Contrast-enhanced mammography improves patient access to functional breast imaging
DB Taylor: MBBS, FRANZCR, FRCPC; MA Kessell BSc, MSc; PM Parizel MD, PhD, FRANZCR.
Summary
Imaging research pathways focus increasingly on the development of individualised approaches to breast cancer detection, diagnosis and management. Detection of breast cancer with X-ray mammography may fail in some cancer subtypes with limited changes in morphology/tissue density and in women with dense breasts. International organisations offer recommendations for contrast-enhanced breast imaging, as it provides superior sensitivity for screening, local staging and assessment of neoadjuvant treatment response, when compared with standard X-ray mammography (including tomosynthesis) and breast ultrasound. Arguably, the evidence base is stronger for contrast-enhanced MRI (CE-MRI). Unfortunately, patient access to breast MRI in rural and remote areas is limited by practical limitations and equipment licensing restrictions. Moreover, breast MRI is an expensive test, likely to be out of reach for many women. Contrast-enhanced mammography (CEM) offers an attractive alternative to improve patient access to functional breast imaging. It is a new type of digital, dual energy X-ray mammography that can be performed on most modern units, following a relatively inexpensive hard- and software upgrade. In this paper, we review the rapidly accumulating evidence that CEM can provide similar diagnostic accuracy to CE-MRI, though at a significantly lower cost and offering greater comfort to the patient. The adoption of CEM can help meet the anticipated increased demand for CE-MRI.
Introduction
For more than 100 years, X-ray mammography has been used as a technique for the detection of breast cancer. Continuous technical improvements (e.g. the development of digital detectors or computer-aided detection (CAD) systems) have transformed mammography to a hi-tech modality capable of detecting breast cancer with unprecedented accuracy. Notwithstanding these innovations, detection of breast cancer with X-ray mammography (including tomosynthesis), relies on demonstrating abnormalities in tissue morphology or tissue density. Such changes may be minimal in some cancer subtypes (e.g. invasive lobular carcinoma) or can be masked by dense breast tissue.
Imaging methods that add functional information (such as breast MRI) improve cancer detection for all breast densities, and particularly for women with dense breasts. Research trials using contrast-enhanced MRI (CE-MRI) of the breast as a screening method in women with extremely dense breasts have provided compelling evidence that MRI enables an important reduction in breast cancer mortality for these women.1, 2 This has prompted the European Society of Breast Imaging (EUSOBI) to issue a statement recommending that women should be informed of their breast density, and that those with extremely dense breasts should be offered breast MRI every 3–4 years.3 Recent Australian studies have indicated that 8.3–12.2% of women have breasts that are almost entirely composed of dense tissue (BI-RADS D); in 2023, the Royal Australian and New Zealand College of Radiologists (RANZCR) has issued a statement recommending women to be informed of their breast density.4
The demand for breast MRI in women at increased risk for breast cancer has increased considerably and is anticipated to increase further in coming years. Given the limited access to breast MRI, particularly for women living in rural and remote areas, and considering the well-known limitations of MRI such as contra-indications (e.g. pacemakers, renal failure) and patient intolerance (mainly claustrophobia), the use of alternative functional imaging techniques that may have similar efficacy to MRI warrants consideration.
Like breast MRI, contrast-enhanced mammography (CEM) can provide functional information (by demonstrating areas of neo-angiogenesis) and identifying subtle changes in tissue morphology. Moreover, CEM delivers a spatial resolution approximately 10 times that of MRI5 and, unlike breast MRI, it can also demonstrate microcalcifications. CEM can be performed on most modern mammography units, following relatively minor hardware and software modifications. Unlike MRI, minimal additional training is needed for interpretation.6, 7 The purpose of this paper is to offer a comprehensive review of the current and future place of CEM in the detection, staging and follow-up of breast cancer.
Technique
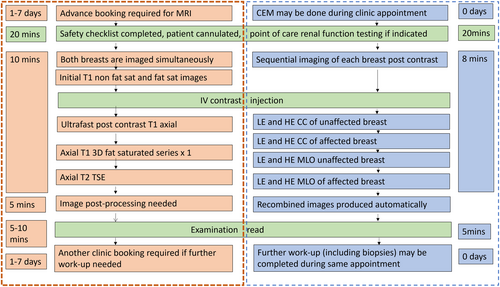
After excluding contraindications (see below) and obtaining informed consent, the patient is given an intravenous (IV) injection of a non-ionic, iodine-based contrast agent (1.5 mL per kg body weight of 350 mg iodine per mL, to a maximum of 125 mL, injected at 3 mL per second), using a power injector, followed by 30 mL of normal saline to flush.
Two minutes later, the breast is compressed, and bilateral craniocaudal (CC) and mediolateral oblique (MLO) views are obtained using dual energy X-ray exposures, below (‘low energy’ (LE) image) and above (‘high energy’ (HE) image) the K-edge of the iodine energy spectrum (33.2 keV),8 Figure 1. There is no agreed standard protocol regarding the order in which the views should be acquired, and there is no evidence to suggest that this affects diagnostic utility.
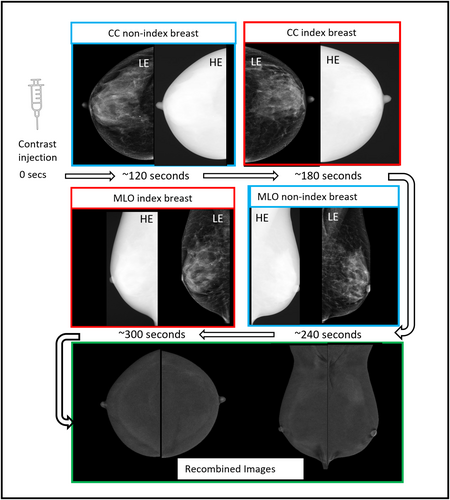
The LE images are typically acquired in the X-ray energy range 26–31 kVp; they are technically and diagnostically equivalent to a standard mammogram,9 with some previous studies suggesting cancer conspicuity could even be higher.10, 11 Volumetric breast density measurements obtained from LE images do not differ significantly from those obtained from standard mammograms.12 The HE images are obtained with X-ray beam energy of 45–49 kVp, well above the K-edge of iodine. They are not individually useful for diagnosis but are used by vendor specific software to produce ‘recombined’ (RC) images, in which background noise is suppressed, and areas of iodine uptake highlighted (Fig. 1). Additional mammographic views can be obtained if needed, all images are usually acquired within 8 min of the injection.
Clinical indications
Clinical indications for CEM are like those for breast MRI (Fig. 2); however, the volume of supportive or mature literature data available for CEM is considerably less. Available data support the use of CEM as an alternative to MRI whenever MRI access and availability are limited, or for patients who have contraindications to MRI or who are unable to tolerate it. The latter is a significant issue, since only 58–59% of women eligible for MRI screening choose to participate.13 Published studies of patients who have undergone both CEM and MRI indicate that, given a choice between having CEM or MRI, most women prefer CEM.13-16
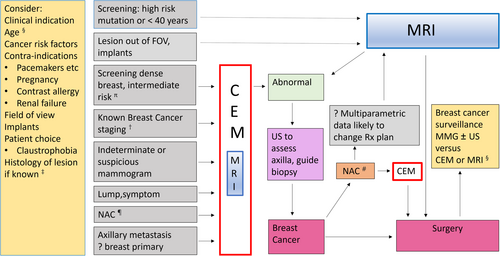
Problem solving
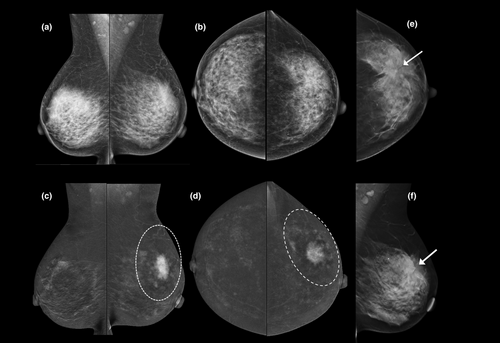
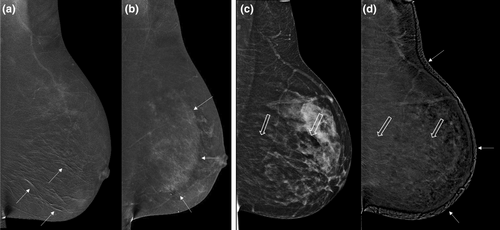
- First-line imaging of symptomatic patients >35 years with a clinically suspicious abnormality (e.g. a palpable lump), instead of standard mammography (adding the latter is unnecessary and only adds radiation dose); for example, see Figure 3.
-
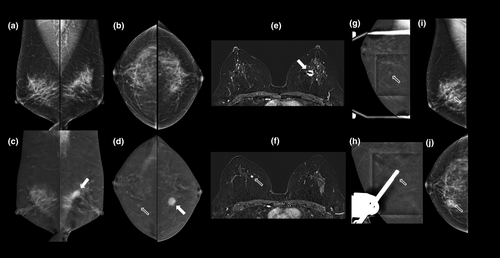
Second-line imaging (instead of standard mammography) for symptomatic patients <35 years with a suspicious abnormality on ultrasound; for example, see Figure 4.
Not only does CEM provide equivalent morphological information to standard X-ray mammography (including microcalcifications), but in addition CEM highlights areas of neo-angiogenesis associated with malignancy (Figs 3-6). The high sensitivity and negative predictive value of CEM facilitate the targeted use of ultrasound to guide biopsy and evaluate the axilla.17, 18 The use of CEM in this context may shorten the patient's diagnostic journey (Fig. 7).
-
Further evaluation of women recalled following mammographic screening.
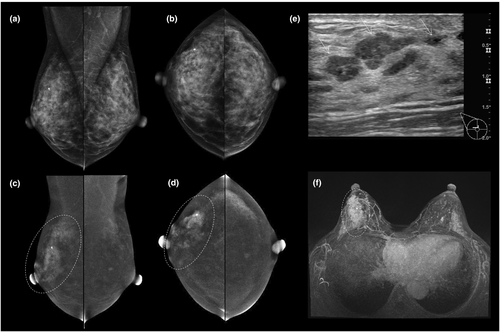
Initial small retrospective studies6, 19 comparing the use of CEM with conventional assessment (further mammography and/or ultrasound) found that CEM gave a higher specificity and positive predictive value, and that a negative CEM virtually excluded breast cancer. In a recent randomised clinical trial,20 the pathways had comparable diagnostic performance; however, using CEM as the primary assessment tool was more efficient (less additional imaging and fewer needle biopsies) and detected more of the otherwise ‘occult’ breast cancers (those not screen-detected with FFDM). For examples, see Figures 5 and 6.
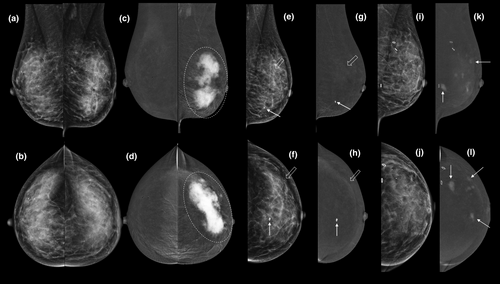
Local staging for patients in whom breast MRI is desirable but contra-indicated (e.g. pacemaker) or unavailable
CEM is more accurate than mammography, tomosynthesis and ultrasound for predicting index lesion size and the local extent of disease (Figs 3-6). Although CEM does not appear to be quite as sensitive as CE-MRI for detection of additional malignant lesions,21, 22 its use in the breast clinic at the time of initial presentation can shorten the patient's diagnostic pathway (Fig. 7), particularly when MRI is not available or contra-indicated.23
Assessing response to neoadjuvant chemotherapy
Early data suggest that CEM has comparable performance metrics to MRI for the prediction of pathological complete response23 (see Fig. 3). Unlike MRI, CEM also demonstrates the extent of any residual microcalcifications that are usually included in planned surgical resection.24
Surveillance following breast cancer treatment
Post-treatment changes make cancer detection on standard mammography difficult (Fig. 3). CEM offers both a higher sensitivity (15.4–29.4 per 1000) and a higher positive predictive value (42.9–47.9%) compared with standard mammography (6.2%–15.1/1000).25, 26 Higher cancer detection rates persist on subsequent surveillance episodes and interval cancer rates are well below those reported for mammographic surveillance.27 Reported recall rates (4.5–10.3%)25-27 are similar to the BreastScreen Australia National Accreditation Standards.28
Screening women at increased risk for breast cancer
Because CEM involves exposure to ionising radiation, MRI remains the screening technique of choice, particularly for women at high risk, that is, women with a faulty DNA-repair gene (e.g. BRCA 1 and 2, ATM, CHEK2, NF1, TP53, PALB2). However, for those women who have MRI contraindications or who are unable to tolerate MRI, CEM can provide a valid alternative. CEM may also be considered for patients whose increased risk relates to a history of thoracic radiotherapy as they have a higher incidence of calcified DCIS.29
Women at intermediate risk (including those with BI-RADS D breast density) are currently ineligible for Medicare-rebatable MRI. Following publication of the results of the DENSE and ECOG-ACRIN 1141 trials,1, 30 indicating that women with dense or heterogeneously dense breasts (10–12% and 40% respectively of the screening population) benefit from screening MRI, and the subsequent European Society for Breast Imaging (EUSOBI) guideline that women aged 50–70 years with extremely dense breasts should undergo screening MRI at least every 4 years,3 the demand for breast MRI is likely to further exceed availability. Installation of more CEM units, at a fraction of the price of a superconductive MRI unit with breast imaging capability, can help to meet this need.
Studies in which CEM has been performed to screen women with dense breasts indicate an incremental cancer detection rate of 6.6–13.1 per 1000 women. While this is significantly lower than the incremental cancer detection figures of 15.2 or 16.5/1000 screens observed for MRI,1, 30 it is significantly higher than what has been reported for supplemental ultrasound (1.8–4.6 per 1000 women).31 Since LE CEM images are equivalent to a standard mammogram and can demonstrate microcalcifications (unlike MRI), CEM could potentially replace standard mammography, at least on an alternating basis.
Axillary adenopathy where standard imaging has failed to show a primary
In some cases, CEM may be helpful in detecting and locating the site of primary breast cancer.32 However, published data for this indication are limited, and MRI remains the gold standard.
Contraindications
The main contraindications for CEM are related to the use of iodinated contrast agents and ionising radiation. They include documented contrast allergy and untreated hyperthyroidism.33 For patients at risk of contrast-induced nephropathy ‘point-of-care’ assessment of renal function can be performed. Patients also need to reasonably mobile, so that they can be positioned, and all mammographic views obtained within 8 min of injection.
Reporting
The CEM BI-RADS lexicon34 was published in 2022 by the American College of Radiology. Non-enhancing findings are reported using the existing mammography lexicon; descriptors like the MRI lexicon are used for enhancing findings on the RC images.
When reading CEM on a reporting workstation, the standard hanging protocol promoted by CEM vendors is to view the LE images first (to assess morphological abnormalities), and then to examine the RC images for additional information. However, a study by van Geel et al.35 found that an inverse hanging protocol (RC images viewed first, then afterwards the LE images) resulted in similar diagnostic performance with an average reduction in reading time per case of 6.2 s, as readers spent less time viewing the LE image. The latter may be relevant if reading a batch of CEM examinations.
The learning curve to report CEM studies is short and steep. Lalji et al.6 performed a study in which radiologists with varying levels of experience with CEM and residents with minimal experience read 199 CEM cases. Diagnostic performance increased significantly with CEM compared with standard mammography regardless of the level of reader experience. The CEM sensitivity scores achieved by the residents and non-experienced readers were equivalent to those of the experienced readers. Cheung et al.7 estimated that a radiologist should read an average of 75 CEM examinations to reach a 90% probability of correct prediction.
Like any imaging modality, false-positive and false-negative findings occur. False-positive findings (Fig. 8) may relate to any condition that is associated with increased vascularity, such as infection or inflammation (e.g. after surgery or radiotherapy), benign lesions (such as fibroadenomas, papillomas, pseudoangiomatous hyperplasia, intra-mammary lymph nodes, skin lesions) and artefacts (such as contrast contamination on the skin).36 Conversely, not all malignancies (e.g. invasive lobular and mucinous carcinomas, ductal carcinoma in situ) will enhance37; therefore, it is essential to review both the LE and RC images when reading a CEM study (Fig. 9). Suspicious findings on LE images (particularly microcalcifications) should undergo biopsy irrespective of whether there is associated enhancement.

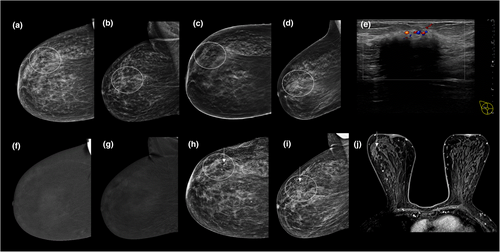
In addition to the non-specific artefacts encountered on standard mammography (e.g. those due to motion, air-trapping, hair and anti-perspirant), artefacts specific to CEM can occur (Fig. 10). They include contrast splatter onto the detector or the patient's skin, ‘breast within breast’, ripple, misregistration, skin line,38 elephant rind and cloudy fat.39 The type, incidence and severity of CEM-specific artefacts differ between vendors, likely due to differences in manufacturer's hardware and the algorithms used to produce the RC images.39
Some physiological enhancement of the normal fibroglandular tissue (background parenchymal enhancement, BPE) is seen on the RC images. This tends to be less marked than seen on CE-MRI at the same stage of the menstrual cycle.40-42 While recent studies suggest that BPE is lowest between days 8 and 14,43 scheduling according to the menstrual cycle is not usually performed. For patients with moderate or marked BPE on prior contrast-enhanced breast imaging, MRI with an initial ultrafast post-contrast sequence can be advantageous by enabling visualisation of malignant lesions, as these often enhance before the onset of BPE,44 see Figure 6.
CE-MRI provides an advantage over CEM in that it offers quantitative information about contrast enhancement kinetics that may be helpful in distinguishing benign from malignant lesions. Limited information on enhancement kinetics can be obtained on CEM by comparing the degree of enhancement of a lesion between the first and the final RC views of the affected breast.45, 46 Some investigators advocate to include an additional delayed (8-min post-injection) dual energy image to extract kinetic information47, 48; however, the diagnostic gain is unlikely to justify the additional radiation dose.
A further difference between CEM and CE-MRI is that the data acquisition and the image presentations currently available with CEM are two-dimensional (with images mimicking axial and lateral post contrast MRI maximum intensity projection images) rather than three-dimensional. MRI acquires a volumetric (3D) dataset; therefore, images can be reconstructed in any plane. Conversely, CEM is a 2D projection technique and lesion localisation can be challenging in some cases.
As with standard mammography, summation of areas of overlapping enhancing normal tissue can result in ‘pseudo-lesions’ (with false-positive recalls) and could also obscure detection of faintly enhancing lesions, particularly in the presence of moderate-marked BPE. Hypothetically, the use of true (dual energy) contrast-enhanced tomosynthesis (CET) could overcome these limitations. However, trials using prototype equipment have not shown a significant improvement in diagnostic accuracy for CET compared 2D CEM versus CE-MRI. One vendor provides equipment that allows standard two-view low-energy tomosynthesis and dual energy CEM to be performed during the same episode of breast compression.49 This may be helpful when targeting a lesion for tomosynthesis guided biopsy; however, true dual-energy CET is not yet commercially available.
Detection and management of ‘contrast-only’ lesions
Additional lesion detection in patients undergoing CEM at breast assessment has been reported to be 19%.50 In some cases, a correlation can be found with second look ultrasound (31% in a study by Coffee et al.),51 or mammography. However, in 7.7–24.2% of patients,52-54 the additional lesion is only visible after intravenous contrast injection (so-called ‘contrast-only’ lesions).
Until recently, MRI guidance was the only reliable method available to biopsy contrast-only lesions. Today, stereotactic CEM-guided biopsy is available (see Fig. 6), and early clinical results suggest high efficacy, shorter procedure times and improved patient tolerance when compared with MRI guided biopsy.55-59
Additional lesions detected on CEM should not be dismissed without further work-up. A study by Kim et al.,54 of patients in whom a CEM had shown an enhancing lesion, found 49% of these lesions to be malignant. Most of the enhancing malignant lesions had an associated finding on LE images; however, contrast-only lesions should be further investigated as 26% were malignant.54
Diagnostic performance of CEM compared with other imaging modalities
Mammography including digital breast tomosynthesis (DBT)
CEM is more sensitive for detection of malignancy than standard mammography even with the addition of ultrasound. In a multireader study of 110 women with 148 breast lesions, Dromain et al.60 found the average per lesion sensitivity across all readers was significantly higher for standard full field digital mammography (FFDM) + ultrasound (US) + CEM, than for FFDM +US (0.78 vs. 0.71, P = 0.006). All readers improved their clinical performance with the addition of CEM and the average area under the ROC curve was significantly higher for FFDM +US+CEM than for FFDM +US (0.87 vs. 0.83, P = 0.045).60
Studies assessing the performance of CEM with digital breast tomosynthesis (DBT) have shown variable results. Cozzi et al.61 reported that a CEM-based approach outperformed standard assessment (i.e. DBT or additional mammographic views) in 220 women. Yang and Gong62 compared the diagnostic accuracy of these modalities in 115 women before and after neoadjuvant chemotherapy. CEM was more effective than DBT in patients ≤50 years of age and premenopausal patients, while no significant difference was found in patients >50 years of age and postmenopausal patients.62 Siminiak et al.63 performed a randomised, prospective study on women recalled for evaluation of an abnormality detected on screening reported that the ROC curve analysis proved a significant advantage of both CEM and DBT over FFDM, however, there was no significant difference between the diagnostic accuracies CEM and DBT.
Ultrasound
Following a negative CEM examination, the need for additional breast ultrasound is questionable. In a study of 611 women at intermediate risk for breast cancer, CEM proved to be significantly more sensitive than standard digital mammography for detecting breast cancer. There was no benefit from ultrasound in women with negative results.64 Another study of 953 patients who had both CEM and ultrasound (US) concluded that if CEM showed suspicious findings, subsequent US and biopsy of the lesion was appropriate. If the CEM was reported as BI-RADS 3, correlation with ultrasound was recommended, followed by biopsy depending on the sonographic findings. If the CEM results were labelled as BI-RADS 1 or 2, routine use of ultrasound may lead to unnecessary biopsies and cost. By using CEM alone, 40% of the benign biopsies, which were due to false positives on ultrasound, could have potentially been spared. Ultrasound may even be misleading in the case of malignancy: in 8% of malignant cases, CEM findings were suspicious (BI-RADS 4, 5), but US findings were not (BI-RADS 1, 2, 3).65
Unlike standard mammography and tomosynthesis, and like MRI, the sensitivity of CEM is not limited by breast density.7, 66 Cheung et al.7 compared CEM with mammography in 89 women with dense breasts and found CEM had higher sensitivity (93% vs. 72%), specificity (68% vs. 52%) and accuracy as measured by area under the ROC curve (0.858 vs. 0.659).
MRI
We currently lack mature, standardised large-scale data on the performance of CEM relative to MRI. Initial studies indicate that CEM has a similar sensitivity and a higher specificity than MRI, with fewer false-positive recalls. However, three subsequent meta-analyses have produced discrepant and highly variable results.44-46 Xiang et al.67 reported a pooled sensitivity of 97% for both modalities. Pooled specificity for CEM was 66% and for breast MRI 52%. The pooled diagnostic odds ratio (DOR) for CEM was higher than for MRI (60.15 vs. 31.34). Pötsch et al.68 reported a pooled sensitivity for CEM of 91% and for breast MRI 97%. The pooled specificity for CEM was 74% and for MRI 69%. The overall diagnostic performance of breast MRI (pooled DOR: 73.0) was higher than that of CEM (pooled DOR: 30.4). The most recent meta-analysis by Neeter et al.69 reported pooled sensitivity values of 96% for CEM and 97% for MRI, with a specificity of 77% or both. The pooled DOR for breast MRI was much higher (122.9 instead of 31.34) and exceeded the pooled DOR for CEM (79.5). These discrepancies likely reflect the use of differing eligibility criteria for the studies that were included in the analyses.
Disadvantages of CEM compared with MRI
Contrast safety
CEM is contra-indicated in patients with a history of prior reaction to iodine-based contrast agents, renal failure and untreated hyperthyroidism. A detailed review of contraindications can be found in the RANZCR contrast guidelines.33 While the risk of a reaction to IV contrast injection is higher for CEM than for MRI, reactions are uncommon (<1%), and usually mild, and self-limiting.70 Nevertheless, practices that perform CEM must have staff capable of managing contrast reactions and the appropriate equipment and medications to do so on-site. Moreover, in the unlikely event of an allergic reaction, the patient undergoing CEM is much more readily accessible than when an untoward event happens in a patient lying prone in the tunnel of a superconducting MRI unit.
Radiation exposure
CEM involves exposure to ionising radiation and should be avoided in pregnancy and in women at high risk for development of breast cancer due to faulty DNA repair genes (e.g. BRCA 1 and 2, TP 53). The radiation dose from bilateral CEM remains within the acceptable limits for diagnostic mammography. The added HE exposure per view yields an overall 20–40% increase in radiation dose, compared with standard digital mammography71 and for some vendors, is similar to the dose for DBT,72 a technique that is increasingly being used for diagnosis and screening. There are significant differences in the radiation dose delivered by different equipment manufacturers,73-75 likely relating to differences in the X-ray target and filter materials.
Breast implants
While CEM images can be obtained using implant displaced views,76, 77 the implant needs to be kept out of the field of view to avoid causing artefacts, which limits coverage of the breast tissue. MRI is the technique of choice for women with implants.78
Lesions outside the field of view of mammography
Tissues close to the chest wall, the inframammary fold, the pre-pectoral zone, and the axillary tail may not be shown on CEM and are better assessed with MRI. Axillary lymph nodes are better assessed with ultrasound or other cross-sectional imaging.79
Background parenchymal enhancement (BPE)
Lesion enhancement on CEM is usually less intense than on MRI. For similar stages of the menstrual cycle, the degree of BPE observed on CEM is usually lower than it is on MRI.80 However, for women with high BPE, ultrafast post-contrast MRI may be advantageous in demonstrating the very rapid (usually <10 s) contrast enhancement that often occurs with breast cancer, prior to the onset off BPE81 (see Fig. 6).
Lactation
Iodinated- or gadolinium-based contrast agents can safely be administered to breast-feeding women82; however, associated physiologically high levels of BPE can make either CEM or MRI difficult to interpret. If either test is to be performed, the breasts should be emptied as much as possible first.
How to get started with CEM
Many vendors of X-ray mammography equipment now offer relatively inexpensive hardware and software upgrades, enabling CEM to be performed on existing equipment. Units capable of performing CEM are a lot cheaper than MRI to buy, install and maintain and therefore have the potential to be more readily available to women than MRI.83, 84 CEM-guided biopsy (Fig. 6) is quicker and easier to perform than MRI-guided biopsy (median of 15 min58 compared with up to 30 minutes for MRI85), and is therefore likely to be better tolerated by patients and cheaper to perform.
On a practical level, a pre-contrast screening algorithm for contraindications and renal impairment and staff able to perform patient cannulation are required. Resuscitation equipment and medications must be available, and staff training in management of contrast reactions is essential. Radiology practices that already perform CT scans, however, will already have these provisions in place. CEM takes longer than a standard mammogram to perform. Mammography staff will need time to become used to giving patients intravenous contrast.
Current factors limiting the uptake of CEM
From a billing perspective, there is currently no Medicare item number for CEM. This is true for Australia and New Zealand, and it also holds true for many other countries in the world. Some radiology groups use the Medicare item number for mammography or tomosynthesis supplemented with an additional patient-out-of-pocket charge to cover the costs of IV cannulation and contrast.
While there are several Medicare item numbers for breast MRI,86 the current licensing system for MRI scanners in Australia renders it difficult for many patients to access MRI services without very significant out-of-pocket costs. This is particularly the case for women in remote and regional areas.
Ongoing clinical trials and research
- CMIST study87: to determine if CEM can detect more cancers with fewer false positives than digital breast tomosynthesis (DBT) in women with dense breasts.
- BRAID: Breast Screening – Risk Adapted Imaging for Density trial, a randomised, multicentre UK study assessing the impact of Supplementary Imaging (automated breast ultrasound, abbreviated MRI, CEM) in the detection of breast cancer in women aged 50–70 years with dense breasts (BIRADS c or d) on screening mammography in the National Health Service Breast Screening Programme.88
- PROCEM study: added value of preoperative contrast-enhanced mammography in staging malignant breast lesions – a prospective randomised multicentre study.89
Machine learning and the use of AI for interpretation of CEM are other exciting areas of active research.90-93
Summary
CEM uses dual-energy X-ray exposures obtained 2 min after IV injection of an iodine-based contrast agent. Diagnostic performance is significantly superior to standard X-ray mammography and breast ultrasound and is not limited by the presence of dense breast tissue. Disadvantages include the risks of ionising radiation, contrast reactions and the non-volumetric data acquisition.
While we are awaiting the results of large-scale studies of CEM, all currently available data support the use of CEM for problem solving, pre-operative local staging and in assessing response to neoadjuvant chemotherapy, particularly if MRI is not available or contra-indicated.
CEM can be performed on most modern mammography units following a relatively inexpensive hard- and software upgrade. A power injector and presence of staff trained in intravenous cannulation and management of contrast reactions are essential elements that are already in place in the many radiology practices that are routinely performing CT studies.
Increased uptake of CEM by radiologists across the world may help address an increasing demand for functional breast imaging that is unlikely to be able to be met by currently available MRI resources.
Funding information
No funding was received for this manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Open Research
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




