Morphology of the wings and attachment apparatus in the evolution of the family Hippoboscidae (Diptera)
Abstract
Using a complex analysis of the molecular genetics, morphological, and ecological characteristics of Hippoboscidae flies, the phylogenetic structure and trends in the evolution of morphological characters that contribute to the ectoparasitic lifestyle of hippoboscid flies of the north of Eurasia were studied for the first time. The research was carried out on 26 Palearctic species from 10 genera. The analysis of molecular phylogeny revealed the levels of clustering of the family with the species predominantly parasitizing mammals or birds, the time of cluster formation, and the divergence of species in the Palearctic conditions. An independent adaptation to birds occurred in the genera Icosta, Pseudolynchia, Ornithoica, and others. Bird parasites are characterized by bifid tarsal claws, long hooks on pulvilli, and long empodium setae (except genus Ornithoica). Mammalian parasites are characterized by simple tarsal claws, short lobes of hooks on pulvilli, and zones on empodium with short setae. Specialization in empodium and pulvillus morphotypes and wing reduction are higher diverged in mammalian parasites than in bird parasites. The decrease of flight ability and wing reduction independently arose in different subfamilies of Hippoboscidae flies. Our results assume that the tribe Ornithomyini is a paraphyletic group, since, according to the complex of morphological features, the genus Ornithoica can be considered a separate lineage of evolution.
INTRODUCTION
Hippoboscidae Samouelle, 1819 is a specialized group of blood-sucking ectoparasites with three related taxa: Glossinidae Theobald, 1903; Streblidae Kolenati, 1863; and Nycteribiidae Samouelle, 1819 (Hennig 1973). With a worldwide distribution (Doszhanov 1980), hippoboscid flies transmit many dangerous diseases both in mammals (Bequaert 1954; Doszhanov 1980; Ganez et al. 2002; Farajollahi et al. 2005; Buss et al. 2016; Lee et al. 2016; Zabashta et al. 2017; Werszko et al. 2020; Tiawsirisup et al. 2023) and birds (Khametova et al. 2018; Bazsalovicsová et al. 2023), such as borreliosis and West Nile virus. Hippoboscidae act as specific carriers of Haemoproteus Kruse, 1890 and mechanical inoculators of Bacillus anthracis Cohn, 1972, the causal agent of anthrax (Matyukhin et al. 2017). Frequent findings of bird louse flies on mammals and, conversely, mammalian keds on birds suggest a variety of biocenotic contacts between animals and, accordingly, their great epidemiological and epizootological significance (Gavrilyuk 2012). Additionally, louse flies transport other parasites: phoretic mites of the family Epidermoptidae (Fain 1965; Hill et al. 1967; Philips & Fain 1991) and feather lice (de Moya 2019; Lee et al. 2022), which in turn carry even more diseases (Dubinin 1953; Gilardi et al. 2001). Hippoboscidae is suggested as a monophyletic group (Petersen et al. 2007) with a sister group Glossinidae (Hennig 1973; Petersen et al. 2007). The family is divided into three subfamilies (Maa & Peterson 1987). Lipopteninae and Hippoboscinae are mainly restricted to mammalian hosts, while Ornithomyinae parasitizes birds (Doszhanov 1980, 2003; Dick 2006).
The subfamily Hippoboscinae is represented by the genera Struthiobosca Maa, 1963 and Hippobosca Linnaeus, 1758. Lipopteninae is divided into tribes Lipoptenini, including Lipoptena Nitzsch, 1818 and Noelipoptena Bequaert, 1942, and Melophagini, including Melophagus Latreille, 1802. The subfamily Ornithomyinae is divided into tribes Olfersini, represented by the genera Austrolfersia Bequaert, 1953, Icosta Speiser, 1905, Ortholfersia Speiser, 1902, Olfersia Leach, 1817, Phthona Maa, 1969, Pseudolynchia Bequaert, 1926, and Microlynchia Lutz, 1915, and Ornitomyini, including Allobosca Speiser, 1899, Crataerina von Olfers, 1816, Ornithoica Róndani, 1878, Ornithophila Róndani, 1879, Ornithomya Latreille, 1802, Ornithoctona Speiser, 1902, Myophthiria Róndani, 1875, Proparabosca Theodor & Oldroyd 1965, and Stilbometopa Coquillett, 1899 (Doszhanov 2003).
Hippoboscidae spend all or most of their lives in the fur or feathers of their hosts. These flies have a large number of unique morphological and physiological adaptations, most of which, as Petersen et al. (2007) proposed, are closely related to their ectoparasitic mode of life. The attachment apparatus is located on the pretarsus and consists of claws, empodium, and paired pulvilli covered with bristle-like formations (Petersen et al. 2018), associated with reliable attachment and efficient movement on the host, especially for species with partially or completely reduced wings (Kemper 1951).
There are a sufficient number of studies on the different taxonomic morphological features (Bequaert 1954; Hennig 1965; Maa 1966a,b, 1969a,c; Doszhanov 1980, 2003; Andreani et al. 2020). Many authors have noted taxonomic differences in the structure of the wings of Hippobosca: The subfamily Ornitomyinae is divided by the number of transverse veins; species of the genus Ornithomya are divided by the area of the wing covered by microtrichia; the absence of microtrichia on the wings is typical for the genera Ornithophila and Crataerina (Doszhanov 1980; Hutson 1984; Liu et al. 2019). The morphological investigations on the four studied hippoboscid species by Andreani et al. (2020) suggest that the development or the regression of important insect body structures has allowed or induced adaptive strategies. The functional morphology of the attachment apparatus (claws and pulvilli) was studied by Hayer et al. (2022) and Yatsuk et al. (2022).
Phylogenetic relationships within Superfamily Hippoboscoidea using different molecular markers were reconstructed by Peterson et al. (2007). Later, a molecular phylogenetic dating analysis was performed by de Moya investigating the temporal origins of bird-specialized Hippoboscidae to provide an estimate of the maximum age of feather louse phoresis (de Moya 2019). Now there are a number of controversial assumptions regarding the evolution of this family relating to the initial host for the hippoboscid flies and their adaptations to other hosts (Bequaert 1954; Maa 1966b; Hennig 1965; Petersen et al. 2007; de Moya 2019), evolutionary origins of different groups, and phylogenetic relationships between the genera (Hennig 1973; Petersen et al. 2007; de Moya 2019).
Thus, it can be summarized that previous studies have focused on common taxonomic and evolutionary patterns, but there are almost no data considering morphological features in terms of the evolution of the Hippoboscidae family, especially directly related to ectoparasitism. The purpose of this research is to retrace the morphological changes of the wings and the structures of attachment apparatus such as pulvilli, empodia as well as host–parasitic relationships during the evolutionary process of the Hippoboscidae family.
MATERIALS AND METHODS
The imago specimens were obtained from the collections of the A.N. Severtsov Institute of Ecology and Evolution (Moscow, Russia) and Zoological Institute (Saint Petersburg, Russia), Russian Academy of Sciences. The specimens were used for the research including 25 Palearctic species of hippoboscid flies from 10 genera: Crataerina hirundinis (Linnaeus, 1758); C. pallida (Olivier in Latreille, 1812); Hippobosca equina Linnaeus, 1758; H. longipennis Fabricius, 1805; H. rufipes von Olfers, 1816; Icosta ardea (Macquart, 1835); Lipoptena cervi (Linnaeus, 1758); L. fortisetosa Maa, 1965; Melophagus ovinus (Linnaeus, 1758); Ornithoctona australasiae (Fabricius, 1805); O. erythrocephala (Leach, 1817); Ornithoica aequiesenta Maa, 1966; Or. exilis (Walker, 1861); Or. stipituri (Schinner, 1868) (we are of the opinion of Soós and Hůrka (1986) that Or. momiyamai Kishida, 1932 is a synonym of Or. stipituri); Or. turdi (Latreille, 1812); Or. unicolor Speiser, 1900; Ornithomya avicularia (Linnaeus, 1758); Orn. anchineura Speiser, 1905; Orn. biloba (Dufour, 1827); Orn. candida Maa, 1967; Orn. chloropus (Bergroth, 1901); Orn. comosa (Austen, 1930); Orn. fringillina (Curtis, 1836); Ornithophila gestroi (Róndani, 1878); Pseudolynchia canariensis (Macquart, 1840). Data on newly collected specimens of species in the family Hippoboscidae are presented in Table 1. Louse flies were collected during the annual bird ringings. Keds were collected from living deer Cervus elaphus sibiricus Severtzov, 1873. All flies were fixed in 96% ethanol. The fly species were identified using the keys of Maa (1965), Doszhanov (1980, 2003), and Salvetti et al. (2020). All animal sample collection and usage comlied with current protocols and guidelines of the UNESCO Intenational Committee on Bioethics, bioethics commission of A.N. Severtsov Institute of Ecology and Evolution and laws of the Russian Federation.
| Species | Collection date | Number of individuals and sex | Place | Collector |
|---|---|---|---|---|
| Lipoptena cervi | 2013 | 3 females | Russia: Far East | A. Matyukhin |
| Ornithoica stipituri | 2020 | 3 individuals. Sex unknown | Russia: Far East | A. Matyukhin |
| Ornithoica unicolor | 2020 | 1 female, 1 male | Russia: Far East | V. Shokhrin |
| Ornithomya avicularia | 2016 | 1 female | Russia: Rostov-on-Don | M. Zabashta |
| Ornithomya avicularia | 2020 | 1 female | Russia: Curonian spit | A. Shapovalov |
| Ornithomya avicularia | 2020 | 1 male | Russia: Moscow | A. Matyukhin |
| Ornithomya avicularia | 2020 | 1 female | Russia: Far East | V. Shokhrin |
| Ornithomya biloba | 2021 | 1 female | Russia: Curonian spit | A. Shapovalov |
| Ornithomya candida | 2021 | 1 female | Russia: Kuril Islands | Ya. Red'kin |
| Ornithomya chloropus | 2019 | 1 female, 1 male | Russia: Karelia | A. Matyukhin |
| Ornithomya chloropus | 2020 | 1 female | Russia: Dagestan | A. Matyukhin |
| Ornithomya comosa | 2020 | 1 male | Russia: Irkutsk | A. Gryaznova |
| Pseudolynchia canariensis | 2020 | 1 female | Russia: Moscow | A. Matyukhin |
The morphology of pulvilli and empodia was studied in the species M. ovinus, O. australasiae, and Ornit. gestroi according to the method of Yatsuk et al. (2023). Photos of the attachment apparatus were obtained using a scanning electron microscope housed at the Joint Usage Center “Instrumental methods in ecology” at the IEE RAS: S150A Sputter Coater (Edwards, UK) with manual gold sputtering (irradiated ion current—10 mA, process time during ion sputter coating—3.5 min) and electron microscope TESCAN MIRA 3 LMH (TESCAN, Czech Republic) with the AZtecOne X-act energy dispersive analysis system (Oxford Instruments, UK) and the Schottky cathode (the accelerating voltage of SEM, 10 kV). To analyze the features of the attachment apparatus, the previously identified morphotypes of hooks on pulvilli and empodia were used (Yatsuk et al. 2023). Morphotypes of the bilobed hooks of the bristle-like outgrowths on the pulvilli: 1—long hooks with rounded inner edges (1P); 2—long hooks with rounded outer edges (2P); 3—long hooks with rounded inner edges, bristle-like formations are collected in bundles (3P); 4—hooks with short lobes (4P). The empodia morphotypes: 1—The empodium is covered with long identical setae (1E); 2—the empodium is covered with long, identical setae and transverse folds (2E); 3—the setae of the basal and apical parts of the empodium differ in morphology and length (3E); 4—the empodium has both short and long setae interspersed (4E); 5—there are edge and central areas on the empodium, the setae on which have different morphology and length (5E).
Wing and claw morphology data and data on empodius and pulvillus morphotypes for some species were obtained from the literature. The wing morphology was studied based on Maa (1965, 1966a, 1969a,b,c), Doszhanov (1980, 2003), and Liu et al. (2019). The tarsal claws morphology was studied based on Hayer et al. (2022) and Doszhanov (1980, 2003), and pulvillus and empodia morphotypes based on Yatsuk et al. (2023).
Molecular research
The total DNA was extracted from whole flies using Diatom-200 reagents (Isogen, Moscow) according to the manufacturer's manual. Polymerase chain reaction was performed using primers LCO1490 and HCO2198 (Folmer et al. 1994). Thermal cycling consisted of an initial denaturation step at 94°C for 1 min, followed by six cycles of 1 min at 94°C, 1 min at the annealing temperature of 45°C, and 1 min at 72°C; followed by 40 cycles of 1 min at 94°C, 1.5 min at the annealing temperature of 55°C and 4 cycles of 1.5 min at 72°C; with a final extension at 72°C for 6 min. The amplification product was purified by the method of precipitation by ethyl alcohol solution with the addition of 5M sodium acetate. Electrophoresis and reading of amplification product nucleotide sequences were carried out on an automatic ABI PRISM 3130 sequencer (Applied Biosystems, USA) using BigDye Terminator reagent kit 3.1 (Applied Biosystems). The study was conducted using “Joint Usage Center Instrumental methods in ecology” at the IEE RAS.
We analyzed 39 sequences of 24 species of hippoboscid flies, of which 23 sequences were obtained by us, 13 sequences were taken from GenBank databases (www.ncbi.nlm.nih.gov/genbank), and 3 sequences were taken from BOLDSystems (https://www.boldsystems.org). To analyze the phylogenetic relationships in Hippoboscidae, data processing was carried out using the MEGA 11 (Tamura et al. 2021) using the following methods: neighbor-joining, maximum likelihood, and maximum parsimony. To clarify the time of divergence of individual genera of hippoboscid flies, we used BEAST ver. 2.4 (Bouckaert et al. 2014) software package with molecular clock Relaxed Clock LogNormal and Yule models (Yule 1925). For comparative analysis and calibration of the divergence time, sequences of the homologous region of mtDNA of Diptera species from three superfamilies were additionally taken from GenBank: Physocephala tibialis (Say, 1829); Glossina fuscipes fuscipes (Newstead, 1911); Drosophila melanogaster Meigen, 1830; Drosophila simulans Sturtevant, 1919; and Drosophila yakuba Burla, 1954. All sequence data are presented in Table 2. The time scale was determined by the time of divergence between Dr. melanogaster and Dr. simulans at 5.1 Ma (Tamura et al. 2004) and the expected time of divergence between Hippoboscidae and species from the genus Glossina Wiedemann, 1830 is 34 Ma (Misof et al. 2014).
| Family | Genus and species name | Sequences number | Data source | Data link |
|---|---|---|---|---|
| Hippoboscidae | Crataerina hirudinis | MW590962 | NCBI | Lehikoinen et al. (2021) |
| Hippoboscidae | Crataerina hirudinis | MW590970 | NCBI | Lehikoinen et al. (2021) |
| Hippoboscidae | Crataerina pallida | EF531196 | NCBI | Petersen et al. (2007) |
| Hippoboscidae | Hippobosca equina | EF531207 | NCBI | Petersen et al. (2007) |
| Hippoboscidae | Hippobosca equina | OR054157 | NCBI | The current study |
| Hippoboscidae | Hippobosca longipennis | MK405667 | NCBI | Mihalca et al. (2019) |
| Hippoboscidae | Hippobosca rufipes | EF531207 | NCBI | Petersen et al. (2007) |
| Hippoboscidae | Icosta ardea | OR064837 | NCBI | The current study |
| Hippoboscidae | Icosta ardea | OR064838 | NCBI | The current study |
| Hippoboscidae | Lipoptena cervi | ON858177 | NCBI | The current study |
| Hippoboscidae | Lipoptena cervi | ON858179 | NCBI | The current study |
| Hippoboscidae | Lipoptena cervi | ON858181 | NCBI | The current study |
| Hippoboscidae | Lipoptena fortisetosa | MK405668 | NCBI | Mihalca et al. (2019) |
| Hippoboscidae | Melophagus ovinus | NC_037368 | NCBI | Liu et al. (2017) |
| Hippoboscidae | Ornithoctona erythrocephala | JQ246707 | NCBI | Marinho et al. (2012) |
| Hippoboscidae | Ornithoica aequisenta | HIPMJ021-19 | BOLD | ZSM Collection of Manfred Sommerer |
| Hippoboscidae | Ornithoica exilis | HIPMJ022-19 | BOLD | ZSM Collection of Manfred Sommerer |
| Hippoboscidae | Ornithoica momijamai | OR045887 | NCBI | The current study |
| Hippoboscidae | Ornithoica momijamai | OR045888 | NCBI | The current study |
| Hippoboscidae | Ornithoica momijamai | OR045889 | NCBI | The current study |
| Hippoboscidae | Ornithoica stipituri | HIPMJ018-19 | BOLD | ZSM Collection of Manfred Sommerer |
| Hippoboscidae | Ornithoica turdi | OR064834 | NCBI | The current study |
| Hippoboscidae | Ornithoica unicolor | OR064840 | NCBI | The current study |
| Hippoboscidae | Ornithoica unicolor | OR064841 | NCBI | The current study |
| Hippoboscidae | Ornithomya anchineura | MZ261718 | NCBI | Levesque-Beaudin and Sinclair (2021) |
| Hippoboscidae | Ornithomya avicularia | OR064829 | NCBI | The current study |
| Hippoboscidae | Ornithomya avicularia | OR064830 | NCBI | The current study |
| Hippoboscidae | Ornithomya avicularia | OR064831 | NCBI | The current study |
| Hippoboscidae | Ornithomya avicularia | OR064832 | NCBI | The current study |
| Hippoboscidae | Ornithomya biloba | OR054213 | NCBI | The current study |
| Hippoboscidae | Ornithomya biloba | MF496010 | NCBI | Sochova et al. (2017) |
| Hippoboscidae | Ornithomya candida | OR064839 | NCBI | The current study |
| Hippoboscidae | Ornithomya chloropus | OR054225 | NCBI | The current study |
| Hippoboscidae | Ornithomya chloropus | OR064835 | NCBI | The current study |
| Hippoboscidae | Ornithomya chloropus | OR064836 | NCBI | The current study |
| Hippoboscidae | Ornithomya comosa | OR064833 | NCBI | The current study |
| Hippoboscidae | Ornithomya fringillina | MW590981 | NCBI | Lehikoinen et al. (2021) |
| Hippoboscidae | Ornithophila gestroi | KJ174684 | NCBI | Gutierrez-Lopez et al. (2015) |
| Hippoboscidae | Pseudolynchia canariensis | OR054156 | NCBI | The current study |
| Glossinidae | Glossina fuscipes fuscipes | KP979584 | NCBI | Votypka et al. (2015) |
| Drosophilidae | Drosophila melanogaster | KT896664 | NCBI | Unpublished |
| Drosophilidae | Drosophila simulans | AY518674 | NCBI | Ballard (2004) |
| Drosophilidae | Drosophila yakuba | KF824901 | NCBI | Llopart et al. (2014) |
| Conopidae | Physocephala tibialis | JF868566 | NCBI | Unpublished |
RESULTS
Phylogenetic analysis of the family Hippoboscidae
All of the constructed dendrograms match the key nodes. The divergence of superfamilies agrees with the scheme of phylogenetic relations of superfamilies of the infraorder Myiomorpha presented by Rodendorf (1964) and the modern developments on the hippoboscid fly phylogeny (Petersen et al. 2007; Liu et al. 2017; de Moya 2019). The dendrograms show that there are two clusters within the family Hippoboscidae, one of which, in turn, is divided into two subclusters (Figs 1, 2). A hypothetical ancestor, dating back to 30 million years ago (Mya) (Fig. 2), originated two branches. One branch gave rise to species of the genus Ornithoica with the formation of a separate cluster, and the other one gave rise to all other genera presented. The cluster formed by the genus Ornithoica is subdivided into two groups of species, one of which is formed by closely related species: Or. momiyamai (= Or. stipituri), Or. exillis, and Or. aequisenta. The second group includes Or. unicolor and Or. turdi.
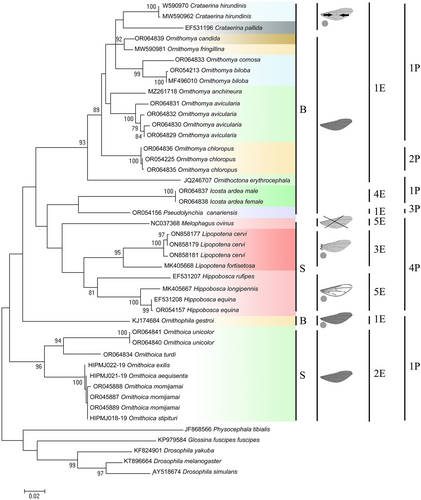
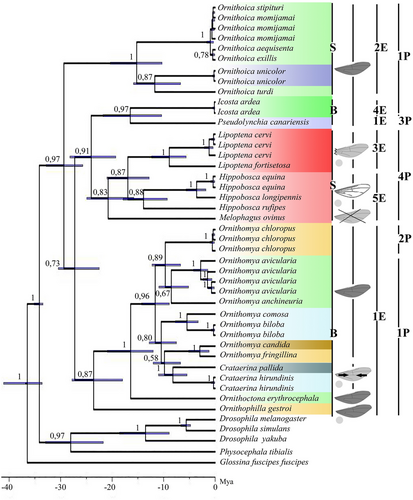
The formation time of two main subclusters of the second cluster is almost the same and dates back to 23–24 Mya. One of them includes the genera Crataerina, Ornithoctona, Ornithomya, and Ornithophilla. In this subcluster, the species Ornit. gestroi separated the earliest. Another subcluster is formed by representatives of the subfamily Hippoboscinae, Lipopteninae, and of the tribe Olfersini, the time of divergence of which is dated slightly later than the group of Hippoboscinae and Lipopteninae species (16 and 21 Mya, respectively). Melophagus ovinus earlier than others diverged from a hypothetical ancestor in this cluster.
Wings and attachment apparatus morphology
Analysis of the literature showed that the parasites of birds differ from the parasites of mammals in the more complicated structure of venation and the presence of microtrichia (Figs 1, 2). The comparison of wing morphology with molecular phylogeny showed that the wing reduction independently arose in different subfamilies of hippoboscid flies (Figs 1, 2). For species from the genera Ornithoctona and Ornithophila, it was found that hooks on pulvilli and empodia belong to morphotypes 1Р and 1Е, respectively (Fig 3a,b). We assume that the identified morphotypes of empodius and pulvillus hooks of O. australasiae are the same for all Ornithoctona species; the morphotypes of Ornit. gestroi—for the whole genus Ornithophila; morphotypes 4P and 5E of H. equina—for the whole genus Hippobosca; morphotypes 1P and 2E of Or. stipeturi, Or. turdi, and Or. unicolor—for the whole genus Ornithoica; and morphotypes 1P and 1E identified in Orn. avicularia, Orn. anchineura, Orn. biloba, Orn. candida, Orn. comosa, and Orn. fringillina—for the whole genus Ornithomya (except Orn. chloropus).
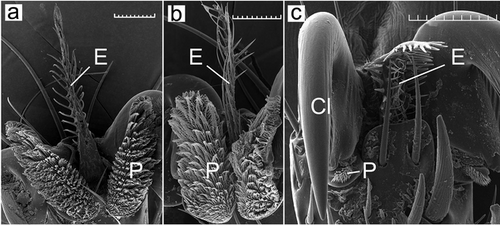
The pulvilli of the species M. ovinus are reduced, but most likely, the hooks on them previously belonged to the morphotype 4P (Fig. 3c). The empodius of M. ovinus belongs to the 5E morphotype (Fig. 3c), but has a structure twice as wide as that of Hippobosca. The pulvillus hook morphotypes 1P, 2P, and 3P differ from each other only by subtle morphological differences but significantly differ from hooks of the 4P morphotype in their elongated lobes. The comprehensive analysis of both molecular–genetic relationships and functional–morphological characteristics of hippoboscid flies demonstrates a unique combination of tarsal claws, pulvilli, and empodia morphology in the genus Ornithoica (first cluster) for Hippoboscidae (Figs 1, 2). All genera from the first subcluster of the second cluster have bifid tarsal claws and 1E and 1P morphotypes (Figs 1, 2). The representatives of the second subcluster from Hippoboscinae have simple tarsal claws and 5E and 4P morphotypes; from Lipopteninae have simple tarsal claws and 3E, 5E, and 4P morphotypes; from the tribe Olfersini have bifid tarsal claws and 1E, 4E, 1P, and 3P morphotypes (Figs 1, 2).
DISCUSSION
Phylogenetic analysis of the family Hippoboscidae
The analysis of the literature showed that although most hippoboscid flies are able to parasitize a wide range of hosts, depending on their ecology and biology (Lehikoinen et al. 2021), groups of preferred host species can still be distinguished. Hosts predominantly associated with certain hippoboscid fly species are shown on the dendrogram (Figs 1, 2). In the analyzed hippoboscid genera from Palearctic, there are several levels of division based on host–parasitic relationships. The first level is parasitism in mammals or birds. Further, the genera are distinguished by the size of the host range. In mammals, these are parasites of Eungulata and Carnivora, for example, Hippobosca spp. A narrower specialization was revealed, for example, in Melophagus. The ked M. ovinus parasitizes the family Bovidae, mainly domestic sheep (Ovis aries Linnaeus, 1758) (Doszhanov 1980, 2003). Highly specialized species (the genus Lipoptena) were found on Cervidae (Doszhanov 2003). Similar regularities were noted in bird parasites. A wide range of hosts is characteristic of many species from the studied genera: Orn. anchineuria (Ratzlaff 2017), Or. aequisenta (Wang et al. 2022), Or. exilis (Nartshuk et al. 2019), Or. stipeturi (Doszhanov 2003), Or. turdi (Doszhanov 2003; Trilar, Kremar 2005), O. australasiae (Doszhanov 2003), O. erythrocephala (da Silva et al. 2021), Orn. avicularia (Doszhanov 2003; Oboňa et al. 2019), Or. unicolor, and Ornit. gestroi (Doszhanov 2003). Some species parasitize a certain order of birds, for example, Passeriformes—Orn. chloropus (Doszhanov 2003; Nartshuk et al. 2020) and Orn. fringillina (Doszhanov 2003; Oboňa et al. 2019; Nartshuk et al. 2020) and Columbiformes—P. canariensis (Doszhanov 2003); some species parasitize only one or two families: Ardeidae, Ciconiidae—I. ardea (Maa 1969d), Muscicapidae—Orn. candida, and Hirundinidae—C. hirundinis, Orn. biloba (Doszhanov 2003; Oboňa et al. 2019), and Orn. comosa (Doszhanov 2003; Nartshuk et al. 2019, 2020). In relatively rare instances, species can be considered highly specialized (C. pallida) (Doszhanov 2003; Lehikoinen et al. 2021).
The comprehensive analysis of both molecular–genetic and host–parasitic relationships of hippoboscid flies demonstrates some evolutionary trends (Figs 1, 2). The first cluster is formed by the genus Ornithoica, and the first subcluster of the second cluster includes louse flies, which parasitize only birds. The second subcluster is formed by two groups: keds, parasitizing mammals, and a younger group of bird parasite species from the genera Icosta and Pseudolynchia.
The question was: did Hippoboscidae flies initially parasitize birds (Bequaert 1954) or mammals (Hennig 1965)? According to Petersen et al. (2007) and de Moya (2019), the ancestor of hippoboscid flies fed on the blood of mammals, while at the same time, the age of the split between mammals and birds (Blair & Hedges 2005) is over 310 Mya and greatly pre-dates the speciation of hippoboscoid flies given that the earliest confirmed calyptrate fossil belongs only to the Oligocene (Michelsen 2000; Petersen et al. 2007).
According to our data received on the evolution of hippoboscid flies in the Palearctic, flies parasitizing birds are divided into three independent branches: the genus Ornithoica, the tribe Olfersini (genera Icosta and Pseudolynchia), and other genera. An early independent origin of the genus Ornithoica was indirectly confirmed by Liu et al. (2017). Parasites of mammals forming a single cluster began to parasitize their hosts later, at least in relation to the genus Ornithoica. It is possible that the tribe Olfersini diverged from the parasites of mammals. This refutes the Petersen et al. (2007) assumption that hippoboscid flies, as parasites of birds, independently evolved at least twice: once in the Ornithomyini group and the second time in Olfersini (data without Struthiobosca Maa, 1963). We believe that this process took place three times: the first in Ornithoica, and then in Ornithomya and Olfersini.
Wing morphology
In 17 studied species of bird parasites, the wings are normally developed (Figs 1, 2) except for Crataerina flies with reduced wings unsuitable for active flight (Doszhanov 1980; Hutson 1984). In laboratory experiments, the C. pallida flies were able to perform only small jumps and glide in the air for very short distances (Eichler 1939; Büttiker 1944). The reduced wings are frequently observed among ectoparasites and are probably correlated with increased mobility on the host (Andersen 1997). Among mammalian parasites, Lipoptena species differ from other genera in breaking off their wings after finding a suitable host. Melophagus has no wings at all. In Hippobosca, the wings are normal but differ (as in Lipoptena) in the reduction of the medial veins (Doszhanov 1980; Hutson 1984; Liu et al. 2019). The more complicated structure of venation and the presence of microtrichia may be caused by the active flight mechanics (Doszhanov 1980; Hutson 1984; Liu et al. 2019). In particular, a large area of the wing filled with microtrichia was marked for Orn. biloba and Orn. comosa; the absence of microtrichia on the wings is typical for the genera Ornithophila and Crataerina. Ornithophila gestroi is additionally distinguished by the location of some wing veins, which confirms its possibly independent adaptation to birds.
Wing morphology may be one of the evolutionary markers for the separation of both higher taxa and genera (Krzeminski 1992). Our results assume an independent decrease in flight ability in hippoboscid flies, which is confirmed by the low flight activity in the species of the genus Crataerina (Liu et al. 2019). In the evolution of Hippoboscidae, but for the species of the genus Ornithophila, the level of wing reduction could be a factor determining the absence of microtrichia. The wing reduction arose either due to living on a large host or due to feeding specialization on a certain host range (Figs 1, 2).
Attachment apparatus morphology
The empodius morphotype 1E (Fig. 3a,b) with long identical setae is present in most of the studied species. This morphotype is closest to the original. This structure is specialized in the genus Ornithoica (2E) and the species I. ardea (4E, hosts Ardeidae), which differs in this feature from the same tribe species P. canariensis (1E, hosts Columbiformes). Special morphotypes of empodius (3E and 5E) are typical for parasites of mammals. Morphological features of the empodius of bird parasites from the Olfersini tribe are close to the empodius morphology of the mammalian parasites, which confirms the assumption of an independent origin of this tribe from the other bird parasites. Also, the specialization to parasitism of Lipoptena species on Cervidae determined the structure of the empodius with setae on the basal and apical parts, differing in morphology and length (3E morphotype). This morphotype is possibly derived from the 5E morphotype of empodius, which has differences in setae on the marginal and inner zones.
Pulvillus hook morphotypes separate parasitic flies of birds (with 1P, 2P, and 3P morphotypes) from mammalian parasitic flies (with 4P morphotype) (Figs 1, 2). The 1P morphotype with long hooks and rounded inner edges is original and typical for most bird parasite louse fly species. Other hook morphotypes are typical for Orn. chloropus flies (2P), the parasite of Passeriformes, and P. canariensis (3Р), the parasite of Columbiformes. This morphological specialization is probably not directly related to the adaptation to a new host since Passeriformes is parasitized by flies from different genera, the hook morphotype of which is 1Р.
The morphology of the tarsal claws clearly separates mammalian and bird parasites. The tarsal claws of the species from the genera Crataerina, Icosta, Ornithoctona, Ornithomya, Ornithophila, and Pseudolynchia are bifid, while Hippobosca, Lipoptena, and Melophagus tarsal claws are simple (Doszhanov 1980, 2003) (Fig. 3c). For Hippobosca, the attachment mechanism to the host hair with the simple tarsal claws was studied as well as the important role of simple claws in the attachment to the host was noted (Hayer et al. 2022). As in the case of the morphology of the pulvilli and empodius, it can be assumed that the change in the shape of claws is associated with the adaptation to birds.
However, among the bird parasites, the genus Ornithoica stands out not only due to the unusual morphology of the empodius but also due to simple claws, as in mammalian parasites (Figs 1, 2). The empodius has morphological structures that distinguish it from all of the other groups. The complex of morphological features confirms the above assumption about the independent origin of the genus Ornithoica from a common hypothetical ancestor of the flies in the family Hippoboscidae.
General patterns in the morphology of the flight and attachment apparatus
Results obtained by overlaying claws, wings, pulvilli, and empodium morphology data on the phylogenetic tree of the family Hippoboscidae in comparison with the host range identified a number of patterns. The separation of large groups and the allocation of some genera correlate with changes in the morphology of claws, wings, and empodius. The morphology of the empodius seems to be more related to the biology of these groups than other structures (Yatsuk et al. 2023). Highly specialized species can be distinguished, in particular, by small changes in the morphology of the pulvilli. It was experimentally shown (Yatsuk et al. 2022) that the hooks of 3P and 4P morphotypes were not able to attach efficiently to the basal cells of down feathers, as compared to hooks of 1P morphotype pulvilli, which attach to all parts of the feather. The functional features of the 1P pulvilli allow flies to parasitize a wide range of birds.
It is suggested that the origin of true ectoparasitism within the superfamily Hippoboscoidea occurred between 62 and 67 Mya (de Moya 2019). Modern birds and mammals began to diversify after the massive Cretaceous–Paleogene extinction event around 66 Mya (O'Leary et al. 2013; Prum et al. 2015). Perhaps, that is why the Ornithomyini species actively evolved along with birds (de Moya 2019), which contributed to the uniformity of the morphology of the attachment apparatus of flies. An independent adaptation to birds occurred in the genera Icosta and Pseudolynchia, which are members of the tribe Olfersini. The tribe mainly consists of tropical species which do not breed in moderate climates. Thus, the host switch, that is, to birds, could occur independently three times.
According to the literature, the genus Ornithoica, which has independently passed to birds, has many plesiomorphies (Maa 1966b), such as seven developed tergites on the abdomen of females and six on the abdomen of males (while other hippoboscid flies show a decrease in the number of tergites) (Maa 1966b; Doszhanov 2003), weakly developed humeral calli, the presence of a well-developed notopleural suture, the completely separated basal antennomere, a short and simple frons, completely developed ocelli, and a concealed inter-antennal area (Bequaert 1953; Maa 1966b). Hennig (1973) even identified the genus Ornithoica as a separate taxon, Ornithoicinae. We marked the following features of the attachment apparatus in this genus: The claws are simple, like those of mammalian parasites, while the empodius is covered with long identical setae and transverse folds, which are not found in species from other genera. The form of hooks on the pulvillus is typical for bird parasites. The genus Ornithoica diverged from the putative ancestor earlier than other genera and retained a number of more generalized characters. Thus, we believe that the tribe Ornithomyini is a paraphyletic group, since, according to the complex of morphological features, the genus Ornithoica can be considered a separate line of evolution.
Based on the analysis of phylogeny and morphology, simple claws, empodius with long setae, and hooks on pulvilli with long lobes can be attributed to the original features of the Hippoboscidae attachment apparatus. In general, bird parasites are characterized by bifid tarsal claws, long hooks on pulvilli, and long empodium setae. Mammalian parasites are characterized by simple tarsal claws, short lobes of hooks on pulvilli, and zones on empodius with short setae. Specialization in empodius and pulvillus morphotypes and wing reduction are more highly diverged in mammalian parasites than in bird parasites since parasitism on big animals helps to minimize the search time for any suitable animal for feeding and reproduction. The patterns obtained confirm the significant role of the analyzed parts of the attachment apparatus and the high effectiveness and versatility of the elements of the legs for the successful functioning of the structures necessary for attachment and movement of the hippoboscid flies on their hosts. We believe that an integrative, comprehensive approach using molecular and morphological techniques expands our knowledge of the morphological changes during the evolutionary process, clarifying disputed issues on taxonomy in the Hippoboscidae family.
ACKNOWLEDGMENTS
We would like to express our profound gratitude to our colleagues from the Center for the Joint Use of «Instrumental Methods in Ecology» at the IEEE RAS for the opportunity to use a scanning electron microscope. We thank Dr. Jacob Wickham, A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, for the English language editing of an advanced draft of this manuscript. The work was performed as part of the State Research Projects of the A.N. Severtsov Institute of Ecology and Evolution (number FFER-2024-0018) and Zoological Institute (number 122031100272-3).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.




