Bluer in the city: urban male lizards exhibit more intense sexual coloration and lower parasite loads than non-urban males
Abstract
Urbanization is a global phenomenon that involves the transformation of natural areas into urban spaces, thereby subjecting organisms to new selective pressures including a wide variety of pollutants and changes in intra- and interspecific interactions. Considering that projections indicate that by the year 2050, 65% of the human population will live in urban areas and that urbanization is a phenomenon with an upward pattern, identifying these phenotypic traits is vital to implementing conservation and management plans for urban fauna. The urban environment may exert different selective pressures on sexually selected traits than more pristine environments, a phenomenon which has been well studied in birds but is less understood in other vertebrates such as lizards, although they are common inhabitants of urban environments. Here, we compare sexual coloration, parasite load, and immune response in Sceloporus torquatus lizards in urban and non-urban environments of Central Mexico. Our study shows that sexual coloration is more saturated (bluer) in male lizards from urban environments, while UV chroma was higher in non-urban lizards. The average parasite load is lower in urban lizards than in non-urban lizards, and we found a negative relationship between hemoparasite count and sexual coloration in male lizards from non-urban environments but not in male lizards from urban environments. Additionally, non-urban lizards exhibited a higher immune response. In female lizards, sexual coloration differed significantly between urban and non-urban environments, but parasite load and immune response did not differ. These results may be useful to improve herpetofauna conservation plans in urbanized environments.
INTRODUCTION
Urbanization is a worldwide phenomenon that involves converting natural environments to spaces suitable for occupation by high-density human populations (Marzluff et al. 2001; French et al. 2014). This process includes infrastructure and building construction, generating the loss of preexisting habitat and reducing biodiversity. Frequently, urban environments are instead dominated by exotic species (Sol et al. 2017). In addition, urbanization reduces ecosystem functioning through the presence of impervious surfaces that do not allow water filtration and through a rise in environmental temperatures (Sol et al. 2011, 2013; Le Roux et al. 2014; Battles et al. 2018). Consequently, it gives rise to new challenges for organisms, encompassing chemical, noise, and light pollution, along with habitat loss, fragmentation, and shifts in species interactions (Miranda et al. 2013; Sol et al. 2013). Currently, 50% of the global human population resides in urban areas, and projections suggest that by 2050, this figure will increase to 65% (United Nations 2019). As urbanization persists as an irreversible trend, it is imperative to understand its impact on the organisms persisting in these environments (McKinney 2008; Dirzo et al. 2014).
Urban environments offer habitat for many species of animals. To predict the impact of urbanization on wildlife, various studies have sought to identify crucial traits for successful colonization or persistence in urban environments (French et al. 2014; Kang et al. 2018; Iglesias-Carrasco et al. 2019; Collins et al. 2021). For example, among birds, researchers have underscored the importance of behavioral flexibility and the incorporation of human-made materials in nest construction (Slabbekoorn & den Boer-Visser 2006; Suárez-Rodríguez et al. 2013, 2017; Marín-Gómez & MacGregor-Fors 2021).
However, knowledge regarding other vertebrates, particularly reptiles, remains somewhat scarce. Studies in this realm have predominantly focused on thermal traits including preferred temperature (Nordberg & Schwarzkopf 2019; Thawley et al. 2019), morphological measurements such as body condition or limb length (Putman & Tippie 2020; Gómez-Benitez et al. 2021), immune activity assessed through the heterophile–lymphocyte ratio (French et al. 2008; Amdekar et al. 2018), dorsal coloration and anti-predatory behavior (Batabyal & Thaker 2017; Pellitteri-Rosa et al. 2017), and social learning (Kang et al. 2018; Batabyal & Thaker 2019).
Sexual selection is a powerful evolutionary force capable of influencing population variability and, consequently, impacting the potential colonization of novel habitats like urban environments (Candolin & Heuschele 2008). The intersexual component of sexual selection frequently relies on the emission of signals, which can manifest visually, chemically, or acoustically (Shuster 2009; Steiger et al. 2011; Baeckens et al. 2018; Buchinger & Li 2023). In urban settings, sound pollution can limit signals emitted by birds and frogs, disrupting the transmission and reception of vocalizations (Marín-Gómez & MacGregor-Fors 2021; Smit et al. 2022). These vertebrates have modified the intensity and duration of their signals to avoid overlap with noise pollution, such as increasing volume or altering emission patterns (Slabbekoorn & Peet 2003; Nemeth & Brumm 2010; Francis et al. 2011). In contrast, lizards’ sexual signals are predominantly visual, with males exhibiting distinctive colorful features like ventral patches. These features convey vital information, enabling males to compete for resources such as territory, food, or mates (Burtt 1979; Cooper & Burns 1987; Pérez I de Lanuza et al. 2013).
An additional effect of urbanization is the modification of species interactions and the host–parasite relationship, which can influence pathogen epidemiology and host susceptibility to infectious diseases (Bradley & Altizer 2007; Delgado & French 2012; Brum et al. 2023). Parasites play a significant role in host population dynamics as they draw resources and diminish the fitness of their hosts (Salathé et al. 2008; Artim et al. 2020; Mendoza-Roldan et al. 2021). They interfere with energy allocation by causing tissue damage and activating the immune system, ultimately affecting host fitness (Meylan et al. 2013). In birds, studies have yielded mixed results. Some indicate a lower parasite load in urban areas (Calegaro & Amato 2014; Giraudeau et al. 2014), while others demonstrate an increased parasite load (Le Gros et al. 2011). In lizards, information is scarce, but prevailing evidence suggests a moderate parasitic increase in urban species (Lazić et al. 2017; Thawley et al. 2019).
The relationship between parasitic load, sexual coloration, and immune response has been explored in several vertebrates (Hamilton & Zuk 1982; but see Megía-Palma et al. 2021). However, this relationship is rarely studied in urban lizards. Investigating the direction of this relationship in both urban and non-urban environments may be valuable for comprehending how lizards, and potentially other organisms, modulate the expression of sexual signals, such as sexual coloration, during the colonization of urban habitats. In this study, we examine sexual coloration, immune response, and parasite load in an endemic Mexican lizard that inhabits both urban and non-urban environments. Our predictions are as follows: (i) lizards from an urban environment will exhibit higher body condition than those from a non-urban environment; (ii) urban males will display a higher parasite load and less intense blue coloration compared to non-urban males; and (iii) coloration in urban and non-urban females will not differ because females do not exhibit the intensely blue sexual coloration typical of males.
Sceloporus torquatus is a lizard endemic to central Mexico that in natural environments lives in extrusive igneous rocks in an elevation range between 1600 and 2700 m (González-Morales et al. 2015). In this lizard, males have blue throat and ventral coloration that has been associated with intra- and interspecific competition, respectively (Rivera-Rea et al. 2024). The low mobility of this endemic species and the fact that urbanization is encroaching on natural populations make it an appropriate model to examine the effect of urbanization on host–parasite interactions and sexually selected traits.
MATERIALS AND METHODS
Ethical statement
This project was conducted under scientific collecting permit number 09/K4-0002/06/23 from the Mexican Secretaria de Recursos Naturales y Medio Ambiente (SEMARNAT). All field and laboratory procedures were performed in accordance with the ethical guidelines of the Mexican government NOM-126-ECOL-2000.
Study area, sampling, and animal husbandry
We studied two populations of S. torquatus with different levels of urbanization in Central Mexico (Fig. 1). We determined urbanization level according to criteria proposed by Marzluff et al. (2001) using residential human and building density as indicators. The urban population was located in Nueva Serratón, Zinacantepec, Mexico State (19°18′42″N, 99°43′41″W) at an elevation of 2642 m. The site consisted of residential housing developments, commercial shops, and busy roads, with lizards primarily using the walls of human houses, human-constructed rock walls, and rocks as basking sites. The non-urban population was located in La Joya, San Pedro Techuchulco, Mexico State (19°07′37″N, 99°29′41″W) at an elevation of 2700 m. The site consists of a pine forest without any human buildings nearby, with lizards primarily using extrusive rocks and pine trees as basking sites. The linear distance between the two populations is approximately 33 km. The locations of these sites are sufficiently far enough from each other that gene flow likely does not occur among populations (Sears 2005; Tucker et al. 2014).
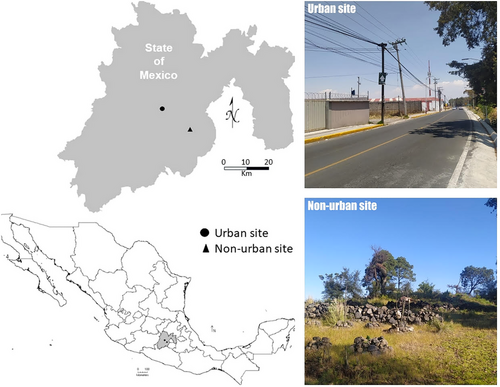
We sampled adult lizards measuring at least 70 mm snout–vent length (SVL), the minimal adult size for this species (Feria-Ortiz et al. 2001). Since parasite load, ventral coloration, and immune response vary by season in S. torquatus (Rivera-Rea et al. 2022, 2024), we only captured lizards during the mating season (October–November) by lasso or by hand. We captured 25 urban lizards (15 males and 10 females) and 22 non-urban lizards (12 males and 10 females). Captured lizards were transported in cotton bags to the Universidad Autónoma Metropolitana Unidad Lerma where they were each kept in an individual terrarium (23 × 41 × 21 cm; width, length, height, respectively) with water and food (Tenebrio molitor larvae) ad libitum for 7 days. Rocks were placed in one corner and peat moss was used as substrate. During the day (8 AM to 6 PM), heat was provided by a 60 W light bulb (temperature range was 20–35°C in each terrarium). Before starting laboratory procedures, we measured body mass with a balance (precision: ±0.01 g) and SVL with a digital caliper (precision: ±1 mm). We calculated the residual values of a linear regression of log-mass versus log-SVL to calculate the body condition index (BCI; Schulte-Hostedde et al. 2005).
Ectoparasite load
The same researcher (JCGM) conducted an ectoparasite (mite) count using a 20× magnification stereoscope (Zeiss). The examination involved systematically assessing the lizards from head to tail, initially on both the dorsal and ventral surfaces and concluding with the examination of the legs. Body mites (excluding those in pockets) exhibit a correlation with total mites that encompass both pocket and overall body mites (R2 = 0.9, P < 0.001). In Sceloporus lizards, mite pockets are anatomical structures located on each side of the neck that concentrate a large number of mites. These structures have been associated with attracting mites to reduce the damage they cause to other parts of the body (Arnold 1986). Mites of the family Trombiculidae are commonly found on Sceloporus species. Distinguished by their red coloration, these mites are easily discernible between the lizard's scales (Guzmán-Cornejo et al. 2018). We categorized the mite count into two groups: mites in pockets and total mites.
Hemoparasite load
We obtained a blood sample (<10 µL) for the quantification of hemoparasites infecting these lizards, employing methods outlined in Rivera-Rea et al. (2024) for this species. Blood was collected from the coccygeal vein in the tail using sterilized needles, ensuring that incisions avoided the hemipenal bulges. The blood drop obtained was collected with a heparinized capillary (BRAND, micro-hematocrit tubes, 75 mm). Subsequently, thin-layer blood smears were prepared, air-dried, and fixed with methanol for 5 min (Rogier & Landau 1975). These blood smears were stained for 40 min using a 1:10 Giemsa/pH 7.2 buffer solution (Schall 1986). Counting 5000 erythrocytes for each blood smear was performed at 1000× magnification in an area with a homogeneous distribution of red blood cells (Merino & Potti 1995), utilizing a BX41TF microscope (Zeiss). Parasite prevalence of ecto- and hemoparasites was calculated as the percentage of infected lizards, while parasite intensity was quantified as the number of parasites per lizard.
Measuring patch coloration
Because coloration in lizards can change due to body temperature (Langkilde & Boronow 2012; González-Morales et al. 2024), before starting the color quantification, each lizard was kept for 3 h at a temperature of 33°C, which corresponds to the preferred temperature previously recorded for S. torquatus in one of the study areas (Rivera-Rea et al. 2018). We quantified the coloration of the lizards’ throat and ventral patches by spectrophotometry within a wavelength range spanning from 300 to 700 nm following a protocol proposed by Rivera-Rea et al. (2024). Measurements were taken from the upper, medial, and lower regions of each blue patch using a Jaz model spectrophotometer equipped with a pulsed xenon light source (all components sourced from Ocean Optics Inc., Dunedin, FL, USA). The light probe was positioned within a black holder to ensure that light readings were 1 mm in diameter, free from ambient light noise, and consistently taken at a distance of 3 mm from the skin surface at a 45° angle. All spectral measurements were referenced against a 99% WS-1 white reflectance standard.
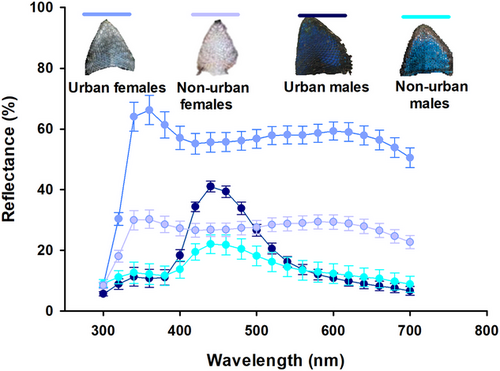
We processed spectral data in R v.4.2.0 (R Development Core Team 2017) using the “pavo” R-package (Maia et al. 2013). We analyzed spectra from the throat and ventral patches separately. We cropped each spectrum between 300 and 700 nm and smoothed it using an interval of 0.2. Then, we extracted colorimetric variables from the spectra of each patch such as luminance, defined as mean brightness; UV chroma, defined as R300–400 nm/R300–700; blue chroma, defined as R400–475 nm/R300–700; and hue, defined as wavelength at the maximum reflectance peak.
Immune response
We followed the protocol by Rivera-Rea et al. (2022) to assess the immune response in the lizards. We used a phytohemagglutinin (PHA) inoculation test to quantify the local non-specific inflammatory response of the skin induced by the inoculation of a plant mitogen (Martín et al. 2008). PHA influences various T cell-mediated immune responses and serves as an indicator of heightened immune cell activity. Consequently, PHA is frequently employed in evolutionary ecology studies as a gauge of immune response (Martin et al. 2008). For the inoculation, we administered 20-µL solutions containing 50 mg of PHA in 10 mL of phosphate-buffered saline (PBS) into the lizards' right hindlimb foot pad. We measured the thickness of the right hindlimb foot pad immediately before and 24 h after PHA inoculation, using the difference as the immune response (IR; Smits et al. 1999; Svensson et al. 2001). In the left hindlimb foot pad of each lizard, we inoculated an equivalent volume of buffered PBS as a sham control and took identical measurements. The sham control exhibited no significant change in thickness before versus after PBS inoculation (Wilcoxon paired test: z = 1.72, P = 0.88). All measurements were conducted in triplicate, and the mean was employed in the analyses. The coefficient of variation between measurements was 3.1%. Lizards did not appear to suffer obvious damage during this test, as they remained active and continued normal feeding and thermoregulatory behavior. When we finished collecting data on each lizard, they were kept in captivity for 2 days to allow them to recover before being released at their original collection site.
Statistical analyses
Before conducting any analyses, we checked the assumptions of normality and homogeneity of variance (Quinn & Keough 2002). We employed the ordinary least square linear model (OLSLM) to compare body mass, SVL, BCI, and immune response using site type (urban or non-urban) and sex (male or female) as fixed factors. Ectoparasite load was compared using a generalized linear model with a Poisson distribution and log as a linking function. Our data on hemoparasite load included many zero values (22/40 lizards), so we fitted a zero-inflated model using the “pscl” R package (Jackman 2012). We used site and sex as fixed factors and BCI as a continuous covariate. As patch coloration is dimorphic in S. torquatus, we analyzed spectral measures separately for males and females (Fig. 2). OLSLM was employed to compare luminance, UV, and blue chroma and hue with site type as a fixed factor and BCI as a covariate, but this variable was not significant for any model (results not shown). When we identified significant effects in OLSLM, GLM, or the zero-inflated model, we conducted Bonferroni post hoc comparisons using the “emmeans” R-package (Lenth 2018). We verified the distributions of all model residuals through visual inspection, utilizing the “DHARMa” R-package (Hartig 2021). Spearman's correlations were employed to assess the relationships between the color components and parasite loads or local immune response. All analyses were performed in R 4.2.0 (R Development Core Team 2017).
RESULTS
Body size and body condition
Morphological parameters and immune response of S. torquatus in urban and non-urban habitats are shown in Table 1. Lizards from the urban habitat exhibited larger SVL than non-urban lizards (F1,43 = 5.85, P = 0.019). Sex and the interaction sex × site type did not have significant effects on SVL (F1,43 = 2.26, P = 0.13 and F1,43 = 1.67, P = 0.20, respectively). The body mass of lizards from urban habitats was heavier than lizards from non-urban habitats (F1,43 = 4.85, P = 0.033). Sex and the interaction site type × sex did not significantly affect body mass (F1,43 = 2.98, P = 0.09 and F1,43 = 0.45, P = 0.50, respectively). No significant differences were observed in BCI across site type (F1,43 = 0.008, P = 0.99), sex (F1,43 = 0.007, P = 0.99), or their interaction (F1,43 = 0.007, P = 0.99).
| Urban | Non-urban | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Body mass (g) | 31.2 ± 2.4 | 29.1 ± 1.71 | 27.7 ± 2.32 | 22.6 ± 1.43 |
| Snout vent–length (mm) | 91.7 ± 2.91 | 92.4 ± 1.67 | 88.6 ± 2.36 | 81.9 ± 1.44 |
| Body condition index (BCI) | 1.33 × 10−6 ± 0.01 | 10.00 × 10−6 ± 0.01 | −2.89 × 10−19 ± 0.01 | 1.60× 10−5 ± 0.01 |
| Immune response (mm) | 0.0125 ± 0.002 | 0.0217 ± 0.001 | 0.0267 ± 0.002 | 0.0268 ± 0.003 |
- Mean ± standard error of the mean (SEM).
Parasite load and immune response
Mite infestation in pockets differed by site type (χ2 = 1019.45, df = 1, P < 0.001; Table 2), sex (χ2 = 22.86, df = 1, P < 0.001), and the interaction site type × sex (χ2 = 68.01, df = 1, P < 0.001). Urban males exhibited higher mite counts in pockets than urban females (z test = −4.73, P < 0.001) but lower than non-urban males (z test = 20.39, P < 0.001), while, non-urban males had higher mite counts in pockets than non-urban females (z test = 4.72, P < 0.001). Total mite counts also differed by site type (χ2 = 290.11, df = 1, P < 0.001), sex (χ2 = 548.11, gl = 1, P < 0.001), and the interaction site type × sex (χ2 = 20.11, df = 1, P < 0.001). Urban males had higher total mites than urban females (z test = −11.37, P < 0.001) but lower than non-urban males (z test = 32.73, P < 0.001), while males from non-urban habitats had higher total mite counts than non-urban females (z test = −22.74, df = 1, P < 0.001). Hemoparasite load did not differ by site type (χ2 = 0.08, df = 1, P = 0.87) but did differ by sex (χ2 = 17.75, gl = 1, P < 0.001). Males had higher hemoparasite load than females (z test = 2.79, P = 0.005). The interaction site type × sex was not significant (χ2 = 3.57, df = 1, P = 0.07). Urban male lizards with higher hemoparasite loads had lower body mass than males with fewer hemoparasites (R2 = −0.58, P = 0.02), but in non-urban lizards, we did not find this relationship (R2 = 0.009, P = 0.98). Immune response varied with site type; urban lizards exhibited lower immune responses than non-urban lizards (F1,42 = 19.38, P < 0.001). Immune response varied by sex; males had lower immune responses than females (F1,42 = 6.50, P = 0.015). The interaction site type × sex was not significant (F1,42 = 2.02, P = 0.16). In female non-urban lizards, the immune response was significantly negatively related to hemoparasite load (R2 = −0.9, P < 0.001); lizards with higher hemoparasite loads had lower immune response. This relationship was not found in male non-urban lizards (R2 = 0.28, P = 0.37) or in urban lizards (R2 = −0.18, P = 0.53)
| Mean ± SEM | Range | Prevalence | ||
|---|---|---|---|---|
| Mites pockets | ||||
| Urban | Male | 4.87 ± 2.2 | 0–30 | 53.3% |
| Female | 0.3 ± 0.2 | 0–2 | 20% | |
| Non-urban | Male | 61.7 ± 22.4 | 1–215 | 100% |
| Female | 81.3 ± 20.3 | 8–160 | 100% | |
| Total mites | ||||
| Urban | Male | 183 ± 42 | 5–536 | 100% |
| Female | 124 ± 28.2 | 33–280 | 100% | |
| Non-urban | Male | 400 ± 80.8 | 40–1046 | 100% |
| Female | 224± 37 | 43–400 | 100% | |
| Hemoparasites | ||||
| Urban | Male | 1.73 ± 0.7 | 0–9 | 46.6% |
| Female | 0.9 ± 0.6 | 0–6 | 30% | |
| Non-urban | Male | 5.58 ± 2.39 | 0–23 | 66.6% |
| Female | 1.9 ± 0.5 | 0–5 | 70% | |
- Intensity values are summarized as mean ± standard error of the mean (SEM) and range (minimum–maximum). Prevalence represents the percentage of lizards in each category with each kind of parasite infestation.
Male coloration
Throat luminance did not differ between urban and non-urban males (F1,23 = 2.90, P = 0.10). Urban lizards exhibited lower values of throat UV chroma than non-urban lizards (F1,23 = 5.26, P = 0.03; Fig. 3a) but higher values of throat blue chroma than non-urban lizards (F1,23 = 12.78, P = 0.001; Fig. 3b). Throat hue did not differ between urban and non-urban lizards (F1,23 = 0.11, P = 0.73). In non-urban lizards, we observed a marginally significant and negative relationship between throat UV chroma and mites in pockets (R2 = −0.68, P = 0.05); lizards with more mites had lower UV chroma. This relationship was not observed in urban lizards (R2 = −0.25, P = 0.4). For urban lizards, total mites were significantly and negatively related to UV chroma (R2 = −0.66, P = 0.01; Fig. 4a); lizards with higher total mites had lower UV chroma values. This relationship was not present in non-urban lizards (R2 = −0.48, P = 0.12). In non-urban lizards, total mites were significantly and positively related to blue chroma (R2 = 0.8, P = 0.003; Fig. 4b); lizards with higher total mites had higher blue chroma. In urban lizards, this relationship was not present (R2 = 0.37, P = 0.2).
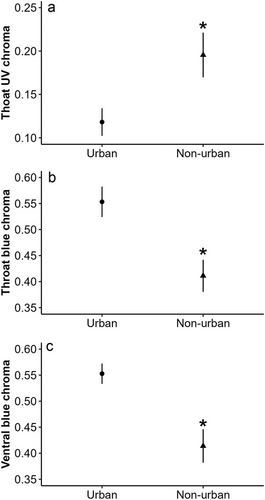
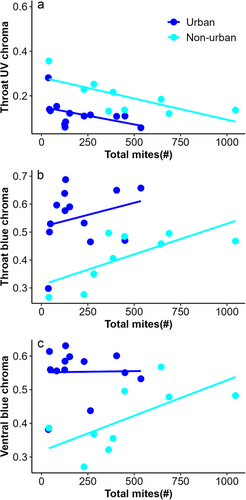
Ventral luminance and ventral UV chroma in urban lizards did not differ from non-urban lizards (F1,23 = 0.95, P = 0.33 and F1,23 = 3.02, P = 0.09, respectively). Urban lizards had a higher blue chroma than non-urban lizards (F1,23 = 12.69, P = 0.001; Fig. 3c). Ventral hue did not differ between urban and non-urban lizards (F1,23 = 0.006, P = 0.93). In non-urban lizards, we found a significant and negative relationship between total mites and ventral UV chroma (R2 = −0.62, P = 0.03); lizards with higher total mites had lower UV chroma. This relationship was not observed in urban lizards (R2 = −0.17, P = 0.55). Similarly, a significant and negative relationship was found between hemoparasite load and ventral UV chroma in non-urban lizards (R2 = −0.61, P = 0.03); lizards with higher hemoparasite loads had lower UV chroma. This relationship was not observed in urban lizards (R2 = 0.07, P = 0.8). Moreover, a significant and negative relationship was identified between total mites and ventral blue chroma in non-urban lizards (R2 = −0.62, P = 0.03; Fig. 3c); lizards with higher total mites had lower blue chroma. This relationship was not observed in urban lizards (R2 = 0.02, P = 0.95).
Female coloration
Female urban lizards exhibited higher throat luminance values compared to non-urban lizards (F1,17 = 55.07, P < 0.001). No differences were found in UV chroma (F1,17 = 0.009, P = 0.92) or blue chroma (F1,17 = 3.03, P = 0.09) between urban and non-urban lizards. However, urban lizards had a lower throat hue than their non-urban lizards (F1,17 = 6.69, P = 0.019). A marginally significant relationship was observed between body mites and throat UV chroma in urban lizards (R2 = −0.64, P = 0.05); lizards with higher total mites had lower UV chroma values. Interestingly, this relationship was not found in non-urban lizards (R2 = −0.38, P = 0.36).
Urban lizards exhibited higher ventral luminance than non-urban lizards (F1,17 = 38.01, P < 0.001). However, no differences were observed in ventral UV and blue chroma based on habitat (F1,17 = 0.005, P = 0.93 and F1,17 = 0.06, P = 0.79, respectively). Ventral hue also did not show differences based on habitat (F1,17 = 0.52, P = 0.48). Ventral spectral variables were not related to any parasite load measure (see Table S1, Supporting Information).
DISCUSSION
This is the first study to examine the effect of urbanization on sexual color expression in lizards, aiming to analyze the interplay between sexual coloration, parasite load, and immune response in both urban and non-urban lizard populations. Our results reveal that urban males of S. torquatus had bluer ventral chroma, lower parasite load, and lower immune response compared to their counterparts in natural habitats. Furthermore, male lizards in the natural environment demonstrated a negative correlation between blue chroma and parasite load, a relationship not observed in urban lizards. However, urban male lizards exhibited a negative correlation between UV chroma and total mites, a relationship not observed in non-urban lizards. Moreover, urban females display elevated ventral luminance compared to non-urban females, although this characteristic was not correlated with either parasite load or immune response.
In this study, urbanization was linked to an increase in body size, but body condition did not vary between urban and non-urban lizards. The increase in body size in urban lizards has been reported in other species and is commonly linked to factors such as habitat composition, food availability, and divergent predator communities (Prosser et al. 2006; Lazić et al. 2017; Gómez-Benítez et al. 2021). Habitat composition significantly influences lizard behavior and performance. In open habitats, lizards may counter higher predator exposure with enhanced running speed, often tied to increased limb length (Garland & Losos 1994; Cooper 1997; Vanhooydonck & Van Damme 1999). However, this relationship is not a common pattern across all lizard species (Prosser et al. 2006; González-Morales et al. 2021). Certain species, like Sceloporus occidentalis and Aspidoscelis costatus exhibit morphological adaptations based on the anthropogenic structures they encounter (Putman et al. 2019; Gómez-Benítez et al. 2021). Conversely, urban habitats are often characterized by reduced predator diversity, potentially leading to an increase in lizard body size due to the relaxation of selective pressures (Eötvös et al. 2018). The body condition of lizards closely correlates with health and fitness, serving as an indicator of nutritional and physical well-being (Labocha et al. 2014; Lazić et al. 2017). Contrary to our initial prediction, urban lizards did not exhibit a higher body condition index than their non-urban counterparts. Putman and Tippie (2020) found that urban lizards showed a higher body condition index compared to those in natural or non-urban habitats. Several non-exclusive hypotheses have been proposed to explain this discrepancy, ranging from increased foraging time to reduced predation pressure (Iglesias et al. 2012; Eötvös et al. 2018; Putman & Tippie 2020). In our study, we did not observe differences in the body condition index. This outcome could be attributed to the fact that we captured lizards during the fall in Mexico, a season when this species is in the reproductive phase. The associated costs linked to courtship behavior and territory defense during this period could limit foraging time (Rivera-Rea et al. 2022, 2024). To understand these lizards’ physiology better, we recommend studies quantifying the quantity and quality of prey in urban and non-urban environments. This information would shed light on how these factors may influence lizards' fat reserves.
Our second prediction was not fulfilled, because urban lizards had fewer ecto- and hemoparasites than non-urban lizards. The parasite–host relationship is not immune to the impacts of urbanization, and outcomes can vary across different taxa, resulting in either an increase or decrease in parasite intensity (Martin et al. 2010). In our study, we observed that urban lizards harbor fewer ecto- and hemoparasites compared to their non-urban counterparts. Factors such as habitat fragmentation, reduced environmental humidity, and increased environmental temperatures in urban settings may contribute to a decreased probability of parasite survival (Schall 1992; Dobson et al. 1992; Riley et al. 2014; Thawley et al. 2019). Additionally, the absence of intermediate hosts in the life cycle of parasites could further diminish the likelihood of infecting urban lizards (Civitello et al. 2015). In natural or preserved environments, dense vegetation cover and high relative humidity elevate the probability of infection in lizards, conditions rarely found in urban areas (Rivera-Rea et al. 2022). While the specific mechanisms by which urban lizards manage to evade high parasite loads remain unclear, there is mounting evidence of parasites' detrimental effects on the quality of signals in vertebrates. Generally, parasites activate the immune system, triggering a resource reallocation that may influence other traits, such as the expression of sexual signals (López et al. 2009; Plasman et al. 2015; Rivera-Rea et al. 2024). Our results indicate that lizards from natural habitats exhibit lower expression of the blue coloration in the ventral zone, coupled with a higher parasite load, possibly indicative of maintaining a robust immune response. Interestingly, lizards from natural areas displayed a higher number of parasites in the mite pockets compared to urban lizards. These mite pockets, characterized by skin invagination, have been associated with effective mite infestation control (Arnold 1986; Salvador et al. 1999). The skin within these structures is hyperplastic and resilient, featuring an accumulation of lymphoid cells that facilitate rapid tissue repair and infection relief (Arnold 1986). Future studies should explore whether these structures influence the honesty of visual signals in lizards.
Vertebrates, including lizards, employ two distinct pigment types to generate sexual coloration, and these may be differentially influenced by urbanization (McGraw 2006a,b). Coloration based on carotenoids cannot be produced endogenously by vertebrates and must be acquired through diet, making it a reliable indicator of body condition and foraging efficiency (McGraw 2006a; Biard et al. 2017). In contrast, structural (or melanin) coloration can be synthesized endogenously by animals and is considered less condition-dependent and more genetically controlled than carotenoid coloration (McGraw 2006b; Roulin 2016). However, structural coloration is energetically costly to produce, limiting its expression to individuals in good condition (McGraw 2006b; Roulin 2016). The throat and ventral patch coloration of S. torquatus exemplifies structural coloration and has been linked to high male quality (Rivera-Rea et al. 2024). In this study, male lizards from urban sites exhibited a higher blue chroma (i.e. more intense blue coloration) than non-urban males. Urban pollution, particularly with metals, can favor darker or bluer coloration phenotypes. Metal pollution may drive blue coloration by increasing circulating testosterone, and some metals such as zinc and calcium can also serve as micronutrients supporting melanogenesis (Laskey & Phelps 1991; Dauwe & Eens 2008; Pacyna et al. 2018). This study is the first to test the interaction of sexual coloration and immune functions in urban and rural lizards for the first time. However, it has been a more recurrent subject in other vertebrates, such as birds (Grunst et al. 2020). In great tits (Parus major), anthropogenic pollution adversely affects carotenoid coloration but not structural coloration (Grunst et al. 2020). Generally, structural coloration is more prevalent in urban sites, as reported by Leveau (2021).
An additional explanation for our results is that urban lizards, with lower parasite loads, may allocate more resources to coloration, resulting in more intense (blue) coloration (Rivera-Rea et al. 2024). In contrast, non-urban lizards, with a higher number of parasites and a robust immune response, may compromise the expression of the blue color (López et al. 2009; Megía-Palma et al. 2021). Our results indicate differential correlations between ectoparasite load and color components in urban versus non-urban environments. In urban lizards, ectoparasite load is related to UV chroma, while in non-urban lizards, it is related to blue chroma. Selective pressures differ between urban and non-urban environments (Sol et al. 2011, 2013; Le Roux et al. 2014; Battles et al. 2018); factors such as predation, parasite type, and species, and pollutants can greatly influence the development of sexual coloration (McLean et al. 2015; Megía-Palma et al. 2016, 2018; Marín-Gómez & MacGregor-Fors 2021; Smit et al. 2022). Further research into signal expression in lizards from urban and non-urban environments is needed to understand better the causes and consequences of the shifts in color components.
An alternative explanation for the observed phenotypic differences between urban and non-urban lizards could be intrinsic variation between populations unrelated to urban environmental pressures (Smyth et al. 2014), although we attempted to minimize this possibility by choosing locations that were geographically close together and located at similar elevations. Another possibility is that only those lizards with high levels of resistance to parasites succeed in colonizing urban environments, founding populations with a high level of local adaptation to parasites, which may subsequently affect the sexual signals present in those populations (Fricke et al. 2010; Gómez-Llano et al. 2020). Finally, the structural arrangement producing the blue color could be subject to developmental modifications, independent of urbanization effects (Jeon et al. 2021; Radovanović et al. 2023). Future studies should include replicate populations to determine if this variation is consistent between environments (Macotela et al. 2023) and utilize common garden experiments to rear lizards from birth under controlled conditions to verify whether color expression remains constant
Our third prediction rested on the expectation that females would not show differences in ventral coloration. This expectation arises from the species-specific feature where males are the primary developers of ventral blue coloration. Our result showed that female urban lizards exhibited higher throat and ventral patch luminance than non-urban female lizards. Research related to sexual coloration often focuses on males, who are generally the ones who express or exaggerate these traits. However, females in many species where males are more brightly colored also use coloration as an indicator of quality and for communication (Kraaijeveld et al. 2007). Females with less intense orange coloration in Alpine newt (Ichthyosaura alpestris) lay eggs more slowly compared to females with more intense orange coloration (Lüdtke & Foerster 2019), while in other species such as convict cichlids (Cichlasoma nigrofasciatum), coloration has been related to levels of aggressiveness (Beeching et al. 1998). In our study, none of the color components was related to the traits we evaluated; however, this does not mean that it does not have a function as such. In juvenile lizards of Sceloporus undulatus, females exhibit a coloration similar to that of males, albeit less intense. This phenomenon has been associated with low levels of corticosterone, which may have implications for the quality of offspring (Assis et al. 2021), so it is necessary to reconsider what this female coloration function could be in future studies.
In conclusion, our results indicate significant differences in color expression, parasite load, and immune response between urban and non-urban lizards. Examining a broader range of species and phenotypic traits in reptiles is essential to understanding the patterns facilitating their colonization and persistence in urban environments. This knowledge will enhance both conservation strategies and urban development plans, ensuring better integration of wildlife considerations in city planning.
ACKNOWLEDGMENTS
Many thanks go to Rodolfo Rodríguez and Edgar Oviedo for field assistance. We are grateful to the Consejo Mexiquense de Ciencía y Tecnología (COMECYT) for the degree scholarship “Programa de Investigadoras e Investigadores COMECYT” for J.C.G.-M. (RCAT2024-0011), J.R.-R. (CAT2024-0062), and G.S.-V. (ESYCA2023-141617). This research was partially financially supported by the Universidad Autónoma Metropolitana–Lerma through project 54302003.
Open access publishing facilitated by Universidad Autónoma Metropolitana, as part of the Wiley–BIDIUAM agreement.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.




