Cryptic diversity begets challenges and opportunities in biodiversity research
Abstract
How many species of life are there on Earth? This is a question that we want to know but cannot yet answer. Some scholars speculate that the number of species may reach 2.2 billion when considering cryptic diversity and that each morphology-based insect species may contain an average of 3.1 cryptic species. With nearly two million described species, such high estimates of cryptic diversity would suggest that cryptic species are widespread. The development of molecular species delimitation has led to the discovery of a large number of cryptic species, and cryptic biodiversity has gradually entered our field of vision and attracted more attention. This paper introduces the concept of cryptic species, how they evolve, and methods by which they may be discovered and confirmed, and provides theoretical and methodological guidance for the study of hidden species. A workflow of how to confirm cryptic species is provided. In addition, the importance and reliability of multi-evidence-based integrated taxonomy are reaffirmed as a way to better standardize decision-making processes. Special focus on cryptic diversity and increased funding for taxonomy is needed to ensure that cryptic species in hyperdiverse groups are discoverable and described. An increased focus on cryptic species in the future will naturally arise as more difficult groups are studied, and thereby, we may finally better understand the rules governing the evolution and maintenance of cryptic biodiversity.
Biodiversity deeply influences the lives of human beings (Díaz et al. 2006). Ecosystem services, the ways other species benefit humankind, are in many ways reliant on species biodiversity, as different species contribute in different ways. Yet, our understanding of global biodiversity remains incipient for many groups, such as arthropods (Stork 2018; Pfingstl et al. 2021).
How many species are there on Earth? In answering this question, the taxonomic challenges posed by cryptic/hidden species are non-negligible, resulting in variable estimates of species richness (Costello et al. 2013). One of the earliest such estimates was 0.4 million, made by Westwood (1833), followed much later by some estimates as high as 30 million or more (Erwin 1982), and more recent estimates have eventually settled on 8.7 million (Mora et al. 2011) and 5.5–8 million species (Stork 2018). Now, we face another wave of even higher estimates, reaching up to 2.2 billion (Li & Wiens 2022). However, as of 2021, roughly 2 million species have been formally described and named (Bánki et al. 2021), and half of them are insects. Even less than 1% of biodiversity may have been described, depending on which estimate of species richness is correct (Laurance 2013; Havermans 2016; Bánki et al. 2021; Li & Wiens 2022). As well, discovering cryptic species will prove essential to estimate the accurate number of species existing on Earth.
With the development of molecular biology technologies, multiple species that could not be discerned via morphology have been identified, and additional difficult species complexes also have become tractable. The concepts of “cryptic/hidden species” and “cryptic/hidden biodiversity” consequently came into being. Li and Wiens (2022) suggest that cryptic species may be widespread and common: that each morphology-based insect species may contain an average of 3.1 cryptic species. For hyperdiverse taxa such as insects, it is to be expected that discovery would be a multi-step process requiring such re-examination. The distribution of cryptic species diversity seems unlikely to be random, and this has important implications for speciation, evolution, biodiversity assessment, and conservation (Schönrogge et al. 2002; Lee & Ó Foighil 2004; Geml et al. 2006; Williams et al. 2012).
This review will introduce the concept and progress of their study, how they are thought to evolve, relevant discovery and research methods, and their significance. Our aim is to convince researchers to pay more attention to cryptic biodiversity and to more deeply understand evolutionary processes.
THE CONCEPT OF CRYPTIC SPECIES
The concept of cryptic species is considered an extension of that of species, and the prerequisite for defining a cryptic species entails defining “species.” In total, more than 30 species concepts were provided for respective purposes (Wilkins 2006, 2009; de Queiroz 2007; Orr et al. 2022). Among them, the biological species concept is the most well-known and widely used, stating that a species is a group of individuals that are reproductively isolated from other groups (Mayr 1963, 1982). The most commonly used concept of a cryptic species is also largely based on the biological species concept, defined as “species that are morphologically similar to known ones but have developed reproductive isolation” (Knowlton 1993; Adams 1998). In addition, some scientists agree the concept of cryptic species and the concept of species are incompatible based on points that “cryptic” itself derives from morphology and many species concepts do not include morphological evidence (e.g. Heethoff 2018; Pfingstl et al. 2021). Thus, we generally accept the theoretical underpinnings of the biological species concept as it pertains to the concept of cryptic speciation, which is the base of this review, although there are long-known major issues in its implementation that prevent its universal application (Ehrlich 1961; Sokal & Crovello 1970; Stamos 2003; de Queiroz 2007; Liu 2016; Orr et al. 2022). Specifically, in this review, we modified the concept of cryptic species as follows: “species owning subtle morphological traits but genetic divergence from the known species” to weaken “reproductive isolation.”
DESCRIPTION AND DEVELOPMENT OF CRYPTIC SPECIES
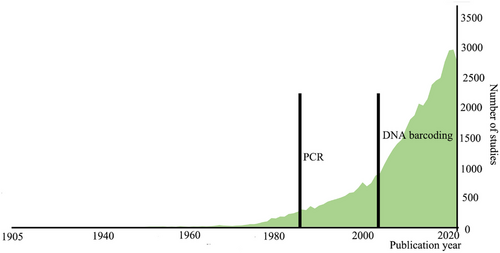
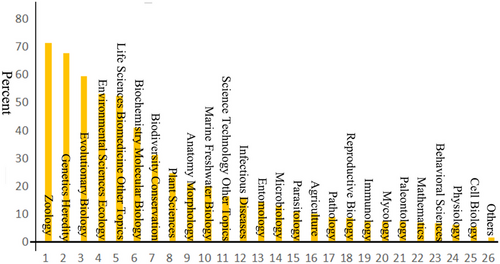
Interestingly, the idea of cryptic species has a long history. William Derham's 1718 study of the bird genus Phylloscopus (Phylloscopidae) may have reported the very first potential cryptic species (Winker 2005). Until the early 2000s, there were few studies about cryptic species, however (Fig. 1). After that, a series of researchers proposed “DNA barcoding” and explicitly extended it to the study of cryptic species (Tautz et al. 2002; Hebert et al. 2003a,b; Blaxter 2004; Xu & Che 2019). This was then a critical time node in the development history of the cryptic species research, being supported by Web of Science queries. Using the keywords “cryptic/hidden species” etc. to search, we found that most (around 95%) of the related research literature was published after this period, beginning to grow rapidly around 2006. Usually, the discovery and confirmation of cryptic species are byproducts of other related biological fields, mainly including zoology, genetic heredity, and evolutionary biology (Fig. 2). Currently, the study of cryptic species has attracted the attention of more biologists, opening a new window for understanding the mechanisms behind speciation and injecting new ideas, methods, and rigor into biodiversity science.
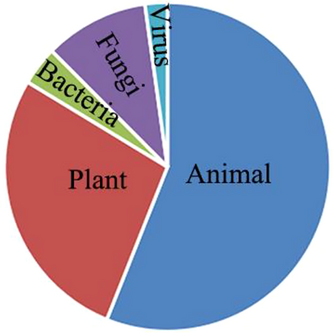
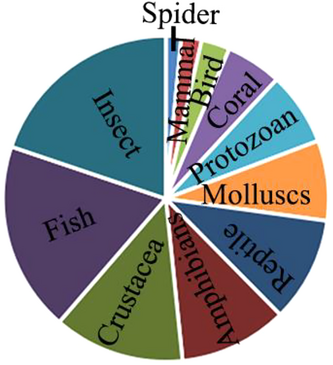
Among all the groups of organisms, one significant bias is the preponderance of animals in published papers (Fig. 3), and almost all animal taxa include cryptic species (Fig. 4). Summarizing these papers, several animal groups were thought to own relatively more common cryptic species, for example, protozoans, arthropods, and frogs. Protozoans, as a paraphyletic conglomeration of single-celled animals, are widely present taxa with many cryptic species due to the lack of clear defining morphological features and the difficulty of collection (Gong et al. 2013; Fan et al. 2021; Liu et al. 2022). Another fascinating group of cryptic diversity is arthropods, many groups of which can be considered as a “taxonomist's nightmare” (Bickford et al. 2007; Pfingstl et al. 2021). Among them, there are groups where the majority of species are likely to be undescribed and difficult to tell apart (Chimeno et al. 2022). There are many possible ways that species might be cryptic, based on characters or habits in addition to typical morphological differentiation. Frogs and some other groups, for example, likely exhibit many cryptic species based on mate selection systems relying on non-morphological features, such as songs (Narins 1983).
HYPOTHESES OF CRYPTIC SPECIATION
In this review, we mainly focus on morphology-conserved cryptic species and how they are formed, which can be best understood as a “special speciation mechanism.” Here, we summarize four non-mutually exclusive evolutionary mechanisms that have been hypothesized to lead to possibly non-random cryptic species (Bickford et al. 2007; Kress et al. 2015; Struck & Cerca 2019; Pfingstl et al. 2021).
Recent divergence
The simplest explanation for cryptic speciation is that they have simply not had time to develop differences in all forms, sometimes called “species on the road to differentiation” (Liu 2016). Species identifications that rely solely on morphology may overlook such recently diverged species (Holland et al. 2004), especially if performed by a non-expert. This idea is based on the notion that morphological traits should follow a neutral evolutionary model (i.e. that morphological differences accumulate linearly over time; Felsenstein 1985). It is posited that in the early stages of speciation, selection pressure may act on physiological, reproductive, behavioral, niche, and other traits, and the effect occurs at a relatively late stage for morphological characteristics (Damm et al. 2010; Cheng et al. 2016; Derycke et al. 2016). There are many related examples. In the case of the genus Rattus (Muridae), which has undergone a recent species radiation (Middle Pleistocene), morphological differences between clades are very small (Rowe et al. 2011). Given the commonness of this phenomenon, much research has gone into alternative lines of evidence.
Molecular evidence in many cases may show significant differentiation in the absence of distinctive morphological characters, resulting in splitting at various levels, such as for insects (Chesters et al. 2012; Jiang et al. 2014; Cheng et al. 2016; Zhou et al. 2017), mammals (Andersen & Light 2012), and protozoans (Shao et al. 2019). For example, the formation of a large number of alpine lakes in the Qinghai–Tibet Plateau and Alps during recent interglacial cycling drove the differentiation of the amphipod Gammarus lacustris; the morphology of each clade was similar, but the genetic distances between clades ranged from 2% to 8% for mitochondrial DNA (Hou et al. 2022).
Some additional special evolutionary phenomena may occur in the early stages of speciation that can profoundly influence morphological evolution and complicate species delineation, such as budding speciation (a consequence of incomplete lineage sorting; Harrison 1991; Funk & Omland 2003; Kruckenhauser et al. 2014; Cheng et al. 2017) and hybridization (Mallet 2005; Weigand et al. 2017). For example, a study of one Tibetan Plateau frog species (Scutiger mamatus) found that both budding speciation and incomplete lineage sorting prompted the formation of a cryptic species (Chen et al. 2009). During hybridization, intermediate morphological populations with both parents' genetic traits in a hybrid zone may form cryptic species (Barton & Hewitt 1985; Harrison 1993; Wu et al. 2011; Gong et al. 2013; Wang et al. 2014).
Morphological stasis
Studies have found that the process of speciation is not necessarily accompanied by morphological changes. In addition to more straightforward biological reasons, such as low mutation rates, or developmental and physiological limitations, selection pressures are important potential causes of morphological stasis (Grundt et al. 2006; Struck & Cerca 2019; Pfingstl et al. 2021). In some environments, selection pressures mainly act on traits such as behavior and physiology, while morphological traits that are not closely related to survival evolve very slowly, and thereby do not necessarily change with speciation. In some parasitic insects, for example, selection pressures may primarily act on traits related to parasitism (Schönrogge et al. 2002), such as the ability to detect hosts or counteract their defenses, while morphological traits unrelated to parasitism may experience morphological stagnation, as changes in them might reduce their parasitic efficiency (Chen 1946; Kaleka et al. 2020).
Morphological stasis is especially common in extreme environments, where organisms do not have many options for adaptation when dealing with harsh living conditions, and physiology and related morphological traits are strongly constrained (Rothschild & Mancinelli 2001; Nevo 2001). This explains why large numbers of cryptic species are found in habitats such as Arctic tundra, underwater karst landforms, caves, and deep-sea environments (Vrijenhoek et al. 1994; Witt et al. 2006; Lefébure et al. 2006; Hou & Li 2010). Cave spiders, for instance, show little morphological variation, due to adaptations such as eye degradation, light body color, and slender legs, and there are consequently many cryptic species only discovered with extensive examination (Ba & Lai 2009). Indeed, species complexes are fairly common in cave ecosystems (Trontelj 2009; Derkarabetian et al. 2010; Hedin 2015; Delić et al. 2017).
Traits may also be strongly conserved by behaviors, such as the use of narrow cavities as nests by the bee family Megachilidae: Nearly all species that make nests carry their pollen on their underside rather than on their legs (Michener 2007), assumedly to avoid pollen rubbing off their legs in these confining environments. Many of the traits that are needed to survive in such specific environments are interlinked with ancillary traits, ultimately inhibiting changes in them that might impact the functionality of the traits needed to survive. Under such circumstances, it is no surprise that morphological stasis may occur.
Nonvisual signals
Cryptic species are commonly found in organisms that distinguish each other by nonvisual signals (Mayr 1963; Ruxton 2009; Cooke et al. 2012). Nonvisual mating signals (i.e. acoustic signals, chemical mating signals) have been used to identify insect relatives or cryptic species (Henry 1994; Marshall & Cooley 2000; Angulo & Reichle 2008; Martinet et al. 2019).
Acoustic signals, such as chirping, are commonly used to distinguish between birds (Cicero 1996; Lei & Payne 2002; Yang & Lei 2008), frogs (Narins 1983), insects (Henry 1994; Henry & Wells 2010), and bats (Jones & Barlow 2004). Perhaps the most famous examples come from birds, where songs play a central role in reproductive isolation between species (Yang & Lei 2008; Lei et al. 2003). Based on differences in songs, several cryptic species were identified, such as the Himalayan cuckoo (Cuculus saturates) and Horsfields cuckoo (C. saturatus and C. horsfieldi) (Lei & Payne 2002). Another important case is among insect songs, which can help entomologists discover cryptic species. For example, two cryptic sibling species of Chrysoperla green lacewings were supported by acoustic niche partitioning (Henry et al. 2003; Henry & Wells 2010).
Convergent evolution and parallel evolution
Although the concepts of convergent and parallel evolution are distinguishing, they can lead to similar morphological characteristics, which are often difficult to distinguish (Belyaeva & Taylor 2009; Pearce 2011; Struck & Cerca 2019). Unlike the recent divergence of speciation hypothesis, where cryptic species are closely related sisters, the convergent evolution hypothesis invokes distantly related species that evolve similar morphological characteristics independently (Holland et al. 2004; Struck & Cerca 2019), such as the evolution of wings in birds, bats, and insects. Similarly, Moen et al. (2013) suggest convergent evolution led to a striking similarity in the morphology of frogs. The convergent evolution hypothesis states that some cryptic species have evolved independently from morphologically distinct ancestors (Swift et al. 2016; Struck et al. 2018). In the early stages of differentiation, such species exhibit similar rates of morphological differentiation. However, at some point in time, some species began to converge in their morphological trajectories, forming cryptic species.
In contrast, the parallel evolution hypothesis involves related species, where descendants of a common ancestor adapt to similar living environments and independently develop similar morphology (Holland et al. 2004; Pearce 2011). A typical example of this hypothesis is that Australian marsupials have similar counterparts to true mammals in Eurasia (Weisbecker & Archer 2008). In an additional example, Yamamoto and Sota (2007) found that wingless females of the family Geometridae evolved independently in three subfamilies, to adapt to climate cooling events in the Oligocene and Early Pleistocene.
DISCOVERY AND CONFIRMATION OF CRYPTIC SPECIES
Technological advances in molecular biology have been central to the study of cryptic species, and a workflow of how to confirm cryptic species derived from molecular evidence is given in Fig. 5. This includes work based on a wide variety of data types and densities, including single mitochondrial genes (COIs), multiple mitochondrial genes, whole mitochondrial genomes, reduced-representation genomes, and genome-wide data (e.g. SNPs). At the same time, morphologists have made great progress in the last several decades, transitioning from classical anatomical morphological characteristics to more detailed morphological studies based on geometric morphometrics, electron microscopy scanning, and micro-CT.
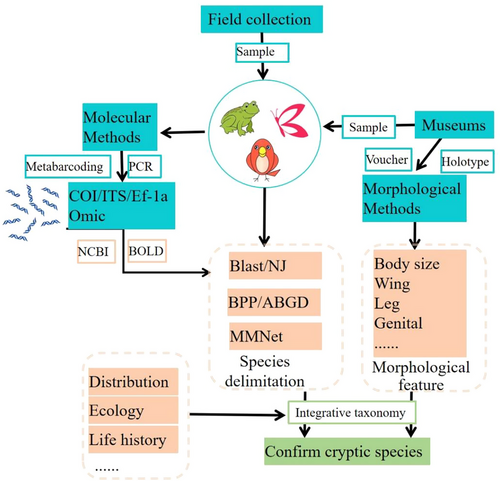
Integrative taxonomy, combining molecular, morphological, ecological, and other sources of evidence, has become key to the synthesis and use of such disparate data types, enabling more effective means of exploring biodiversity and discovering and delimiting cryptic species (Dayrat 2005; Padial et al. 2010; Hartop et al. 2022; Orr et al. 2022). Kergoat et al. (2015) combined ecological, morphological, and molecular data to discover five cryptic species within it, providing a sounder theoretical basis for the precise control of pest species in this genus. In another example, it was long thought that there were only 10 species of the common Chinese white-bellied rat genus Niviventer. However, by integrating external and craniodental measurements with multi-locus molecular data, Ge et al. (2018, 2021) delimited 18 species in China alone. However, the ways in which these data are used, including how different lines of evidence are weighed, can have a substantial impact on the results of such biodiversity investigations. It is therefore vital to understand both the data and how they can reasonably be used.
Here, we focus our methodological review primarily on molecular data and how these may be used to explore cryptic species and cryptic biodiversity.
Single locus or multiple loci DNA barcoding
The most revolutionary development in the study of cryptic species has been the widespread adoption of molecular methods spurred by DNA barcoding and similar initiatives for a system of DNA taxonomy (Hebert et al. 2003a; Tautz et al. 2003). DNA barcoding technology was first proposed in 2003 to rapidly discover cryptic species, delimit species, and reveal their genetic structure through a gene fragment that is highly variable (quickly evolving) and relatively easy to amplify for many animal taxa (Hebert et al. 2003a,b, 2004; Xiao et al. 2004; Peng et al. 2008; Liu et al. 2010). The agreed-upon animal marker, mitochondrial cytochrome c oxidase subunit I (COI), has been widely used in invertebrates, birds, fish, and beyond (Hebert et al. 2003a,b, 2004; Costa et al. 2007; Liu et al. 2010; Shen et al. 2019; Xiong et al. 2022). As an independent line of evidence, COI and similar markers, such as 16S rRNA for bacteria, provide an invaluable link between life stages and dimorphic sexes, while also making it easier to account for complexities such as phenotypic plasticity.
Driven by the International Barcode of Life (iBOL) project and immense community inputs, the BOLD website has so far collected 15 466 718 animal barcode sequences from 247 000 species as of February 7, 2023, with arthropods (13 899 279) representing 89.86%. Based on barcode technology, many cryptic species have been found in almost all animal groups (Hebert et al. 2004; Costa et al. 2007; Wang et al. 2020; Yu & Wang 2021). More recently, the approach has been extended to other braconid wasps to hasten biodiversity discovery and species description of hyperdiverse groups (Sharkey et al. 2021). Another benefit of this approach is, ideally, an inherent standardization of the species delimitation process, such that theoretically, if applied correctly, all species would be recognized in the same, replicable manner within the group (Orr et al. 2022).
In addition, technological advances have made larger-scale data and analyses feasible over the last 20 years. Except traditional acquisition of DNA sequences based on PCR amplification of a single sample, eDNA metabarcoding technology uses high-throughput approaches to obtain batches of amplicon sequences in mixed samples (Ruppert et al. 2019). For mixed insect samples from light traps, pitfall traps, malaise traps etc., metabarcodes have been repeatedly leveraged (Zhou et al. 2013; Tang et al. 2015), and in some cases, even just residual ethanol used to preserve such samples may be sequenced without damaging specimens (Ritter et al. 2019; Mata et al. 2021; Zenker et al. 2020). For example, Huang et al. (2022) found that 95% of the species of the dipteran insect community from Tianmu Mountain based on metabarcodes were unidentified or undescribed species.
There have been many critiques of this approach, however, as minimalist treatments may make it harder to do future taxonomic work on these groups (Fernandez-Triana 2022). Almost complete reliance on molecular data in tropical countries could, for instance, make it much harder for local researchers with limited access to funds and facilities for molecular analysis to identify local species. Another primary issue is the questionable practice of relying solely on a single gene fragment for species definition (Meier et al. 2022), which is especially problematic when the data are not fully public and analyses are not replicable. For example, across varied taxa such as collembolans, spiders etc., the results of species delimitation using COI+ITS2 are better than when using COI alone (Agnarsson et al. 2007).
One suggested fix for this is to use multiple markers, especially to add nuclear markers (Fišer et al. 2018; Després 2019), because maternal mitochondrial genes may prove ineffective in discovering cryptic species undergoing a special evolutionary process (Funk & Omland 2003). For example, the moth cryptic species Limbatoclamys pararosthorni and its sister species L. rosthorni were supported by the nuclear gene (Ef-1a), not by COI (Han et al. 2007; Jiang et al. 2021). Multiple barcoding markers can find the traits of pseudo-cryptic species and try to avoid several complex evolutionary processes such as mito-nuclear discordance and paraphyly. Mito-nuclear discordance is widespread across certain taxa like copepods (Barreto et al. 2018) and insects (Jiang et al. 2021), and the possible reasons include gene introgression, incomplete lineage sorting, and Wolbachia infection within arthropods. For example, Weigand et al. (2017) confirmed two cryptic species with mito-nuclear discordance induced by gene introgression.
Omics-based research on cryptic species
Although many studies of cryptic species are based on DNA barcoding, when facing some complex evolutionary processes, genomic data provide greater promise for the resolution of species. Janzen et al. (2017) suggested that COIs are suited to expose potential cryptic species, whereas nuclear genomes are used to distinguish/confirm cryptic species. Thankfully, commonly cryptic species identified by single mtDNA gene are also supported by omics data (Janzen et al. 2017; de Moya et al. 2019).
However, sometimes omics data rebut cryptic clades identified by single mtDNA genes (Cong et al. 2017; Hinojosa et al. 2019; Hupało et al. 2023). Many potential causes have been discussed previously (introgression etc.). Hinojosa et al. (2019) suggested homogeneity of the nuclear genome via long periods of geographic isolation also can deny the validity of cryptic species based on COI. More cases of mitochondrial genome introgression have gradually been discovered in different animal groups, demonstrating that some molecular cryptic species for barcodes might have been products of introgression (Cong et al. 2017; Ge et al. 2022). For example, several well-studied and emblematic butterfly groups owning striking mtDNA introgression, such as Erynnis (family Hesperiidae), genome data revealed greater reliability than COI (Zakharov et al. 2009; Cong et al. 2017).
Complicating matters, introgression may have even occurred historically with now-extinct species, known as “ghost introgression” (Ottenburghs 2020). This phenomenon can occur when species are undergoing sudden range or habitat expansions, bringing them into contact with other closely related species. With the introduction of new genetic material and its potential fixation, it would then be much harder or even impossible to use that hypothetical marker to accurately delimit species. In some cases, these species might also be morphologically cryptic, in which case genomic methods become truly invaluable.
Facing these complex evolutionary processes, genome scanning is a useful method to discover potential cryptic species by locating key regions of genetic differentiation between candidate species, such as the genomic island of speciation and introgression segments (Utsunomiya et al. 2013; Steane et al. 2015; Chattopadhyay et al. 2016; Després 2019). In the future, pointing omic islands of speciation, containing a series of linked sites related to reproductive isolation, may be one important method to distinguish cryptic species.
APPLIED IMPORTANCE OF CRYPTIC SPECIES RESEARCH
The prevalence of cryptic species has clear and substantial implications beyond the bounds of systematics and evolutionary studies. Nowadays, cryptic species have profound effects on many fields, including biodiversity, conservation, pest management, and so on. Here, we will discuss four such distinctive and important cases.
Important advancements for morphological taxonomy
Classical taxonomy based on morphological features is highly reliant on taxonomic knowledge and verified museum specimens (Orr et al. 2020; Hong et al. 2022; Zhu et al. 2022). Taxonomic knowledge requires substantial training and experience and their practices are often relatively more subjective in comparison across groups; molecular analyses and other lines of evidence spurred in their use by cryptic species discovery can enable more researchers to freely explore species delimitation while offering additional methods for standardization (such as through novel species delimitation algorithms designed for molecular data). Meanwhile, verified reference material, especially type specimens, can be difficult to check for taxonomists across the Global South, as many types of verified specimens are held in Western institutions. Thankfully, barcoding codes are typically more open-source, held on repositories such as BOLD or NCBI.
Molecular data are also invaluable for making associations that might be otherwise unclear based on morphology alone. This is especially important for associating different sexes, larval and adult stages, or those that differ in appearance by season. For example, potential cryptic species of insects and echinoids have been revealed by larval barcoding (Suh et al. 2019; Collin et al. 2020).
A critical role for biodiversity research
The assessment of species diversity is inseparable from the number of species present in an area, and this includes cryptic species. It is plausible that the discovery of cryptic species can significantly increase the diversity of communities, that is, α and β diversity (Vodă et al. 2015a; Darwell & Cook 2017; Mayr et al. 2021). Immense hidden biodiversity is also expected in some specific areas or naturally fragmented habitats, such as oceans, rivers, islands, and caves (Barber et al. 2006; Witt et al. 2006; Liu et al. 2011; Chen et al. 2015; Suh et al. 2019; Wang et al. 2020). For example, in recent years, the diversity of cave ecosystems has caused great concerns due to the discovery of plenty of hidden spider species (Ba & Lai 2009; Hedin 2015; Trontelj 2009; Delić et al. 2017). Zhang and Li (2014) discovered 15 undescribed cryptic species from the spider Pinelema cucurbitina in southern China karst cave, but even some vertebrates such as bats are likely to have many cryptic species (Chornelia et al. 2022).
Targeted protection of rare and beneficial species
Biodiversity conservation is an increasingly important goal for governmental or non-governmental organizations, as well as the general public, and the understanding of cryptic species can dramatically influence how we go about conservation. Nowadays, many biologists have realized the importance of cryptic species for conservation and acknowledged that cryptic species may be more vulnerable to threats than previously thought (Nair et al. 2012; Niemiller et al. 2013; Delić et al. 2017). In recent years, many cryptic species were discovered within flagship protected species, including in the International Union for Conservation of Nature Red List. Examples include giraffes (Fennessy et al. 2016), finless porpoises (Zhou et al. 2018), and giant salamanders (Yan et al. 2018; Chai et al. 2022). Because of the differences in their distributions, population health, and degrees of endangerment among such related species, these cryptic species may decrease the effectiveness of management if not accounted for and increase unnecessary investments. Thus, timely and effective taxonomic efforts are needed to ensure that we are protecting the right species.
Targeted identification and control of pest and invasive species
The accurate identification of pests is crucial for effective pest management strategies. The existence of cryptic species in pest species can decrease management efficiency and increase unnecessary investments (Simmons & Scheffer 2004; Hendrichs et al. 2015; Andrews et al. 2020), and detection of cryptic species in economically important taxon should consequently be cautious. For example, the important agricultural pest thrips Thrips palmi likely contains three cryptic species that are partially sympatric (Tyagi et al. 2017), such that variance in behavior or susceptibility to pesticides might differ between them and require separate management strategies. One important example of cryptic species within invasive species is that of whiteflies. Whiteflies are disastrous pests prone to widespread spread and often require specialized management, but they were considered an untenable species complex until Dinsdale et al. (2010) found at least 24 cryptic species based on molecular data (Luo et al. 2002).
UNSOVLED BUT INTERESTING PHENOMENON ABOUT CRYPTIC SPECIES
Although taxonomic challenges involved in cryptic biodiversity have been recognized for hundreds of years, forming the foundation of challenges in telling species apart by morphology, many fascinating unresolved questions remain (Bickford et al. 2007; Tattersall 2007; Korshunova et al. 2017).
Are cryptic species evenly distributed among different taxa and various geographic regions? Through exploration of the published taxonomic literature, there has always been an illusion that cryptic species are more common in the tropics than in temperate areas, in insects than vertebrates (Bickford et al. 2007; Dyer et al. 2007). This idea is related to the expected high species diversity of tropical areas, as two-thirds of species might exist in such areas (Willig et al. 2003; Pekár 2014). However, inconsistently, most of the published studies focused on cryptic species of temperate organisms (Bickford et al. 2007). This discrepancy is likely a result of efforts made in temperate areas of the Global North, where there has historically been more taxonomic work (Janzen et al. 2005; Dyer et al. 2007). Among different taxa, cryptic diversity is thought to be especially common within insects (Bickford et al. 2007; Murray et al. 2008) because of their complex life history, limited and small morphological features etc. (Van Campenhout et al. 2014). Pfenninger and Schwenk (2007) suggested that cryptic animal species are evenly distributed among various regions and taxa based on one detailed taxonomic list and distribution information of cryptic species reports, but this requires further exploration.
Does cryptic speciation prefer allopatric, parapatric, or sympatric speciation? Cryptic speciation has been reported for all these types of speciation, for example, allopatry (Amor et al. 2014; Fišer et al. 2018), parapatry (Dennis & Hellberg 2010), and sympatry (Scriven et al. 2016). However, because of biases in the interests of researchers, where they work, and what they work on, confirming the relative prevalence of cryptic speciation across these forms of speciation is likely to prove even more challenging than for proportions in groups or areas, as each such case requires greater evidence, including for the specific mode of speciation.
Can cryptic species occur alongside known species? How often does this happen? For cryptic species, an enduring question of active research is whether such species can evolve together and co-occur (Zhang et al. 2004). One point supported no-occurrence between cryptic species based on species interactions (Vodă et al. 2015a,b; Garcia-Robledo et al. 2016; Darwell & Cook 2017). Conversely, others believe that hidden species can and do co-occur, either because the rate of competitive exclusion is low and slow-acting, or because they are not strictly ecologically identical (Scriven et al. 2016; Johnston et al. 2022) that they differ enough to avoid undue impacts. Key to answering this question will be looking for additional lines of evidence by which these species might distinguish themselves.
CONCLUSION AND OUTLOOK
Cryptic species have now been discovered across much of the Tree of Life, from every corner of the globe (citations from above). Going forward, we need to better account for cryptic species in biodiversity research and beyond, and we are constantly developing better methods and frameworks for these purposes. The fields of pest management and invasion biology present especially important arenas for future work. From these and other studies, we are gradually building a better understanding of how both species and cryptic species evolve, a fundamental question in biology. A growing number of studies confirm the importance and reliability of integrative taxonomy using multiple lines of evidence, but taxonomic researchers face more challenges in hiring and funding than ever before, even as awareness of the biodiversity crisis grows. Therefore, in the future, we hope that more scholars will pay attention to cryptic species, participate in this research, integrate various methods, and strive to explore the mechanism and evolutionary mechanisms underlying cryptic species. Cryptic species are ultimately a crux in the current biodiversity conservation framework, and only by understanding them can we effectively know and protect species.
ACKNOWLEDGMENTS
We are thankful to Prof. Keping Ma, Prof. Yibo Hu, Prof. Yue-Hong Yan, Yunxia Luan, and Guannan Wen for their strong support and valuable comments on this paper. This study was supported by the Ningxia Hui Autonomous Region Agricultural Science and Technology Independent Innovation Fund (NGSB20211405), Key Laboratory of the Zoological Systematics and Evolution of the Chinese Academy of Sciences (2008DP173354), and the Survey of Wildlife Resources in Key Areas of Tibet (ZL202203601).
CONFLICT OF INTEREST
The authors declare no competing interests.




