B, D | Site B |
| Naja subfulvab,c (Elapidae)a | Central African Forest Cobra | - | Site D |
| Naja annulifera (Elapidae)a | Snouted Cobra | - | Site B |
| Naja mossambica (Elapidae)a | Mozambique Spitting Cobra | - | Site B, D |
| Philothamnus semivariegatus | Spotted Green Snake | - | Site B |
| Psammophis sp. (Psammophiidae) | African Green Snakes | Site B, D | Site B |
| Pseudaspis cana (Pseudaspididae)a | Mole Snake | Site D | Site B |
| Python natalensis (Pythonidae)a | Southern African Python | Site A–F | Site B |
| Stigmochelys pardalis (Testudinidae)a | Leopard Tortoise | Site B, D, F | - |
| Varanus albigularis (Varanidae)a | Rock Monitor | Site B, C, D, F | Site B, D |
| Varanus niloticus (Varanidae)a | Nile Monitor | Site B, C, D, F | Site B, C, D |
- a Recorded in previous literature.
- b Species not native to South Africa.
- c Naja subfulva was previously identified as N. melanoleuca using DNA barcoding (Mulcahy et al., 2022; Wüster et al., 2018). Sites: A = Pretoria Muthi Shop, Gauteng, province. B = Faraday Muthi Market, Gauteng, province. C = Pietermaritzburg Muthi Shop, KwaZulu-Natal province. D = Warwick Muthi Market, KwaZulu-Natal province. E = Ga-Mokekolwana, Limpopo province. F = Kwa Mai Mai Traditional Market, Gauteng, province.
Traditional health practitioners reported obtaining the herptile specimens they use or sell by either hunting the animals themselves, buying from hunters that regularly go on hunts to supply multiple traditional health practitioners, or taking roadkill and animals that died of natural causes. Practitioners specifically target species that they require at the time of their hunts, while the hunters take orders for specific animals from multiple traditional medicine practitioners and will also opportunistically hunt other species they encounter when hunting to fulfil their list of orders. Traditional health practitioners and the hunters that supply them with herptile species employ the same tissue preservation methods for all animals (not just herptiles) and the specimens are either preserved at home or at the open market. They remove visible body fat and internal organs. The body fat has traditional medicine value and is stored in bottles, while the internal organs are usually discarded. Following removal of fat and internal organs, the carcasses are smothered with ash and/or salt, placed in the sun to dehydrate them and subsequently placed on display at markets/shops once dry.
Dried carcasses of herptiles and other animals are displayed together for customers. According to the traditional health practitioners, customers usually buy body parts or small pieces (relative to an animal's size), and it is uncommon for someone to buy an entire carcass. The pricing for each piece of animal that a customer wants to buy was noted to be uniform among this study's participants with the exception being Pseudaspis cana (Linnaeus, 1758) which was priced at 40% higher than the rest of the herptiles being sold by those participants. The reason provided for this pricing difference was because the participants believed that P. cana preyed on other snake species. Due to how they are sold, herptile specimens were sometimes found missing body parts, and only bones with muscle and skin remained. Carcasses displayed in open traditional medicine markets (in Durban and Johannesburg) were either removed at the end of each business day to be stored together overnight in plastic containers or they were left on the stalls and covered with plastic sheets. All storage of specimens is at ambient temperature; there is no refrigeration.
3.3 DNA barcoding of traditional medicine market samples
The absorbance measurements of the extracted DNA suggest that the traditional health practitioners' preservation of herptiles using salt and/or ash can preserve DNA for molecular identification. Based on the 260/280 nm absorbance ratio, 23 of the 111 (20.7%) extracted DNA samples would not be suitable for further experimental work, while the 260/230 nm ratio suggested that 33 of the 111 (29.7%) samples would not be suitable for further experiments due to their absorbance ratios being either negative or outlier values (Figure 2).
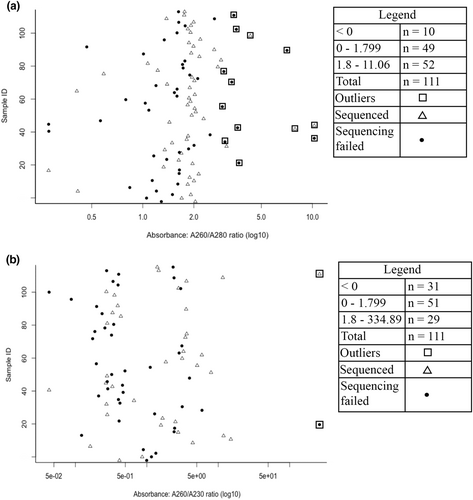
The subsequent amplification and sequencing outcomes were partially in line with the interpretation of absorbance ratios as amplification of DNA fragments was successful from 90 of 111 DNA extracts (based on the PCR products visualized using gel electrophoresis), compared to an estimated success rate of 88 of 111 extracts based on the 260/280 nm absorbance ratio and a success rate of 78 of 111 extracts based on the 260/230 nm ratio. There was, however, deviation from the absorbance ratio guidelines as 25 of the extracted DNA samples with negative or outlier absorbance ratios were successfully amplified and sequenced yet absorbance interpretations suggest that such samples would not be suitable for further experimental work (Table 3). A further 16 extractions with absorbance ratios interpreted to be suitable for further experimental work were not successfully amplified or sequenced. Furthermore, nucleic acid concentration measurements from NanoDrop considered in combination with absorbance did not provide a reliable indicator for whether extracted DNA would be suitable for further experimentation. For example, sample J29 (A260/A280 = 2.049, A260/A230 = 0.355, nucleic acid concentration = −4.970 ng/μL) was successfully sequenced while J28 (A260/A280 = 1.950, A260/A230 = 0.377, nucleic acid concentration = −5.151 ng/μL) could not be successfully sequenced (Data set S1). The succesfully amplified DNA fragments from 81% (90 of 111) of the DNA extracts (based on the PCR products visualized using gel electrophoresis) were subsequently sent out for sequencing. We obtained DNA sequences from 81 samples (73% of total samples), for the remaining 9 of 90 amplicons the sequencing reactions failed. From the 81 DNA sequences, 39 matched with species on the BOLD database with 99.13%–100% similarity (Table 3). An additional three sequences had species-level matches with 99.0% pairwise similarity on the NCBI GenBank database (Ng & Tay, 2004), with an e-value of zero suggesting that there is no better match besides that current result (Metzler, 2006). Lists of nearest matches rather than exact species matches were returned for 12 sequences on the BOLD database with 98.12%–99.09% similarity and one sequence with 98.9% similarity on the NCBI GenBank database (Table 3). Observed morphological traits could be used to supplement identification of these 13 sequences with lists of nearest species matches. A 93.8% similarity to P. phillipsii which is not native to South Africa was returned as the highest match for one of this study's DNA sequences on the NCBI GenBank database. This match to a non-native species was likely due to the sample being obtained from a related native species and supplementing this match with observed morphological traits confirms genus level (Psammophis sp.) identification (Table 2).
| No. | IsiZulu names provided by participants | Molecular identification (% match) | Absorbance | |
|---|---|---|---|---|
| 260/280 nm | 260/230 nm | |||
| J01 | Imfezi (Naja mossambica) | Naja mossambica (100%) | 1.76 | 6.58 |
| J03 | Imamba (Dendroaspis sp.) | Hemachatus haemachatus (98.24%) | 1.90 | 3.56 |
| J04 | Inyoka (Serpentes sp.) | Pseudaspis cana (100%) | 0.38 | −0.08 |
| J05a | Inyoka (Serpentes sp.) | Python natalensis (100%) | 1.77 | −2.31 |
| J06a | Uxam/Imbulu (Varanus sp.) | Procavia capensisb (98.12%) | 1.30 | −0.6 |
| J07 | Imamba eluhlaza (Dendroaspis angusticeps) | Philothamnus semivariegatus (99.48%) | 1.89 | 3.8 |
| J09 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (99.04%) | 1.83 | 3.96 |
| J10 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (99.13%) | 0.55 | −0.07 |
| J15a | Imamba eluhlaza (Dendroaspis angusticeps) | Psammophis sp. (93.8%) | 1.14 | −0.23 |
| J17a | Uxam/Imbulu (Varanus sp.) | Ictonyx striatusb (99.2%) | 2.61 | 0.28 |
| J18 | Isibankwa (Scincidae sp.) | Varanus albigularis (99.09%) | 1 | −0.48 |
| J20 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 2.72 | 0.44 |
| J21 | Imfezi (Naja mossambica) | Naja annulifera (99.8%) | 2.03 | 0.33 |
| J23 | Ibululu (Bitis arietans) | Bitis arietans (99.83%) | 1.81 | 3.89 |
| J24a | Inyoka (Serpentes sp.) | Python natalensis (100%) | 2.48 | 0.4 |
| J25 | Uxam/Imbulu (Varanus sp.) | Varanus albigularis (98.38%) | 1.87 | −1.06 |
| J26 | Uxam/Imbulu (Varanus sp.) | Varanus albigularis (99.03%) | 1.75 | −2.07 |
| J29 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 2.05 | 0.36 |
| J30 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 4.07 | 0.27 |
| J31 | Imfezi (Naja mossambica) | Naja annulifera (99.79%) | 1.79 | 3.02 |
| J32 | Imfezi (Naja mossambica) | Naja annulifera (99.79%) | 1.73 | −9.71 |
| D01 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 1.92 | 0.41 |
| D02 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 1.59 | 0.9 |
| D03 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 1.80 | −2.21 |
| D04 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 0.38 | −0.14 |
| D13 | Imfezi (Naja mossambica) | Naja subfulvac (100%) | −7.16 | 0.16 |
| D16 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (98.9%) | 1.73 | 4.85 |
| D20 | Ibululu (Bitis arietans) | Bitis arietans (100%) | 1.89 | 17.33 |
| D22 | Unwabu (Chamaeleonidae sp.) | Chamaeleo dilepis (99.65%) | 1.78 | 13.58 |
| D23 | Unwabu (Chamaeleonidae sp.) | Chamaeleo dilepis (99.65%) | 2.01 | 2.98 |
| D24 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 0.26 | −0.08 |
| D25 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 1.23 | 1.67 |
| D26 | Imfezi (Naja mossambica) | Naja subfulvac (100%) | 1.84 | 2.72 |
| D33 | Ibululu (Bitis arietans) | Bitis arietans (99.82%) | 1.53 | 1.77 |
| D39 | Unwabu (Chamaeleonidae sp.) | Chamaeleo dilepis (99.65%) | −4.29 | 0.28 |
| D41 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (98.94%) | 1.58 | −11.29 |
| D42 | Imfezi (Naja mossambica) | Naja mossambica (99.79%) | 1.26 | −11.25 |
| D44 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 2.94 | 0.27 |
| D46 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (98.76%) | 1.39 | 0.68 |
| D47 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (98.93%) | 1.70 | −4.89 |
| D48 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (99%) | 1.73 | −1.91 |
| D49 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (99%) | −0.79 | 0.04 |
| D50 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (98.94%) | 7.51 | 0.33 |
| D51 | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (98.85%) | 9.78 | 0.27 |
| D52 | Uxam/Imbulu (Varanus sp.) | Varanus albigularis (99.28%) | 1.34 | −0.17 |
| D53 | Uxam/Imbulu (Varanus sp.) | Varanus albigularis (99.28%) | 2.13 | 0.28 |
| D54 | Uxam/Imbulu (Varanus sp.) | Varanus albigularis (99.28%) | 1.82 | 8.94 |
| D55 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 1.93 | −5.86 |
| D56 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 1.9 | 5.62 |
| D57 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 1.72 | 1.98 |
| D58 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 1.79 | 3.40 |
| P01a | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (98.7%) | 1.80 | 3.93 |
| P02a | Uxam/Imbulu (Varanus sp.) | Varanus niloticus (99%) | 1.87 | 13.25 |
| P06 | Imamba eluhlaza (Dendroaspis angusticeps) | Dendroaspis angusticeps (100%) | 1.29 | 334.9 |
| T01a | Uxam/Imbulu (Varanus sp.) | Crocodylus niloticus (100%) | 1.64 | 1.56 |
| T02a | Uxam/Imbulu (Varanus sp.) | Crocodylus niloticus (100%) | 1.65 | 1.48 |
- Note: Grey highlight indicates mislabelling of specimens by traditional health practitioners. 260/280 nm and 260/230 nm absorbance ratios ≥1.8 generally considered indicative of samples that are suitable for further experimentation (Koetsier & Cantor, 2019).
- a Piece of bone and muscle tissue sold by traditional health practitioners that was difficult to identify based on morphology.
- b Not a herptile species.
- c Species previously identified as N. melanoleuca (Mulcahy et al., 2022; Wüster et al., 2018).
Using molecular identification, supplemented with observed morphological traits, 56 tissue samples collected during this study were matched to one genus and 11 species of reptiles that had already been recorded in earlier literature and two additional reptile species that were not recorded in literature (Table 2). Twenty-four of the 26 remaining sequences had no matches on the BOLD database, but it could be ascertained that they were DNA fragments of reptiles by comparing them to their NCBI GenBank reference sequence matches of reptiles with a similarity of 81.2%–86.9%, using 70% similarity to reference sequences as a threshold below which the results would not be meaningful (Baxevanis et al., 2020). Two of the 26 remaining sequences matched with reference sequences from mammal species: Ictonyx striatus (Perry, 1810) with 99.2% similarity on the NCBI GenBank database and Procavia capensis (Pallas, 1766) with 98.14% similarity on the BOLD database. These mammalian tissues were obtained from pieces of bone and muscle that a traditional health practitioner mislabelled as either uxam or imbulu, IsiZulu names for Varanus spp. (Table 3).
The mislabelled mammal tissue and others (e.g. N. subfulva mislabelled as Naja mossambica Peters, 1854, imfezi in IsiZulu) were among 13 (23%) mislabelled samples from 56 that were identified using DNA barcoding (Table 3). In addition to detecting mislabelling, molecular identification verified IsiZulu names that traditional health practitioners assigned to 43 specimens (Table 3). Some IsiZulu names for the specimens were specific (e.g. Dendroaspis angusticeps (Smith, 1849), imamba eluhlaza in IsiZulu) while other Indigenous names were only accurate to higher taxonomic ranks, for example, specimens named as unwabu (IsiZulu word for members of Chamaeleonidae) were later confirmed to be Chamaeleo dilepis with molecular identification. Further examples of DNA barcoding as a tool for verification of folk taxonomy include specimens broadly labelled as snakes and monitor lizards in IsiZulu (inyoka and uxam/imbulu, respectively) being confirmed up to species level by DNA barcoding as P. cana and Varanus niloticus (Linnaeus, 1766) respectively (Table 3).
4 DISCUSSION
In this study, we aimed to combine visual surveys, literature reviews, DNA barcoding and interviews of traditional health practitioners in documenting and updating the knowledge of herptile use in South Africa's urban traditional medicine markets. This study further provided insights into Indigenous methods for animal tissue preservation and the willingness of some traditional health practitioners to collaborate in conservation initiatives aimed at traditional medicine markets.
4.1 DNA barcoding Indigenous medicine specimens
We observed that animal tissue preservation methods used by IsiZulu speaking traditional health practitioners can preserve DNA for molecular identification. The practitioners' storage and display of multiple species in one place and handling of specimens by multiple customers could lead to DNA contamination. Storage of specimens at ambient temperatures also introduces risk of DNA degradation. Daily temperature and humidity fluctuations of ambient temperature storage can cause degradation of DNA (Asari et al., 2018).
The spectrophotometry we used to measure the absorbance of DNA samples extracted from herptile specimens gave indications of the purity of the extracted DNA in comparison with other coextracted products (DNA vs. other molecules), but not in terms of exogenous DNA contamination (endogenous DNA vs. DNA contamination). These absorbance measurements were sometimes inconclusive about suitability of extracted DNA samples for further experimental work. Some extractions that were expected to be unsuitable for further experimental work, based on their absorbance ratios, provided useful results in further experiments (i.e. amplification was successful and DNA sequences were obtained from them). Additionally, some extractions with absorbance ratios close to that of pure nucleic acid (1.8) were expected to be suitable for further experimental work but they failed in subsequent reactions.
Some of these failures to amplify and sequence extracted DNA could be explained by DNA degradation. Indeed, DNA can be extracted in relatively good quantity and purity but may exclusively consist in fragments that are shorter than the marker targeted by the PCR. Furthermore, a chemical contaminant introduced during extraction such as guanidine hydrochloride in a sample will have similar absorbance to pure DNA in the 260 nm and 280 nm range (Page & Gomez-Curet, 2011), thus giving the false impression that a sample is suitable for further analysis. Some chemical contaminants introduced during DNA extraction act as PCR inhibitors (Page & Gomez-Curet, 2011). Using spectrophotometry as a cost-effective method to obtain an indication of DNA amplification success was sometimes unreliable, potentially due to contamination. In future projects, Qubit fluorometers (Life Technologies) could be used as an alternative for cost-effective DNA quantification (Ponti et al., 2018). Another possible solution to the shortfalls of using spectrophotometry in future research could be to run part of the DNA extract on an agarose gel and check its degradation.
From this study's 81 successfully sequenced samples, 26 could not be identified to species level due to the absence of reference sequences on the BOLD and NCBI GenBank databases. A phylogenetic analysis could be used to understand the taxonomic position of the unidentified DNA sequences thus providing an indication of where they belong in the phylogeny of South African reptiles. Continuation of this research that brings DNA barcoding into studies of herptiles in South Africa's urban traditional medicine markets should also consider obtaining species-level identifications by barcoding the 12S, 16S, ND1, ND2, ND4 or cytochrome b genes which have previously been used in molecular identification of reptiles by Vences et al. (2012). Of the 418 reptile species known to be distributed in South Africa (Uetz et al., 2022), only 86 had COI reference sequences on the NCBI GenBank and/or BOLD databases at the time of drafting this text (in June 2022). The results further showed that from the 34 herptile species recorded in previous literature as being offered in the South African urban traditional medicine circuit, eight did not have COI reference sequences on either NCBI GenBank or BOLD databases (Table 1).
Another possible solution to this study's shortcomings with amplification and sequencing is to use metabarcoding for the multiple samples collected. Metabarcoding with high-throughput sequencing can amplify, sequence and identify multiple samples simultaneously (Ficetola et al., 2019). As traditional health practitioners have shown willingness to collaborate, it would be possible to re-survey traditional medicine markets to obtain a larger sample size of herptile tissue and use metabarcoding for identification. Given that our study only included one participant from the estimated 220–300 practitioners at the Faraday Muthi Market (Williams & Whiting, 2016), there is opportunity for increased sampling of herptile specimens, provided participation from more traditional health practitioners.
4.2 DNA barcoding compared to morphology-based identification
We used DNA barcoding to confirm 11 species and one genus of reptiles from the 33 reptile species reported in previous literature which did not use molecular identification. Morphology-based identifications have a supplementary role in this study as we were not given consent to photograph specimens and publish those photographs as an additional data set. Previous literature provided morphology-based identification of one anuran species from traditional medicine markets whereas no anurans were found during this study. Philothamnus semivariegatus (Smith, 1840) was identified in this study using DNA barcoding (Table 2), and Whiting et al. (2011) previously recorded a genus-level identification (Philothamnus sp.) of a morphologically similar species. Inconsistencies that exist between molecular identifications and those based on morphology show a need for additional barcoding studies of Indigenous medicine specimens (Veldman et al., 2020). Species richness of traditional medicine markets is likely underestimated due to difficulties with morphology-based identification when distinctive features of specimens are not sufficiently preserved (Whiting et al., 2011). Identification based on morphology is valuable for fast identification of threatened species with visible distinctive traits in traditional medicine markets before tissue samples are obtained for molecular identification. Additionally, morphology-based identification can be relied upon on when traditional health practitioners do not provide consent for collection of tissue from specimens in their possession as was the case in this study (Table 2).
The mislabelling or confusion of culturally important animal tissue based on morphology shown in this study (Table 3) has also been recorded in other studies (Gombeer et al., 2021). Mislabelling could be intentional when practitioners substitute specimens to meet customer expectations (Bitanyi et al., 2012). However, cryptic morphology of some species can result in confusion. Tissue might be deliberately mislabelled so the practitioners can charge higher prices for them, but such intentional substitution was not detected in this study as there was no price difference between mislabelled and correctly labelled specimens. Hunters who also rely on morphology-based identification may have mislabelled the animals they sold, and practitioners subsequently used those names supplied with the specimens they bought. Substitution or confusion of morphologically identified species in Indigenous remedies is said to pose human health risks when toxic plants are the substitute species (Ouarghidi et al., 2012). No acute health issues have been associated with ingestion of herptile tissue (Anthony & Bellinger, 2007; Du Preez & Cook, 2004; Ngwenya, 2001); thus, no immediate health concerns from substitution of herptile tissue are apparent. There is, however, zoonotic risk associated with ingestion of herptile tissue and it is worth investigating the magnitude of this risk for increased understanding of the human health effects of using animals in traditional medicine. Some of the possible zoonotic infections include Salmonella spp. associated with eating crocodile (Crocodylus spp.) meat and a chance of zoonotic parasitic disease caused by nematode species belonging to Gnathostoma in the undercooked flesh of frogs and reptiles (Magnino et al., 2009). Furthermore, people can be accidental hosts of some herptile parasites (Pantchev & Tappe., 2011), and herptiles are reservoirs of zoonotic parasites which may be considered a public health concern (Mendoza-Roldan et al., 2020).
4.3 Conservation issues
South Africa's urban traditional medicine markets rely more on reptile species than frog species (34 reptile species vs. 1 frog species were jointly recorded by this and other studies). This trend of greater dependence on reptiles than amphibians for Indigenous remedies is global (Alves et al., 2013). The underestimation of species richness at some of South Africa's urban traditional medicine markets highlighted by Whiting et al. (2011) makes it difficult to estimate the proportion of herptile species that are considered to have traditional medicine value and the underestimation of endangered species in particular could lead to the traditional medicine markets' conservation impacts being misevaluated. It has previously been difficult to estimate the number of individuals per species harvested for traditional medicine markets and their impact on wildlife populations as traditional health practitioners were reluctant to talk about their practices (Whiting et al., 2011). There is hope for lessening this reluctance as practitioners that participated in this study expressed willingness to collaborate with researchers or conservation practitioners. With such collaboration, the species at traditional medicine markets can be comprehensively documented and identified using molecular and morphology-based identifications.
A collaborative approach to managing conservation issues arising from traditional medicine markets would not only be just but it is also legally required. South Africa's overarching environmental management legislation is supportive of collaborative conservation planning as it states that decisions relating to the natural environment must account for the interest, needs and values of interested parties and recognize all forms of knowledge including Indigenous knowledge (Republic of South Africa, 1998). Collaboration will of course require additional research resources as efforts to find synergies between Indigenous cultures and modern practices will require extra field days and additional ethics approvals to protect Indigenous knowledge and its custodians from exploitation. Within the context of South African environmental legislation, Indigenous medicinal uses of wildlife (as a form of Indigenous knowledge) should not be dismissed by conservation practitioners. This environmental legislation further states that management of the environment should equitably provide for people's needs and their cultural interests (Republic of South Africa, 1998). Functional collaborations with traditional health practitioners have been demonstrated by modern health professionals both in South Africa (Nkhwashu et al., 2021) and other parts of the African continent (Kayombo et al., 2007) despite disagreement between the two parties, thus providing hope that collaborations with conservation practitioners are achievable. With collaboration, the traditional health practitioners can be encouraged to openly substitute endangered species with abundant species of lower conservation priority thus reducing negative impacts of Indigenous remedies. The manner in which animals are bought for Indigenous remedies can be considered to limit negative conservation impacts (albeit unwittingly); pieces of animal tissue are bought, rather than the entire carcass, thus allowing an individual animal to be used by multiple people instead of one individual animal being used by only one person.
Traditional health practitioners would make suitable conservation ambassadors due to the respect they have from people that follow Indigenous cultural practices (Simelane & Kerley, 1998), and these practitioners' choices influence which species are collected for traditional medicine markets through their hunts or outsourcing to dedicated hunters. Another prospect for lessening conservation pressure on traditional medicine markets, but not necessarily their accumulation of endangered species, is that traditional health practitioners are willing to take animals that died due to accidental or natural causes (Whiting et al., 2011). There is perhaps opportunity for collaboration between traditional health practitioners with initiatives that monitor and collect roadkill on busy roads to lessen the hunting pressure on species used in Indigenous remedies.
Studies of this nature that focus on the urban areas of developing countries can contribute to increased understanding of urban sustainability in such countries and can also contribute to policymaking when such research is published in high visibility journals (Nagendra et al., 2018). This study represents the first attempt in South Africa to combine DNA barcoding, morphology-based identifications and folk taxonomy to comprehensively identify herptile species at urban traditional medicine markets. This study transcends disciplines by combining Indigenous knowledge with DNA barcoding and social science methodology for outcomes that can be used for socially inclusive conservation planning. The wide applicability of the mixed-methods approach employed here is demonstrated by Gombeer et al. (2021) using site visits, molecular identification and focus group discussions to identify illegally imported wildlife meat (also referred to as bushmeat) from West African countries to Belgium and highlight prevalence of Indigenous cultural practices in the urban centre of a developed country. Incorporating molecular identification in the introduction of collaborative monitoring of traditional medicine markets is likely to improve understanding of their species richness and prevent over-exploitation of herptile species while being considerate of Indigenous practices that make use of animals. A collaborative and mixed-methods approach is also necessary because in its absence, the use of herptiles (and other animals) has continued unmonitored while Indigenous practices and their custodians have continually been excluded from conservation planning.
AUTHOR CONTRIBUTIONS
Fortunate M. Phaka carried out fieldwork, conducted laboratory analysis and wrote the initial draft of the manuscript; Fortunate M. Phaka, Louis H. du Preez, Maarten P. M. Vanhove, Jean Hugé designed the project, acquired funding and revised the initial manuscript; Edward Netherlands, Maarten Van Steenberge provided training and supervision for laboratory analysis, revised the initial manuscript; Erik Verheyen and Gontran Sonet contributed to the project design and revised the initial manuscript.
ACKNOWLEDGEMENTS
The Traditional Healers Organisation of South Africa and the traditional health practitioners who are members of this organization are thanked for their participation in this study and allowing access to their specimens. This research is made possible by a bilateral scientific cooperation between North-West University and Hasselt University. Financial support for F Phaka was provided by the National Research Foundation (UID: 114663; 130501), South African Institute of Aquatic Biodiversity, Youth 4 African Wildlife NPC and the Flemish Interuniversity Council (VLIR) Global Minds program (Contract Number: R-9363). M Vanhove is supported by the Special Research Fund of Hasselt University (BOF20TT06) and by Research Foundation–Flanders (FWO-Vlaanderen) research grant 1513419N.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The Traditional Healers Organisation of South Africa agreed to its members being approached for participation in this research project. Informed consent according to North-West University's Health Research Ethic Committee's guidelines was obtained from the participants of this study after the first author explained the purpose of this research and that they could revoke their consent to participate at any point. Participants also consented to the results of their participation being published in a thesis and scientific articles, but they did not consent to their names being made public. Explanation of this study prior to obtaining consent was in either SePedi or IsiZulu which were languages preferred by the participants. The scope of human participation in this study was approved by North-West University Biodiversity and Ecology Research Ethics Committee. Ethics approval for this bilateral study between North-West University and Hasselt University was obtained from the North-West University Animal Care, Health and Safety Research Ethics Committee (Ethics number: NWU-00185-18-S5) and Hasselt University Social-Societal Ethics Committee (Reference: REC/SMEC/VRAI/189/127). The research conducted complies with the Nagoya Protocol on Access and Benefit-sharing (UID: ABSCH-IRCC-ZA-257320-1).
BENEFIT SHARING
Benefits Generated: The research conducted provides a first step to collaborative conservation planning for use of herptiles in traditional medicine contrary to current approaches that scarcely consider that South African legislation allows the country's citizens to utilize its natural resources within the confines of laws that provide for Indigenous knowledge systems to be considered in conservation planning.
Benefits Generated: This research provides details about the practices of traditional health practitioners and also their willingness for collaboration in managing the use of herptiles in traditional medicine. Conservation practitioners can utilize these details to make their planning more integrative.
Open Research
DATA AVAILABILITY STATEMENT
Sequence data are publicly accessible on the NCBI GenBank database under the accession numbers [OQ360757-OQ360811]. Other data collected during sampling are available within the article and its supplementary materials.




