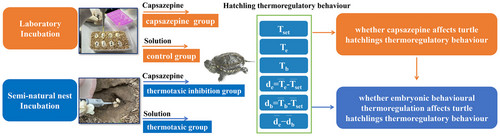
Does embryonic behavioral thermoregulation enhance thermoregulatory capacity of turtle hatchlings?
Graphical Abstract
We found that embryonic behavioral thermoregulation could not enhance the thermoregulatory capacity of turtle hatchlings. Our study is not only the first to provide experimental evidence regarding the impact of embryonic behavioral thermoregulation on offspring thermoregulation but also falsifies the play behavior hypothesis that suggests thermotaxis by embryos allows them to practice thermoregulatory tactics at later life stages.
Dear Editor,
Traditional studies on animal behavior mainly focus on the post-hatching phase of the life history, assuming that embryos are works-in-progress, despite most animals spending a significant time developing embryos inside an egg or mother's body (Du & Shine 2022). Although embryos are thought to be passive to their environment, several studies have demonstrated that behavioral thermoregulation occurs in embryonic reptiles and birds (Du et al. 2011; Zhao et al. 2013; Li et al. 2014). Behavioral thermoregulation may enable embryos to develop at suitable temperatures, thereby increasing their developmental success (Du et al. 2011). Second, behavioral thermoregulation by embryos is expected to produce hatchlings with better performance and higher fitness (Ye et al. 2019; Liu et al. 2023). For example, in species that exhibit temperature-dependent sex determination, behavioral thermoregulation may enable embryos to select suitable temperatures to fine-tune sexual differentiation, thereby expanding the range of nest temperatures that produce equal offspring sex ratios (Ye et al. 2019). However, experimental evidence on how embryonic behavioral thermoregulation affects offspring traits and thereby fitness is still limited. In this study, we experimentally manipulated embryo thermotaxis to test whether embryonic behavioral thermoregulation affects the thermoregulatory behavior of offspring. We used the drug capsazepine to block the ion channels that constrain thermotaxis in turtle embryos and measured the thermoregulatory parameters of the hatchlings in laboratory and semi-natural settings in a semi-aquatic turtle (Mauremys reevesii), in which the embryo exhibited behavioral thermoregulation in the semi-natural nests (Ye et al. 2019).
To determine whether capsazepine affects the thermoregulatory behavior of M. reevesii hatchlings, we collected 60 freshly laid turtle eggs from a turtle farm in Jiaxing (30.77°N, 120.88°E), Zhejiang Province, China. All eggs were selected from the different clutches and immediately transported to our laboratory. Eggs were weighed (ML204, Mettler Toledo, Switzerland, ±0.001 g) and incubated individually in 80-mL jars containing moist vermiculite (−220 kPa). The eggs were assigned to a constant temperature of 29°C, which produces an even hatchling sex ratio in M. reevesii (Ye et al. 2019) in a Binder incubator (KB400, Binder GmbH, Tuttlingen, Germany). When embryos reached stage 13, half of the eggs were randomly selected to be treated with 5 µL of capsazepine (0.37 µg µL−1; MedChemExpress, China) dissolved in a solution of 80% saline, 10% ethanol (EtOH, Sigma-Aldrich, USA), and 10% Tween80 (Amresco, USA) (capsazepine group); the other half of eggs were treated with an equal amount of the solution without capsazepine (control group). Sixteen hatchlings from each treatment were used to measure their selected body temperature in a thermal gradient inside the laboratory and field body temperatures in three outdoor enclosures (see details in Materials and Methods in Supporting Information). We found capsazepine did not affect the upper limit, lower limit, or mean of the selected body temperatures (Table S1, Supporting Information). Capsazepine did not affect the Tb of hatchlings in the field enclosures (F1,20.1 = 0.087, P = 0.772), although Tb fluctuated throughout the day (F8,121.7 = 56.413, P < 0.001) (Fig. S1, Supporting Information). In addition, capsazepine did not affect the thermoregulatory accuracy (db) or effectiveness (de − db) of the hatchling turtles (Table S1, Supporting Information).
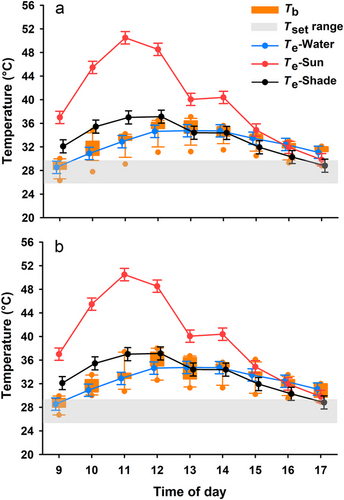
To determine whether embryonic behavioral thermoregulation could influence hatchling thermoregulatory behavior, we conducted a semi-natural incubation experiment in an outdoor pond (10 × 10 m) with breeding turtles at a turtle farm in Jiaxing. In late May 2021, 30 nests were established, and a split-clutch design was used to randomly apply capsazepine (thermotaxic inhibition group) or solution (thermotaxic group) to eggs within each clutch, as described above for the laboratory experiments. Embryonic behavioral thermoregulation did not affect the selected body temperatures of hatchling turtles (Table 1). The mean Tb of hatchlings in field enclosures did not differ between the thermotaxic inhibition and thermotaxic groups (F1,32.9 = 0.061, P = 0.806) and showed similar fluctuation patterns (F8,173.2 = 0.441, P = 0.895), although Tb fluctuated on a daily basis (F8,173.2 = 145.036, P < 0.001; Fig. 1). Moreover, we did not detect significant differences in the thermoregulatory accuracy or effectiveness of hatchlings between the thermotaxic inhibition and thermotaxic groups (Table 1).
| Thermotaxic group | Thermotaxic inhibition group | Statistical results | |
|---|---|---|---|
| Tset upper limit (°C) | 29.4 ± 1.1 | 28.9 ± 1.1 | F1,18.1 = 0.176, P = 0.680 |
| Tset lower limit (°C) | 25.9 ± 0.8 | 25.0 ± 0.8 | F1,30 = 0.585, P = 0.451 |
| Tset mean (°C) | 27.6 ± 0.9 | 27.2 ± 0.9 | F1,18.7 = 0.170, P = 0.685 |
| de | 5.9 ± 0.7 | 6.4 ± 0.7 | F1,52 = 0.247, P = 0.621 |
| db | 3.3 ± 0.3 | 4.0 ± 0.3 | F1,25.8 = 2.490, P = 0.127 |
| de − db | 2.3 ± 0.2 | 2.6 ± 0.4 | F1,23.2 = 0.755, P = 0.394 |
Our findings are contradictory to the prediction of the play behavior hypothesis, which suggests that the thermotaxic behavior of embryos should promote the thermoregulatory capacity of hatchlings (Telemeco et al. 2016; Cordero et al. 2018). Therefore, our results do not support the suggestion that thermotaxis by reptile embryos may represent play behavior rather than thermoregulatory behavior (Telemeco et al. 2016). Instead, the thermotaxic behavior represents behavioral thermoregulation in its own right, which benefits embryos themselves and offspring as well, because this behavior of reptile embryos can improve the developmental rate, hatching success, and hatching synchronization of embryos and affect the offspring sex ratio (Du et al. 2011; Ye et al. 2019; Liu et al. 2023).
Why does embryonic behavioral thermoregulation not affect hatchling thermoregulatory capacity? This could be due to the small change (<1.5°C) in development temperatures induced by behavioral thermoregulation of reptile embryos (Ye et al. 2019). The change is too insignificant to affect thermoregulatory tactics of hatchlings compared with those incubation experiments showing significant temperature effects at a larger difference of over 4°C (Blouin-Demers et al. 2000). Ultimately, it is reasonable that embryonic behavioral thermoregulation does not affect offspring thermoregulation in reptiles because this embryonic behavior is absent in small lizards (Li et al. 2014), which consist of over 60% reptile species and highly rely on behavioral thermoregulation to achieve suitable body temperatures. Given that many reptiles need to behaviorally thermoregulate immediately after hatching (Deeming 2004), it is likely that the sensory-motor navigation system for thermotaxis developed successfully during the embryonic stages and subsequently triggered thermoregulation behavior in ectotherms (Mori & Ohshima 1995; Ye et al. 2021). Nonetheless, given that hatchling thermoregulatory behavior was assessed at approximately 4 weeks after hatching, our study cannot exclude one possibility that the influence of embryonic behavioral thermoregulation on hatchling thermoregulatory behavior might be transitory, that is, present in newly hatched hatchlings but absent in older hatchlings. Therefore, additional tests on younger hatchlings, such as 1-week or even 1-day-old, are necessary to validate this hypothesis.
Notably, the developmental temperature of embryos significantly affects the selected body temperature of hatchlings in some reptile species. For example, juveniles from eggs incubated at colder temperatures have higher body temperatures than those from eggs incubated at warmer temperatures in some turtles and lizards (O'Steen 1998; Qualls & Andrews 1999; Rhen & Lang 1999), but this pattern is reversed in some snakes and crocodiles (Lang 1987; Blouin-Demers et al. 2000). In contrast, embryonic developmental temperatures did not affect selected body temperatures of hatchlings in some reptiles, such as the black rat snake (Elaphe obsoleta), the veiled chameleon (Chamaeleo calyptratus), desert tortoise (Gophorus agassizii), and the eastern three-lined skink (Bassiana duperreyi) (Spotila et al. 1994; Blouin-Demers et al. 2000; Andrews 2008; Du et al. 2010). In addition, embryonic developmental temperatures affect not only selected body temperatures but also the thermoregulatory precision of hatchlings. For instance, hatchlings from eggs incubated at lower temperatures thermoregulate more precisely (i.e., lower standard deviations of observed body temperatures) in one lizard (Sceloporus virgatus) but less precisely in another species of Sceloporus lizard (S. occidentalis) (Buckley et al. 2007). The causes of these between-species differences in the effects of developmental temperature on offspring thermoregulatory capacity are unknown but could be related to different temperature treatments between studies and potential between-sex differences in thermoregulatory capacity in species with temperature-dependent sex determination. In addition, most of the above studies were constant-temperature incubation experiments in the laboratory; further ecologically relevant experiments or field experiments are needed to determine whether developmental temperatures affect offspring thermoregulation.
In conclusion, we found that embryonic behavioral thermoregulation could not enhance the thermoregulatory capacity of turtle hatchlings. Our study is not only the first to provide experimental evidence regarding the impact of embryonic behavioral thermoregulation on offspring thermoregulation but also falsifies the play behavior hypothesis that suggests thermotaxis by embryos allows them to practice thermoregulatory tactics at later life stages.
ACKNOWLEDGMENTS
We thank Yanxuan Zhu, Shurui Xu, and Hang Liu for their assistance with laboratory work. Weiguo Du was supported by funding from the National Natural Science Foundation of China (Grant Nos. 32030013 and 31821001). Yongpu Zhang and Shuran Li were supported by funding from the National Natural Science Foundation of China (Grant Nos. 31971419 and 32171486).
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
ETHICS STATEMENT
This study was under the supervision of the Animal Ethical and Welfare Committee of Wenzhou University (Approval No. WZU-049).