The difference and variation of gut bacterial community and host physiology can support adaptation during and after overwintering in frog population
Abstract
The hibernation of amphibians can offer a unique window into overwintering adaptation processes and host–gut microbiota interactions through changes in metabolic availability and homeostasis. We attempted to identify differences in the physiology and gut microbiome during and after hibernation in Japanese wrinkled frogs (Glandirana rugosa), an aquatic overwintering amphibian. After hibernation, the high alpha and beta diversity of the gut bacterial community appears to reflect the more diverse and complex environmental conditions. During winter, Proteobacteria dominated the majority of the gut bacterial community, likely due to high oxygen saturation. After hibernation, Firmicutes and Bacteroidetes increased, which are supportive of host metabolism by gut microbiota. Corticosterone also showed high values and variances after hibernation, presumably allowing the population to remain adaptable across a broad range of environmental gradients. Innate immunity was high after hibernation but exhibited low variation among populations, which supports the idea of a prioritized investment in immunity after hibernation. Blood biochemistry suggests that aquatic overwintering frogs have a mechanism to adapt through overhydration and regulate homeostasis through water excretion associated with the kidney and urine after hibernation. Frog populations exhibit variations and adaptability in gut microbiota and physiology during and after hibernation: Through this, they may demonstrate an adaptive response that regulates metabolic availability in preparation for unpredictable environmental changes. We also propose that the maintenance of Proteobacteria during hibernation can support the colonization of Firmicutes and Bacteroidetes after hibernation, underscoring the need to study the complex effects of gut microbiota across multiple life stages.
INTRODUCTION
Amphibians, typically ectothermic animals found in both aquatic and terrestrial habitats, exhibit a range of physiological, biological, and behavioral traits that are dependent on temperature (Wells 2007a). Hibernation, a periodic phenomenon in temperate regions, represents a significant adaptation to temperature-constrained environments. For these animals, hibernation is not optional but an essential adaptation, as their body temperature drops in response to cooler climates, prompting extensive changes across all biological systems (Wells 2007a). During hibernation, movement and metabolic rates decrease substantially, the gut remains vacant, and physiological states, including homeostasis, are altered to sustain a low-energy state (Costanzo et al. 2013; Gao et al. 2015; Niu et al. 2022). This state allows amphibians to evade the need for reproductive activities and foraging during times of scarce resources, thereby reducing energy demands throughout their lifecycle (Pough 1980).
However, hibernation comes with its costs. Depending on the type of hibernation, amphibians may have to endure freezing, hypoxia, and dehydration, as well as the threat of winter predators (Storey 1990; Pulliainen & Ollinmäki 1996; Tattersall & Ultsch 2008). They must manage the energy reserved before hibernation effectively to sustain a low-energy state for prolonged periods. For example, many species experience a reduction in immunity during winter because maintaining an immune system is energetically expensive (Rollins-Smith & Woodhams 2012). Instead, they convert energy into glycogen, storing it in the liver and tissues for winter survival (Jenkins & Swanson 2005; Tattersall & Ultsch 2008). Additionally, they undergo drastic changes in homeostasis; terrestrial species maintain a hyperosmotic state to minimize water loss (Costanzo & Lee Jr 2008), while freeze-tolerant species use substances like urea, glucose, or glycerol as antifreeze to reduce their body's freezing point (Costanzo & Lee Jr 2005; Costanzo et al. 2013). Aquatic hibernators reduce ion exchange and skin permeability to avert overhydration and ion loss (Tattersall & Ultsch 2008).
The reactivation of metabolism and the restoration of homeostasis post-hibernation are also critical for amphibians but have been underrepresented in hibernation research. We argue that these processes are indicative of successful hibernation. With the rise in temperatures after hibernation, energy demands escalate, and physiological systems vital for metabolism and survival, such as the immune and homeostatic regulation systems, are likely prioritized for recovery (Costanzo & Lee Jr 2008; Rollins-Smith & Woodhams 2012). Some species immediately engage in breeding using stored energy post-hibernation, while others reserve energy for later reproduction (Saenz et al. 2006; Wells 2007b). Given the various hibernation strategies among amphibians and their considerable intraspecies plasticity, fully grasping their physiological adaptations remains a formidable challenge. Moreover, most studies have been conducted under controlled laboratory conditions, highlighting the need for further field studies on overwintering.
The role of gut microbiota in host interactions has recently become a focal point of interest. The gut microbiota is thought to influence the host's physiology and metabolism, including digestion, energy absorption and efficiency, immune regulation, and disease susceptibility (Kosiewicz et al. 2011; Nicholson et al. 2012). Alterations in the gut microbiome during hibernation, due to decreased metabolic rates, prolonged low temperatures, fasting, and dramatic physiological shifts, may affect its diversity and interaction with the host. Understanding these interactions can deepen our knowledge of hibernation physiology. The gut microbiome may help meet energy needs or adapt to energy availability during and after hibernation (Tong et al. 2020, 2023). It is possible that the gut microbiota, maintained and altered during hibernation, may induce complex effects and physiological adaptations not only within a single life stage but across multiple stages (Macke et al. 2017). While there has been extensive research into the gut microbiome in endothermic animals, a more comprehensive examination is needed to better understand host–microorganism interactions.
In this study, we aimed to investigate the overwintering physiology of adult Japanese wrinkled frogs (Glandirana rugosa) that hibernate underwater. We hypothesized that frog populations would encounter more complex metabolic challenges post-hibernation than during it. To explore this, we focused on changes in the diversity and composition of the gut microbiota. We also considered changes or variations in physiological states related to energy trade-offs. We investigated the recovery of innate immunity, important for post-hibernation disease dynamics (Demas et al. 2011), and examined corticosterone, a hormone essential for glucose metabolism, survival, and adaptive responses (Narayan et al. 2019). Additionally, we used blood biochemistry to identify metabolic and homeostatic regulation during hibernation. Despite the close relationship between gut microbiota and host physiology, no studies to date have concurrently examined them to understand amphibian hibernation physiology. We believe our study will contribute to a better understanding of physiological adaptive responses and host–microbe interactions within the context of amphibian hibernation.
MATERIALS AND METHODS
Field investigation
Japanese wrinkled frogs (G. rugosa) were collected from Gongju City in South Korea from early January to early February 2022 (during hibernation: winter group). The frogs were captured from three small streams, with 8 to 12 individuals collected per site. The frogs were found hibernating under stones in gently flowing areas without stagnant water. After collecting water from each site and adding an ice pack to maintain its cold temperature, the captured frogs were immediately placed into plastic cages. A total of 55 frogs (26 males and 29 females) were collected during the winter hibernation. This particular species of frog exhibits pronounced sexual dimorphism (Khonsue et al. 2001). However, as we did not aim to analyze complex relationships (such as comparing pre-hibernating males to post-hibernating females) that would not align with our research objectives, we separated the genders and compared them independently. The collected frogs were transported to the animal laboratory at Kongju University within 1 h.
Subsequently, frogs were recaptured from the same locations from late March to late April 2022 (after hibernation: spring group). Similar to the winter sampling, 8 to 12 individuals per site were collected in the spring. The frogs had emerged from hibernation and were found in rock crevices or the grass at the water's edge. Unlike the winter collection, the frogs gathered in spring were placed in plastic cages where the water's temperature from the site was maintained without an ice pack. A total of 56 frogs (25 males and 31 females) were collected post-hibernation in the spring. These frogs were transported to the animal laboratory at Kongju National University within 1 h and were used for hormone assays, innate immunity assays, blood biochemistry, and analysis of gut bacterial composition. Experimental procedures involving animals were conducted following the regulations and with the approval of the Experimental Animal Ethics Committee of Kongju National University (KNU_2022-01).
Saliva and blood extraction
Considering that salivary corticosterone concentrations can respond within 3 min (Romero & Reed 2005), saliva extraction was conducted immediately after capturing the frogs in the field, during both hibernation periods. Upon collection, a frog's mouth was carefully opened with a sterile pipette tip while wearing latex gloves, and a saliva sample was collected using a sterile, dry cotton swab for 40 s. The swabs for saliva extraction were pre-weighed to the nearest 0.001 g using an electronic balance (A & D Company Limited, Fx-200i, Japan) and stored in 1.5 mL microcentrifuge tubes. All salivary cotton swabs were kept in an icebox until transported to the laboratory. Once in the laboratory, the cotton swabs for saliva extraction were reweighed to calculate the amount of saliva, which was then used to determine the concentration of corticosterone per mass.
The frogs brought to the laboratory were anesthetized in ice-cold water, and blood was collected via cardiac puncture. The collected blood was placed into a heparinized vacuum tube and centrifuged at 3000 × g for 5 min to obtain plasma. The plasma was analyzed to assess bacterial killing ability (BKA), serving as an indicator of innate immune capacity, and to measure various plasma biochemical components. Post blood extraction, the snout–vent length (SVL), an indicator of amphibian body size, was measured using a digital caliper to the nearest 0.01 mm, and the body weight was recorded using a digital scale to the nearest 0.01 g.
Analysis of gut bacterial community
We euthanized the frogs, whose length and weight had been measured, by decapitation while they were still under ice anesthesia. The frogs were dissected using sterilized equipment such as scissors and forceps on a clean bench to prevent cross-contamination among individuals. The section from the beginning of the small intestine to the end of the large intestine was excised and transferred to a tube containing beads, as provided by the Qiagen DNeasy Powersoil Pro Kit (Qiagen, Hilden, Germany). Following this, the scissors were sterilized in a flame, disinfected with alcohol, rinsed with sterile water, and then used to cut the intestinal sample several times within the tube. The euthanized frogs were preserved in alcohol for 2 months for subsequent analyses.
The genomic DNA of the gut bacterial community was extracted according to the instructions of the Qiagen DNeasy Powersoil Pro Kit. The sample was homogenized for 5 min at a speed of 30 m s−1 using a TissueLyser II (Qiagen, Hilden, Germany) after adding a lysis solution to the tube containing the sample and beads. Then, genomic DNA was extracted by performing the binding, washing, and elution processes sequentially, following the manufacturer's instructions. We quantified the extracted DNA using a DeNovix-QFX fluorometer (DeNovix Inc., Wilmington, DE, USA) and a QFX dsDNA High Sensitivity Assay Kit. The quality of the genomic DNA was also checked by electrophoresis, and no abnormalities were observed in any of the samples.
For the metagenomic analysis of bacterial communities, we added the overhang adapter sequence provided by the Illumina protocol to two primers targeting the 16S ribosomal DNA (16S rDNA) region: 515F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3′). We purified the primary amplified PCR products using the Agencourt AMPure XP PCR purification system (Beckman Coulter, Brea, CA, USA), which was also used for the secondary index PCR products. The Nextera XT Index Kit (Illumina, San Diego, CA, USA) was utilized for index PCR. Finally, the concentration of the purified index PCR product was measured using a DeNovix-QFX fluorometer with a QFX dsDNA High Sensitivity Assay Kit, and electrophoresis was performed to confirm the size of the PCR product. All samples were diluted to 10 nmol L−1, pooled, further diluted to 1 nmol L−1, and analyzed on an Illumina Miniseq system.
Paired-end data in FASTQ format were processed using QIIME2 (v.2022.2) and associated plugins. The DADA2 plugin was used for quality filtering, denoising, paired-end merging, and feature-table construction. The trimming parameters were established based on demux visualization. Taxonomic assignment was conducted using the default settings of the feature-classifier plugin for QIIME2 against the EzBioCloud 16S reference database (accessed on February 9, 2023). The dataset was filtered to contain only bacterial sequences. Alpha diversity (observed operational taxonomic units [OTUs], Chao1 index, Shannon index, and Simpson index) and beta diversity analyses of the gut bacterial community from the frogs were conducted using the microeco package in R software version 4.2.0 (Liu et al. 2021). Bacterial taxonomic data were transformed into Hellinger distance, and beta diversity was assessed using the Bray–Curtis distance metric.
Hormonal and immune assay
Cotton swabs used for saliva extraction were stored in a freezer at −40°C for 2 weeks to facilitate mucin precipitation. The hormonal assay was conducted by referring to previously reported salivary hormone analysis protocols (Hammond et al. 2018). Specifically, the swabs were transferred to perforated microcentrifuge tubes. After adding 150 μL of ELISA buffer to each cotton swab and centrifuging at 5000 × g for 10 min, the extracted solution was used for analysis. As previous studies have indicated that interfering proteins in saliva can affect the analysis of corticosterone hormones in some species (Hammond et al. 2018; Park & Do 2022), we employed trichloroacetic acid (TCA) to precipitate these interfering proteins. We added 1.5 μL of TCA per 10 mg of saliva mass, vortexed the solution, then incubated the samples at 25°C for 15 min. Subsequently, the samples were centrifuged at 3000 × g for 8 min to collect the supernatant. Samples with blood on the saliva swab or an unclear solution after resuspension in ELISA buffer were considered contaminated and excluded from the experiment.
The salivary corticosterone concentration was analyzed according to the manufacturer's instructions for a 96-well corticosterone enzyme-linked immunoassay kit (501 320, Cayman Chemical, Ann Arbor, MI, USA). The absorbance of the plate was measured using a microplate spectrophotometer at a wavelength of 412 nm, and these data were used to calculate the concentration. Each sample was measured in duplicate on three different plates, and the average value of the three measurements was used as the representative value for each individual. All experiments were conducted simultaneously, and the inter-assay coefficient of variation (CV) for each sample was less than 13.7%, which is considered suitable for analysis.
The bacterial killing assay was performed according to the protocol of a previous study (Assis et al. 2013). Briefly, plasma samples were diluted 1:20 with amphibian Ringer's solution (ARS). Then, 10 μL of a non-pathogenic Escherichia coli working solution (Microbio-Logics #24 311-ATCC 8739, St Cloud, MN, USA; approximately 104 microorganisms) was added to the diluted plasma sample (10 μL plasma: 190 μL ARS). A total of 210 μL of ARS was used as a negative control, and a mixture of 200 μL of ARS with 10 μL of the E. coli working solution served as a positive control. All samples and controls were incubated at 37°C for 60 min, mixed with 500 μL of tryptic soy broth, and transferred in duplicate to a 96-well plate in 300 μL aliquots. The plate was incubated at 37°C for 2 h, and the absorbance of the mixed solution was measured at 600 nm using a microplate spectrophotometer at 1-h intervals for a total of six measurements. The BKA was calculated at the start of the bacterial exponential growth phase using the formula [1 − (optical density of the sample/optical density of the positive control)], which indicates the percentage of non-pathogenic E. coli killed in the plasma samples relative to the positive control.
Blood biochemistry analysis
We conducted blood biochemistry analysis on the extracted plasma using a clinical chemistry automated analyzer (Hitachi Automatic Analyzer 7020, Japan). Eight parameters were assessed to understand the frogs' physiology: aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose, total protein (TP), albumin, total globulin (TGB), blood urea nitrogen (BUN), and creatinine. AST is a non-specific indicator of hepatic stress that increases with damage to the liver, heart, and skeletal muscle. ALT, however, is liver-specific and only rises with hepatic damage; it is also highly sensitive. Consequently, these indicators can be used in tandem to evaluate the condition of hepatic cells (Divers & Cooper 2000). Glucose is a parameter for evaluating endocrine disruption, body osmotic pressure, metabolism, and nutritional status (Costanzo et al. 1993; Divers & Cooper 2000). Variations in TP, albumin, and TGB help assess blood loss, nutritional status, homeostasis, and hepatic and renal function (Cathers et al. 1997; Divers & Cooper 2000). BUN, produced during protein metabolism, and creatinine, produced during muscle metabolism, are parameters that can jointly determine renal function (Casey & Greenhaff 2000; Salazar 2014).
Statistical analysis
Our objective was to identify and compare the response of the gut bacterial community and physiological condition relative to hibernation within each sex, not to compare differences between males and females. Therefore, we made no intersexual comparisons. Intrasex comparisons were conducted between frogs collected in the winter season and those collected in the spring season. The Mann–Whitney U test was utilized to compare four indices of diversity: Bray–Curtis distance, the relative abundance of the gut bacterial community, body length and weight, plasma bacterial killing ability, salivary corticosterone concentration, and eight plasma biochemical components during and after hibernation within each sex. We analyzed differences in the composition of the gut bacterial community using multivariate analysis of variance (MANOVA) and visualized these differences with principal coordinates analysis (PCoA). Statistical significance was set at P < 0.05, and all statistical analyses were performed using JASP version 0.16.4.0 (Love et al. 2019).
RESULT
Alpha and beta diversity of gut bacterial community
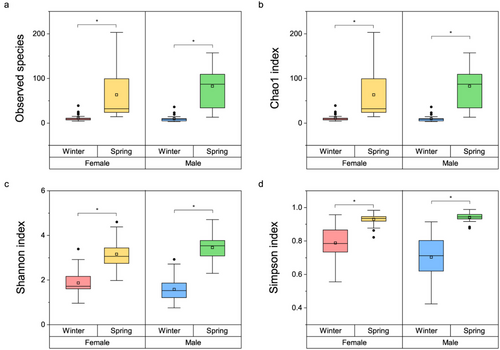
In the female groups, the observed OTUs (W = 841, P < 0.001), Chao1 index (W = 841, P < 0.001), Shannon index (W = 845, P < 0.001), and Simpson index (W = 835, P < 0.001) in the spring season were significantly higher than in the winter season. Similarly, in the male groups, the observed OTUs (W = 587, P < 0.001), Chao1 index (W = 587, P < 0.001), Shannon index (W = 580, P < 0.001), and Simpson index (W = 576, P < 0.001) in the spring were higher than in the winter (Fig. 1).
The Bray–Curtis distance, representing dissimilarity, showed similar results. For both males and females, the beta diversity of the gut bacterial community in frogs from the spring season was higher than in those from the winter season (Fig. 2a). MANOVA revealed significant differences in the gut bacterial composition among the four groups (F = 6.933, P < 0.001). PCoA separated the gut bacterial communities of frogs, particularly between the winter and spring seasons (Fig. 2b). Principal coordinates 1 (PCo1) accounted for 19% of the variance and separated the gut bacterial communities of frogs from those of the spring and winter seasons. PCo2 explained 11.5% of the variance but did not separate the groups.

Unique OTUs were most numerous in frogs from the spring season for both males and females (Fig. 2c,d). Specifically, male frogs in the spring season had the highest number of unique OTUs at 350, while male frogs in the winter season had only 20 unique OTUs. This indicates an influx of new bacterial groups due to the seasonal change. Female frogs showed 40 unique OTUs in the winter season, whereas 177 unique OTUs were observed in female frogs in the spring season, suggesting that new bacterial groups may dominate diversity and composition. There were also 39 OTUs common to all four groups.
Composition of gut bacterial community
Although we obtained the relative abundance of the gut bacterial community from the kingdom level to the species level, we analyzed only the results from the two most critical levels, phylum and genus, to avoid complexity.
At the phylum level, the gut bacterial composition varied markedly between winter and spring (Fig. 3a). Proteobacteria (females: W = 15, P < 0.001; males: W = 71, P < 0.001), Firmicutes (females: W = 832, P < 0.001; males: W = 565, P < 0.001), Bacteroidetes (females: W = 772, P < 0.001; males: W = 539, P < 0.001), and Actinobacteria (females: W = 730, P < 0.001; males: W = 518, P < 0.001) showed significant differences in both female and male frogs. Proteobacteria were the dominant taxa in the winter (females: 76.52%; males: 74.56%), followed by Firmicutes (females: 16.42%; males: 18.29%) and Bacteroidetes (females: 5.14%; males: 5.35%). In contrast, in the spring, Firmicutes (females: 62.32%; males: 59.12%), Bacteroidetes (females: 18.48%; males: 17.46%), and Proteobacteria (females: 14.39%; males: 16.05%) were dominant.
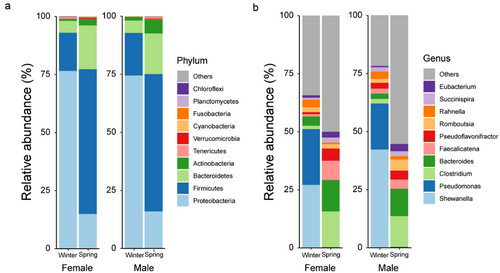
At the genus level, both males and females showed significant differences for Shewanella (females: W = 144, P < 0.001; males: W = 65, P < 0.001), Pseudomonas (females: W = 126, P < 0.001; males: W = 86, P < 0.001), Clostridium (females: W = 710, P < 0.001; males: W = 546, P < 0.001), and Bacteroides (females: W = 764, P < 0.001; males: W = 580, P < 0.001) (Fig. 3b). Shewanella (females: 27.08%; males: 42.18%) and Pseudomonas (females: 24.02%; males: 19.08%), which were dominant in the winter, were nearly absent in the spring. Conversely, Clostridium (females: 15.39%; males: 13.20%) and Bacteroides (females: 13.47%; males: 11.77%), which were dominant in the spring, were scarce in the winter.
Comparison of physiological conditions
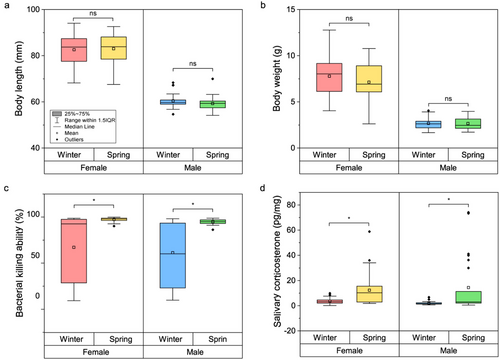
There was no significant difference in body length (females: W = 474, P = 0.725; males: W = 255, P = 0.190) or weight (females: W = 383, P = 0.331; males: W = 300, P = 0.647) between the seasons for either female or male frogs (Fig. 4a,b). However, female frogs in the spring season exhibited higher BKA (W = 733, P < 0.001) and corticosterone concentrations (W = 689, P < 0.001) compared to those in the winter season. Similarly, male frogs in the spring season also demonstrated higher BKA (W = 534, P = 0.001) and corticosterone concentrations (W = 494, P = 0.001) than those in the winter season.
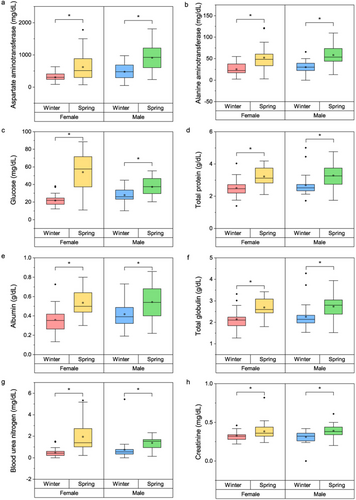
Parallel results were observed in blood biochemistry (Fig. 5a–h). Levels of AST (females: W = 655, P = 0.002; males: W = 532, P < 0.001), ALT (females: W = 745, P < 0.001; males: W = 545, P = 0.002), and glucose (females: W = 814, P < 0.001; males: W = 492, P = 0.002), which may indicate hepatic stress and glucose metabolism; TP (females: W = 748, P < 0.001; males: W = 498, P = 0.001), albumin (females: W = 758, P < 0.001; males: W = 463, P = 0.009), and TGB (females: W = 739, P < 0.001; males: W = 500, P = 0.001), which suggest homeostasis within the body; and BUN (females: W = 818, P < 0.001; males: W = 552, P < 0.001) and creatinine (females: W = 622, P < 0.001; males: W = 503, P < 0.001), which can indicate renal function, were all elevated in the spring season compared to the winter season for both sexes.
DISCUSSION
The alpha and beta diversity of the gut bacterial community was significantly higher after hibernation, which was expected due to the reduced body temperature, decelerated metabolism, and prolonged fasting that occur during overwintering (Tong et al. 2020). Notably, the high individual variation in alpha diversity and the increased beta diversity of gut bacterial groups from the spring season likely reflect the varied and complex environmental conditions encountered post-hibernation. The environment during hibernation may be more consistent than that experienced after emerging from hibernation. Although there may be subtle differences in underwater environmental gradients during overwintering, frogs generally exhibit minimal movement, remaining in a confined space with low temperatures and slow metabolism (Tattersall & Ultsch 2008). As ectothermic acclimatizers, frogs depend on microhabitat selection to regulate their physiological systems (Wells 2007a).
After hibernation, the timing of awakening, as well as the physical and physiological conditions of frogs, may vary among individuals, influenced by their metabolic rate and energy reserves (Kristín & Gvoždík 2014). Frogs emerging from hibernation display a spectrum of behaviors, including feeding, predator avoidance, stress reduction, and habitat shifting, which are based on individual differences (Hirai & Matsui 2000; Grayson & Wilbur 2009; Kelleher et al. 2017). In these scenarios, both individual frogs and their populations are likely to face a broader variety of threats or environmental conditions (Beebee 2013; Lenhardt et al. 2015). Where unpredictable environmental factors are at play, increased gut microbial diversity may bolster individual stability by enhancing the microbiome's resistance or resilience to the various environments encountered by the host (Lozupone et al. 2012). Not only at the individual level but also at the population level, the elevated beta diversity of the post-hibernation gut microbiome suggests it may support the adaptability of these populations across a wider array of environmental gradients (Couch et al. 2021).
Another aspect worth mentioning is the variation in immunity and corticosterone levels. During winter hibernation, individual variation in corticosterone was quite low (SD of males = 1.29; SD of female = 2.28), likely due to the stable environmental state that nearly halted their metabolism, akin to the response of gut bacterial diversity described earlier. After hibernation, there was a substantial inter-individual variation in corticosterone (SD of male = 21.92; SD of female = 12.49), which might enable populations to adjust their energy investments flexibly in response to different conditions such as energy reserves, resource availability, and predator presence (Romero & Wikelski 2001; Cote et al. 2006). In contrast, the pattern of innate immunity was reversed: low immunity but high individual variation during the winter season, and heightened immunity but low individual variation post-hibernation. This pattern indicates a scaling down of immunity during overwintering to varying degrees according to individual differences, followed by a concerted investment in immunity after winter (Rollins-Smith & Woodhams 2012). The prioritized restoration of immunity in the spring may be a critical energy investment for frogs, signaling that not all physiological systems display high variation in the same environment. Remarkably, the variability in immunity showed that some frogs maintained approximately the same level of immunity in winter as in spring, while others exhibited significantly reduced immunity, ranging from nearly half (50%) to almost none. This discrepancy could be due to factors such as individual immune differences prior to hibernation, the extent of disturbance in microhabitats during hibernation, differences in energy allocation for reproduction by sex or individual post-hibernation, and physiological stability related to variations in body weight or surface area. However, our results do not elucidate much about these factors, necessitating further detailed research. Variability in physiology can offer insights into adaptive mechanisms, such as energy trade-off systems (Park & Do 2022).
The gut microbiota can potentially reflect environmental adaptations during and after hibernation while also facilitating physiological adaptations in the host (Tremaroli & Bäckhed 2012). The bacterial composition within the gut undergoes significant changes from hibernation to post-hibernation periods, with Proteobacteria becoming dominant during the winter, in contrast to the spring season. As some of the most common facultative anaerobes in freshwater, Proteobacteria are known for their ability to dominate microbial communities in oxygen-rich gut environments (Guaraldi & Salvatori 2012; Shin et al. 2015). This dominance is particularly evident in aquatic amphibians overwintering in gently flowing streams, which provide a continuous oxygen supply essential for successful hibernation (Tattersall & Ultsch 2008).
During hibernation, the gut bacteria are predominantly composed of the Proteobacteria group, including genera such as Shewanella and Pseudomonas. While these bacteria are associated with diseases like diarrhea and infections in humans and animals (Shin et al. 2015), they may also play a role in disease processes in hibernating frogs. Further research is needed to explore this potential link. However, it is more likely that these microbes are responding to the host's metabolic state and environmental changes rather than being directly associated with diseases in frogs.
After hibernation, the prevalence of Firmicutes and Bacteroidetes, which are closely associated with the metabolism of frogs, becomes more pronounced. Firmicutes assist in carbohydrate fermentation and improve nutrient absorption and efficiency (Ramakrishna 2013), while Bacteroidetes play crucial roles in food digestion and maintaining gut homeostasis in animals. They are involved in the breakdown of polysaccharides, maintenance of gut mucosa, and development of the immune system (Bäckhed et al. 2004). This shift in gut bacterial composition is expected, as post-hibernation, the Japanese wrinkled frog prioritizes energy absorption, storage, and digestion in preparation for reproduction.
We conjecture that the presence of Proteobacteria during hibernation contributes to successful overwintering and subsequent life stages. Rather than affecting hibernation directly, Proteobacteria may facilitate the successful colonization of Firmicutes and Bacteroidetes, which are crucial for the host's metabolism after hibernation. In early human neonates, the gut bacterial community is typically unstable, but this instability is accompanied by the colonization of facultative anaerobes such as Proteobacteria. They predominate in the neonatal gut microbiota due to the high oxygen content (Guaraldi & Salvatori 2012). By consuming oxygen, producing carbon dioxide and nutrients, and modifying pH and redox potential, Proteobacteria create a conducive environment for the colonization of Firmicutes and Bacteroidetes (Wilson 2005; Chow & Lee 2006). Although the biological processes differ in endothermic animals, Proteobacteria may play a similar role in frog hibernation. They can dominate and stabilize the gut bacterial community during hibernation and then support the colonization of Firmicutes and Bacteroidetes post-hibernation, contributing to the host's metabolism. This suggests a need to shift the research focus toward the functional roles of Proteobacteria, beyond their potential pathogenicity. Further research is essential to understand how interactions with microbial communities impact the complex life histories of ectothermic animals, such as amphibians, not only in their present stages but also throughout their life stages.
Immunity is typically heightened in the spring as compared to the winter. Overwintering often results in a decreased immune system function due to the high cost of maintaining immunity at low temperatures. This reduction in amphibian immunity is known to reverse within a few weeks post-hibernation in the spring (Rollins-Smith 2017; Le Sage et al. 2021). At the same time, the elevated corticosterone levels observed in the spring are likely in response to an increased need for energy mobilization (Romero & Butler 2007). While winter hibernation is characterized by a reduced metabolic state and suppressed energy mobilization, the reverse is true for the spring season. This is corroborated by elevated blood glucose levels, which reflect increased corticosterone levels. The rise in corticosterone may facilitate flexible environmental adaptation through an upsurge in metabolism following hibernation (Moore & Jessop 2003). Although chronic elevation of corticosterone is typically associated with prolonged stress and reduced immunity due to maladaptive energy allocation (Marketon & Glaser 2008), our findings indicate simultaneous high immunity and corticosterone levels. This suggests that the springtime increase in corticosterone is not indicative of a severe stress response but rather supports metabolic and adaptive responses during and after hibernation.
Corticosterone, a glucocorticoid, is a steroid hormone that targets the liver to boost glucose metabolism (Thrall et al. 2012; Zaytsoff et al. 2019). This results in increased enzymatic reactions in the liver, potentially leading to raised levels of AST and ALT (Qian et al. 2015). The concurrent elevations in AST, ALT, and glucose appear to stem from increased metabolism driven by elevated corticosterone levels, rather than from hepatic cell damage.
Three types of blood proteins, BUN, and creatinine—markers of body homeostasis and renal function—also increase post-hibernation. Aquatic overwintering frogs undergo changes in homeostatic and osmoregulatory mechanisms due to decreased ion exchange through the skin and a temporary cessation of urine production or bladder excretion as temperatures fall (Tattersall & Ultsch 2008). These mechanisms are essential for preventing overhydration and ion loss. When immersed in cold water, frogs in dilute aquatic environments may rapidly become overhydrated due to water influx, disrupting their osmolarity and homeostasis (Parsons & Lau 1976). This could potentially lead to ion efflux, which needs to be prevented. To address this, frogs reduce skin permeability and progressively close ion channels to block continuous water inflow and ion loss (Koefoed-Johnsen & Ussing 1974).
Although there are no documented examples of aquatic overwintering amphibians, some terrestrial overwintering amphibians maintain homeostasis by excreting urine at the end of hibernation. Terrestrial overwintering frogs demonstrate rapid increases in glucose concentrations during hibernation to prevent freezing, and they re-establish homeostasis by excreting glucose through urine post-hibernation (Layne et al. 1996). Whether underwater hibernating frogs restore homeostasis through urine excretion was not determined in our study. Nonetheless, the elevated levels of blood proteins, BUN, and creatinine suggest swift homeostatic adjustments via renal filtration and urine excretion. Our study is among the few field investigations examining homeostatic changes in aquatic overwintering amphibians. We advocate for additional field research on the mechanisms of homeostatic regulation during hibernation in underwater hibernating amphibians, alongside laboratory-based studies.
In our study, we observed a more pronounced increase in OTUs in males compared to females. This could potentially be attributed to the significant sexual dimorphism present in the Japanese wrinkled frog species, where females are notably larger than males (Khonsue et al. 2001). Body size could be directly associated with energy stability (Rip & McCann 2011), suggesting that sexual dimorphism might provoke distinct gut microbial responses. However, in amphibian species characterized by absent or only subtle sexual dimorphism, the patterns of energy metabolism or gut bacterial community responses may differ.
CONCLUSION
After hibernation, frogs exhibited increases in both alpha and beta diversity of the gut bacterial community. Individual variation in corticosterone, which is significant for metabolism, followed a similar upward trend. It appears that frogs are capable of enhancing their metabolic capacity in preparation for the various environmental changes they may face post-hibernation. However, this is not universally true for all variables; components critical for survival, such as immunity, typically escalate to higher levels, albeit with reduced individual variation due to intensive resource allocation. Differences in gut bacterial composition, hormone levels, immunity, and blood biochemistry have enriched our understanding of the processes that occur during and after hibernation. We propose that Proteobacteria, which predominate during overwintering, may contribute to hibernation not by directly supporting the hibernation process itself but by facilitating the colonization of microbial communities associated with post-hibernation metabolism. This underscores the need for further research into the lasting effects of early-life microbiota on later-life stages in ectothermic animals. Our study also provides a field study-based framework for comprehending how aquatic overwintering amphibians maintain homeostasis during hibernation. Integrating findings related to gut microbiota and host physiology can deepen our understanding of the metabolisms involved as well as the adaptations and responses of animals to various environments. Unraveling the potential relationships between gut microbiota and host physiology, particularly in different environmental conditions of amphibians, could be a very interesting and significant endeavor. Future research could focus on these associations to underline their importance.
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A2C1004240) and by the Korea Environmental Industry and Technology Institute (KEITI) through the Wetland Ecosystem Value Evaluation and Carbon Absorption Value Promotion Technology Development Project, funded by the Korea Ministry of Environment (MOE) (No. 2022003640001).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data will be made available by the corresponding author upon acceptance.




