Chest computed tomography findings among adult Aboriginal Australians with bronchiectasis in the Top End Northern Territory of Australia
SS Heraganahally: , FRACP; T Howarth, PhD; C Gibbs, MBBS; SS Heraganahally, MBBS; L Sorger, FRANZCR.
Conflicts of interest: All authors declare no conflicts of interest for this study.
Abstract
Introduction
There is limited evidence in the literature illustrating chest computed tomography (CT) characteristics among adult Aboriginal Australians with bronchiectasis. This retrospective study evaluates the radiological characteristics of bronchiectasis in Aboriginal Australians residing in the Top End, Northern Territory of Australia.
Methods
Patients aged >18 years with chest CT-confirmed bronchiectasis between 2011 and 2020 were included. Demographics and relevant clinical parameters were collected. Alongside confirming bronchiectasis, chest CT reports were assessed for (i) lobar location (ii) unilateral or bilateral involvement and (iii) bronchiectasis type when available.
Results
A total of 459 patients were identified with chest CT-confirmed bronchiectasis, with a median age of 47 years, and 55% were females. Bronchiectasis was predominantly recorded in the left lower lobe (LLL) (73%), followed by the right lower lobe (RLL) (62%) and the left upper lobe (LUL) was least common (22%). Females recorded the right middle lobe (RML) affected significantly more often than males (50 vs. 34%, P = 0.012). Bilateral involvement was common (74%), with the strongest pairwise correlation associated between the right upper lobe (RUL) and LUL (P < 0.001). Cylindrical (50%) and cystic (28%) types were most common. The RML and LLL showed positive correlation with cylindrical and LUL with cystic bronchiectasis. Neither lobar location nor bronchiectasis type showed any significant association with lung function parameters other than RML, Lingula and LUL involvement being associated with better percent predicted values of diffusing capacity for carbon monoxide. There were no significant associations between sputum culture and type or lobar locations of bronchiectasis except for non-Aspergillus fungus culture prevalence was higher with cystic or cylindrical types.
Conclusion
The results of this study may be an avenue to develop CT bronchiectasis severity scale in the future specific for Aboriginal Australians.
Introduction
Bronchiectasis is a chronic respiratory condition characterised by recurrent lower respiratory tract infections and airway inflammation, dilatation of the bronchial airways and impaired mucociliary clearance.1 The pathogenesis of bronchiectasis is complex and multifactorial, though typically arising via a pathophysiologic combination of recurrent lower respiratory infections, smoking, airway inflammation, impaired ciliary function and other chronic lung diseases such as emphysema.1 Globally, there has been an observed relationship between socio-economic status and the prevalence of bronchiectasis. Higher socio-economic groups have increased bronchiectasis in the elderly population, whereas socially disadvantaged populations demonstrate a higher prevalence of bronchiectasis that presents at a younger age.2 Bronchiectasis is more prevalent among Indigenous populations residing in English-speaking Organisation for Economic Cooperation and Development (OECD) countries compared to non-Indigenous populations, such as the Alaskan and Native Americans, First Nations New Zealand Māori and Pacific Islanders and Aboriginal Australians3, 4 (from here on “Indigenous” is used to refer to global First nations populations, while “Aboriginal Australian/people/population” is used to specifically refer to Australia's' First Nations population). Despite known longstanding higher prevalence of bronchiectasis among these groups,5 the morphological and radiological characteristics of bronchiectasis have not previously been described.
The exact influence of pulmonary morphological characteristics on bronchiectasis outcomes is a matter of ongoing debate and research. The presence of multiple lobar involvement is included in prognostic tools, such as the Bronchiectasis Severity Index (BSI) or FACED scale, with multi-lobar involvement associated with more exacerbations and hospitalisations.6, 7 The influence of bronchiectasis type upon lung function parameters, however, is controversial, with some research showing no significant effect,8 and others showing a significant difference between types.9 Moreover, micro-organism cultures appear to differ between various lobes affected by bronchiectasis.10
The lobar distribution and type of bronchiectasis differ across global geography, although, broadly, the lower lobes are the most affected.11 In Israel, 20.7% of patients were identified with right lower lobe (RLL) and 20.4% with left lower lobe (LLL) involvement10 compared with 52.3% and 64.8%, respectively, in Turkey,9 and 60% and 62% respectively in the USA.12 The reason underlying the relationship between geographical location and lobar involvement is unclear. However, it may be due to environmental factors, micro-organisms, prevalence of comorbidities or other factors.
Currently, chest computed tomography (CT) is the gold standard in the accurate diagnosis of bronchiectasis.13 Adult Aboriginal Australians residing in the Top End Health Service (TEHS) regions are reported to have a significantly higher prevalence of bronchiectasis in comparison to non-Aboriginal Australians.14 A recent study from the Northern Territory (NT) of Australia demonstrated that 23% of Aboriginal adults undergoing chest CT scans showed evidence of bronchiectasis.15 Other research has shown a disparity in bronchiectasis prevalence and mortality for adult Aboriginals between various health districts within the TEHS region.16 Given the lack of data in the literature regarding the morphological characteristics, it is timely to describe chest CT scan findings among adult Aboriginal Australians diagnosed with bronchiectasis residing in various TEHS health districts in the NT. Therefore, in this study we examined the chest CT features among an Aboriginal Australian cohort with bronchiectasis over a 10-year period (2011–2020).
Methods
Setting and study participants
This study was conducted at the respiratory and sleep division based at the Royal Darwin Hospital (RDH), a university-affiliated tertiary care teaching hospital and Darwin Respiratory and Sleep Health, Darwin Private Hospital, within the TEHS, NT region of Australia. This study is a part of a broader research project investigating various clinical outcomes in an Aboriginal population aged >18 years, and included all patients with chest CT confirmed bronchiectasis between 2011 and 2020 in the TEHS, NT health districts, namely; Darwin Urban (DU), Darwin Rural (DR), East Arnhem (EA) and Katherine region (KR). Further details on the setting and study participants are detailed in a recent report from our centre.16
Ethics
This study was approved as a part of the bronchiectasis research project by the Human Research Ethics governance/committee of the TEHS, NT and Menzies School of Health Research (Reference: HREC; 2019-3547).
Clinical and chest CT data
Details of patients' usual place of residence, demographics and relevant clinical information were recorded. Lung function test results and sputum microbiology data were collected when available. Chest CT scan findings were recorded as per the reporting radiologist based at the RDH. All chest CT reports were assessed for (i) lobar location, (ii) unilateral or bilateral involvement and (iii) bronchiectasis type, when available.
Statistical analysis
Continuous data was displayed as median (interquartile range (IQR)) and categorical data as number (%). Differences in CT findings (lobal location, bronchiectasis type and bilaterality) between sexes were assessed via univariate logistic regression and between administration regions via ANOVA. Pairwise correlations between lobar location for patients with ≥2 lobes affected and between lobar location and bronchiectasis type were assessed via Pearson's pairwise correlation. The influence of lobar location, bilaterality and bronchiectasis type on lung function results (forced vital capacity (FVC), Forced expiratory volume in 1 second (FEV1), FEV1/FVC, diffusing capacity of carbon monoxide (DLCO) and total lung capacity (TLC)) were assessed in univariate and multivariate quantile regression models, adjusted for age, sex, comorbid COPD and comorbid asthma, with results reported as beta (95% confidence interval (CI)). The influence of sputum cultures on odds of bronchiectasis type were assessed via multivariate logistic regression, adjusted for age, sex, comorbid COPD and comorbid asthma, reporting odds ratios (ORs) (95% CIs). All regression analyses were adjusted for multiple hypotheses testing via Romano Wolf correction with 250 bootstrap replications, while pairwise correlation analyses were adjusted with the Bonferroni method. Alpha was set to 0.05 throughout, and all analyses were conducted in STATA IC 15 (StataCorp, College Station, TX, USA).
Results
Study participants
A total of 459 patients were identified with chest CT-confirmed bronchiectasis during the study window, with a median age of 47 years, and 55% were females. Further clinical details of the study participants included in this study are described in a recent report.16
Chest CT data
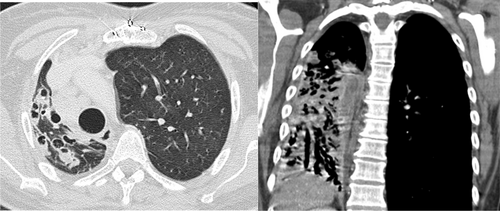
Bronchiectasis was predominantly recorded in the LLL (73%), followed by the RLL (62%). The upper lobe (LUL) was least commonly affected (22%) (Table 1). Females were significantly more likely to have right middle lobe (RML) involvement (Fig. 1) than males (50 vs. 34%, P = 0.012). Bilateral involvement was common (74%), with no significant difference between the sexes. When the type of bronchiectasis was available to be assessed from the radiologist reports, cylindrical or tubular (50%) and cystic (28.1%) types were the most common. No significant differences in the type of bronchiectasis were noted between the sexes.
| Total (n = 459) | Female (n = 254) | Male (n = 205) | P-value | |
|---|---|---|---|---|
| CT- Lobar location of bronchiectasis | ||||
| RLL | 285 (62.1%) | 148 (58.3%) | 137 (66.8%) | 0.427 |
| RML | 196 (42.7%) | 126 (49.6%) | 70 (34.1%) | 0.008* |
| RUL | 126 (27.5%) | 67 (26.4%) | 59 (28.8%) | 0.955 |
| LLL | 337 (73.4%) | 184 (72.4%) | 153 (74.6%) | 0.955 |
| Lingula | 130 (28.3%) | 73 (28.7%) | 57 (27.8%) | 0.955 |
| LUL | 100 (21.8%) | 52 (20.5%) | 48 (23.4%) | 0.932 |
| Type | ||||
| Cylindrical or tubular | 80 (50%) | 51 (54.3%) | 29 (43.9%) | 0.775 |
| Cystic | 45 (28.1%) | 28 (29.8%) | 17 (25.8%) | 0.955 |
| Traction | 35 (21.9%) | 16 (17%) | 19 (28.8%) | 0.502 |
| Type not stated | 299 (65.1%) | 160 (63%) | 139 (67.8%) | 0.852 |
| Laterality | ||||
| Bilateral | 339 (73.9%) | 181 (71.3%) | 158 (77.1%) | 0.712 |
- P-values obtained via logistic regression, adjusted for multiple hypotheses testing via Romano-Wolf method. LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
- *Statistically Significant.
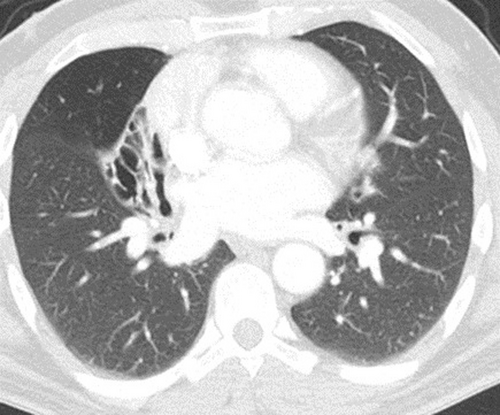
There were minor differences noted between health district regions in the CT lobar involvement and for the type of bronchiectasis (Table S1). RLL involvement was the most common in DU (67.6%), while LLL involvement was most common in each of DR (78.8%), EA (62.7%) and KR (76.3%) regions. The LLL and Lingula involvement were significantly more common in DR compared to the DU region (uncorrected P = 0.032 and P = 0.044, respectively). Among those patients where a type of bronchiectasis was reported, traction bronchiectasis was the most common in DU region (33.3%), cylindrical bronchiectasis in DR (38.4%) and KR (33.3%) regions, and both traction and cystic bronchiectasis in EA (34.5% each). Bilateral involvement did not significantly differ between regions. In multivariate analysis, the prior noted differences in lobar involvement between regions were attenuated.
Pairwise correlations between lobar location of bronchiectasis
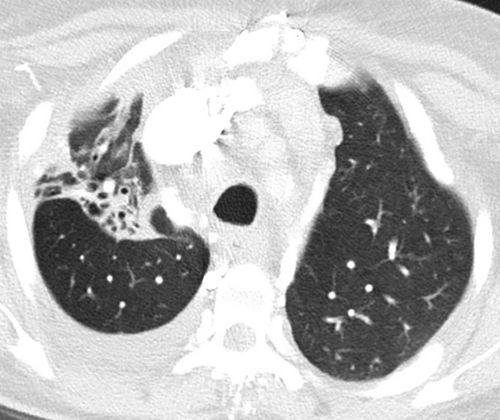
For 337 (73.4%) patients, bronchiectasis affected more than one lobe. The strongest correlation was noted between the right upper lobe (RUL) (Fig. 2) and LUL, R2 of 0.539 (P < 0.001) (Table 2). However, moderate correlations (R2 > 0.4) were also noted between the Lingula and the LUL (R2 0.418) and between the Lingula and the RML (R2 0.415). The RML and LLL showed a significant positive correlation with cylindrical or tubular type bronchiectasis (R2 0.265 and 0.260, respectively) and a negative correlation with traction type bronchiectasis (R2 –0.247 and −0.253, respectively). LUL involvement showed a significant positive correlation with cystic type bronchiectasis (R2 0.270) (Table 3).
| CT- Lobal location | RLL (n = 285) | RML (n = 196) | RUL (n = 126) | LLL (n = 337) | Lingula (n = 130) | LUL (n = 100) |
|---|---|---|---|---|---|---|
| RLL | ||||||
| RML | 129 (45.3%) | |||||
| 0.066 (1.000) | ||||||
| RUL | 90 (31.6%) | 78 (39.8%) | ||||
| 0.118 (0.167) | 0.239 (<0.001) | |||||
| LLL | 248 (87.0%) | 138 (70.4%) | 86 (68.3%) | |||
| 0.394 (<0.001) | −0.059 (1.000) | −0.072 (1.000) | ||||
| Lingula | 87 (30.5%) | 98 (50%) | 60 (47.6%) | 103 (30.6%) | ||
| 0.063 (1.000) | 0.415 (<0.001) | 0.263 (<0.001) | 0.083 (1.000) | |||
| LUL | 67 (23.5%) | 63 (32.1%) | 73 (57.9%) | 80 (23.7%) | 64 (49.2%) | |
| 0.053 (1.000) | 0.217 (<0.001) | 0.539 (<0.001) | 0.079 (1.000) | 0.418 (<0.001) |
- Data displayed as number (%) followed by Pearsons R2 (Bonferroni adjusted P-value). Bolded cells indicate correlations with P < 0.05. LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
| Bronchiectasis type | RLL (n = 100) | RML (n = 69) | RUL (n = 61) | LLL (n = 120) | Lingula (n = 48) | LUL (n = 41) |
|---|---|---|---|---|---|---|
| Cylindrical | 59 (59%) | 45 (65.2%) | 25 (41%) | 69 (57.5%) | 25 (52.1%) | 17 (41.5%) |
| 0.232 (0.056) | 0.265 (0.013) | −0.142 (1.000) | 0.260 (0.016) | 0.027 (1.000) | −0.100 (1.000) | |
| Cystic | 29 (29%) | 23 (33.3%) | 23 (37.7%) | 35 (29.2%) | 21 (43.8%) | 20 (48.8%) |
| 0.025 (1.000) | 0.101 (1.000) | 0.167 (0.621) | 0.040 (1.000) | 0.228 (0.068) | 0.270 (0.011) | |
| Traction | 17 (17%) | 7 (10.1%) | 17 (27.9%) | 19 (15.8%) | 9 (18.8%) | 7 (17.1%) |
| −0.152 (0.983) | −0.247 (0.029) | 0.114 (1.000) | −0.253 (0.022) | −0.050 (1.000) | −0.068 (1.000) |
- Data displayed as number (%) followed by Pearsons R2 (Bonferroni adjusted P-value). Bolded cells indicate correlations with P < 0.05. LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Chest CT location of bronchiectasis to lung function parameters and sputum microbiology
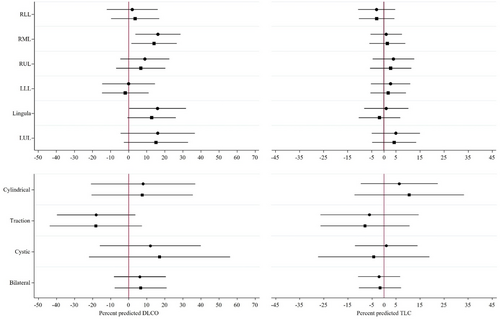
Neither lobar location nor bronchiectasis type showed any significant association with lung function parameters either for FEV1, FVC or FEV1/FVC in univariate or multivariate models (Figure S1). However, RML, Lingula and LUL involvement were associated with significantly greater percent predicted values of DLCO in univariate regression (16.2 (95% CI, 3.76, 28.64), P = 0.023, 16 (95% CI 0.29, 31.71), P = 0.023 and 16.1 (95% CI −4.47, 36.67), P = 0.023 respectively), though the associations were attenuated on multivariate regression (14.08 (95% CI 1.72, 26.44), P = 0.092, 12.75 (95% CI −0.45, 25.94), P = 0.095 and 15.1 (95% CI −2.61, 32.82), P = 0.067, respectively) (Fig. 3). No CT parameters showed any significant association with TLC.
Sputum microbiological cultures were available for 147 (91.9%) patients for whom the type of bronchiectasis was also reported. In multivariate modelling, presence of non-Aspergillus fungus in sputum was associated with significantly reduced odds for traction type bronchiectasis (RW P = 0.0319). There were no other significant associations between sputum culture and type of bronchiectasis (Table S2) nor between sputum culture and lobar location of bronchiectasis (Table S3).
Discussion
To the best of the authors knowledge, this is the first comprehensive study assessing chest CT data in an adult Indigenous population with bronchiectasis, especially from the Top End, NT of Australia. In line with previous studies, lower lobes are predominantly affected, and bilateral involvement is common. Although data pertaining to the subtype of bronchiectasis was limited, the available data demonstrated that cylindrical or tubular types of bronchiectasis are most common in this population. We identified that RML involvement is significantly more common in females and that there are differences in chest CT findings based on geographic location. Furthermore, we identified better lung function values for DLCO with RML, Lingula and LUL involvement.
Currently there is sparse literature detailing chest radiology data for Indigenous populations globally,17, 18 and for the Australian Aboriginal population in particular.19-24 In other global non-Indigenous populations, chest CT findings of bronchiectasis patients and their association with other clinical parameters such as potential aetiology, sputum microbiology and lung function parameters have been well described.11 Chest CT characteristics of bronchiectasis secondary to tuberculosis (TB) are documented in previous reports from geographic regions with high incidences of TB.21, 22 However, the incidence of TB in Australia is much lower (including among Aboriginal Australians) as compared to certain global geographic regions.23 Hence, it is unlikely that the vast majority of the CT findings of bronchiectasis observed among our study participants are sequalae secondary to pulmonary TB. Similarly, chest CT characteristics of non-tuberculosis mycobacterial (NTM) infections are recognised to be associated with RML involvement.11 The incidence of NTM infections, however, are lower in Aboriginal Australians in comparison to their non-Aboriginal counterparts.24 In our study, a significantly higher proportion of females were noted to have bronchiectasis affecting the RML. However, in multivariate regression, there was no significant association between NTM and RML involvement. It is unclear at this stage the reasons for heightened RML involvement among Aboriginal Australian females in our study cohort. Therefore, it is reasonable to speculate that previously unexplored or unknown etiologic factors may be contributing to bronchiectasis in global Indigenous populations and in Aboriginal Australians.
We observed that cylindrical or tubular (50%) and cystic (28%) bronchiectasis were the most common types among Aboriginal Australian patients. Variations in the lobar involvement and the type of bronchiectasis among patients residing in differing health districts/urban versus rural and Aboriginal Australian communities were also noted. The reason for the observed geographical variations in the chest CT characteristic was not explored in this current study, as that was beyond the scope of this study. However, future studies may be useful to explore the reasoning behind geographic variation. Previous studies have demonstrated that the presence of cystic bronchiectasis is associated with a higher incidence of pseudomonas infections.25 We found no such association in the current study; however, non-Aspergillus fungus was positively associated with cylindrical types (though the P-value was attenuated following Romano-Wolf correction) and negatively associated with traction-type bronchiectasis.
One important observation we noted in this study is that a significant proportion of the study patients demonstrated extensive bronchiectasis in association with significant parenchymal lung architectural distortion (Fig. 4). This suggests that the pathological damage to the pulmonary parenchyma affects the smaller airways and alveoli as well as larger airways that could give rise to substantial respiratory physical impairment. Indeed, shortness of breath and chronic cough with mucous production are noted to be the most common clinical manifestations among adult Aboriginal patients presenting with respiratory disorders in day-to-day clinical practice.4, 14 Nonetheless, functional radio-pathological correlation will require further research in this area.
Lung function parameters in relation to chest CT findings among patients with bronchiectasis are documented in the literature for non-Indigenous patients, showing a correlation of bronchial wall thickening against FEV1 values,25 and greater lung function impairment among patients with cystic bronchiectasis.9, 26 Moreover, significant impairment in DLCO is reported to occur among patients with bronchiectasis.27 However, there is limited data on the correlation of specific lobar involvement against lung function impairment, more specifically for DLCO. Previous studies have shown significant impairment in lung function parameters among adult Australian Aboriginal patients with chronic airway disease, including among patients with bronchiectasis.4 Moreover, DLCO values are noted to be lower for Aboriginal Australian patients in comparison to Caucasian counterparts.28 In this study, we noted that the presence of bronchiectasis in the RML, lingula or LUL was associated with a higher DLCO, although these associations were attenuated to borderline significant in multivariate models. The RML, Lingula and LUL also demonstrated significant pairwise correlations, although their strength was weak to moderate (R2 0.21–0.41). Exactly what this means for patients in clinical sense, and the potential mechanisms underlying these findings require further exploration.
This study has demonstrated radiological manifestations of bronchiectasis in an adult Aboriginal Australian cohort and the results of study may be useful in the future to develop predictive models in bronchiectasis severity classifications in global Indigenous and among Aboriginal Australians. The burden of chronic respiratory disorders is significantly higher in Aboriginal Australians, contributing to higher overall morbidity and mortality.4, 29 Therefore, it is imperative that future prospective studies investigate other various clinical conundrums30 associated with chest radiology findings and clinical outcomes in Indigenous/Aboriginal peoples, including innovative strategies to address chronic respiratory burden in regional and remote Aboriginal communities.
Limitation
The authors acknowledge that there are several limitations in this retrospective study, Firstly, chest CT scan results in this study are limited to Aboriginal Australian people residing in the TEHS region of the NT of Australia and absent confirmatory data, it is unclear whether they will be able to be generalised to the wider Aboriginal Australian populations. Secondly, our study relied on existing radiology reports with numerous primary indications for undergoing a chest CT other than for bronchiectasis, so it is understandable that the reports do not all elaborate on the type of bronchiectasis. Thirdly, lung function data was not available in all patients. Moreover, this study being retrospective in nature, has its inherent limitations. Nevertheless, this is the first study to describe chest CT data in a large Aboriginal Australian cohort and this may facilitate to compare our data to other Indigenous populations globally and in Australia.
In conclusion, this study has demonstrated that among adult Aboriginal Australians, chest radiology evidence of bronchiectasis disease burden is substantial, usually bilateral and often being muti-lobar. There is also a disparity in the type and extent of bronchiectasis within the region and communities. The results of this study may be of use to develop Indigenous-specific chest CT bronchiectasis severity scale in the near future.
Acknowledgement
We thank the Thoracic Society of Australia and New Zealand (TSANZ) research grant assessment committee members for providing support through the – Robert Pierce Grant-In-Aid for Indigenous Lung Health. We also thank Associate Professor Linda Ford – an Indigenous Australian woman, a Mak Mak Marranunggu descendent from the Delissaville, Wagait Larrakia Aboriginal Land Trust and the Gurudju Aboriginal Land Trust in the NT for the support and facilitating Mrs Adriana Ticoalu from the Northern Institute, Faculty of Arts & Society, Charles Darwin University, Darwin, NT, Australia to assist with population data collection for this study. We also extend our sincere apparition to Dr Asanga Abeyaratne, Dr Shiidheshwar Ravichandran and Dr Davaadorj Erdenebayar for their help during this study. We would like to thank our respiratory clinical nurse consultants, Mrs Raelene Messenger and Mrs Siji Issac from the respiratory chronic disease unit at the RDH, including, rural and remote community Aboriginal health workers, all the RDH medical services, in particular radiology and pathology services, including patients travel division for coordinating care for Aboriginal people living in the remote and rural communities. We also thank Dr Edwina Binacardi, respiratory physician at the RDH, for reviewing this manuscript. Open access publishing facilitated by Flinders University, as part of the Wiley - Flinders University agreement via the Council of Australian University Librarians.
Funding
This research received the Thoracic Society of Australia and New Zealand (TSANZ) - Robert Pierce Grant-In-Aid for Indigenous Lung Health. The TSANZ did not have any role in the study design, data collection, analysis or interpretation.
Author contributions
All authors contributed to the conception and design, interpretation of the data, drafting of the paper, revising it critically for intellectual content and final approval of the version to be published and agree to be accountable for all aspects of the work.
Ethics statement
This study was approved by the local Human Research Ethics Committee.
Open Research
Data availability statement
Research data are not shared.