Molecular evolution of wound healing-related genes during cetacean secondary aquatic adaptation
Abstract
The marine environment presents challenges for wound healing in cetaceans, despite their remarkable recovery abilities with minimal infections or complications. However, the molecular mechanism underlying this efficient wound healing remains underexplored. To better understand the molecular mechanisms behind wound healing in cetaceans, we investigated the evolutionary patterns of 37 wound healing-related genes in representative mammals. We found wound healing-related genes experience adaptive evolution in cetaceans: (1) Three extrinsic coagulation pathway-related genes—tissue factor (F3), coagulation factor VII (F7), and coagulation factor X (F10)—are subject to positive selection in cetaceans, which might promote efficient hemostasis after injury; positive selection in transforming growth factor-beta 2 (TGF-β2), transforming growth factor-beta 3 (TGF-β3), and platelet-derived growth factor D (PDGFD), which play immunological roles in wound healing, may help cetaceans enhance inflammatory response and tissue debridement. (2) Coagulation factor XII (F12) is the initiation factor in the intrinsic coagulation pathway. It had a premature stop codon mutation and was subjected to selective stress relaxation in cetaceans, suggesting that the early termination of F12 may help cetaceans avoid the risk of vascular blockage during diving. (3) Fibrinogen alpha chain (FGA) and FIII, which were detected to contain the specific amino acid substitutions in marine mammals, indicating similar evolutionary mechanisms might exist among marine mammals to maintain strong wound-healing ability. Thus, our research provides further impetus to study the evolution of the wound healing system in cetaceans and other marine mammals, extending knowledge of preventing coagulation disorder and atherosclerosis in humans.
INTRODUCTION
Effective wound healing is one of the basic biological processes required for mammalian survival (Cano Sanchez et al. 2018). On the one hand, the wound must be exposed to enough oxygen to promote an inflammatory reaction and neovascularization (Bishop 2008). On the other hand, the wound must be kept sterile to prevent infection during wound healing (Guo & Dipietro 2010). However, it is difficult to meet these requirements in the marine environment: saltwater has extremely little dissolved oxygen and lots of bacteria, which poses a severe challenge to wound healing in marine animals (Bergh et al. 1989; Suttle 2007; O'Boyle et al. 2009).
According to previous studies, due to marine mammals living in a fluid environment, which is different from terrestrial mammals, researchers have tried to find the specific mechanism of marine mammals. Studies have shown that since fish live in a fluid environment, it is even more urgent that they are able to rapidly re-epithelialize their wound. Fish's epidermal cells could rapidly respond to a wound in vivo, while the immune system of fish is also different from other mammals (Griffeth et al. 2014; George & Martin 2022). Meanwhile, cetaceans are frequently injured by predation (Corkeron et al. 1987) and boat propellers (Bloom & Jager 1994), and a stronger and more efficient wound-healing ability is required for surviving in the harsh marine environment, for cetaceans. Studies have also found that cetaceans also have extremely strong wound-healing abilities (Zasloff 2011); thus, we hold the view that the marine mammals’ wound-healing ability is stronger than that of terrestrial mammals. The analysis of the convergent amino acid (AA) substitutions among marine mammals might provide some new clues for further study about the different wound-healing abilities between marine mammals.
Several studies have reported that cetaceans tend to show outstanding wound-healing abilities; the wound always heals within as few as 30 days, even in the case of severe injuries like exposed muscle tissue (Corkeron et al. 1987). Meanwhile, Greenwood et al. (Greenwood et al. 1974) found that cetaceans heal faster than terrestrial mammals. Previous research has found that the wound could stop bleeding within 2 min and heal over in about 7 days in bottlenose dolphins (Tursiops truncates) (Bruce-Allen & Geraci 1985). Interestingly, cetaceans are rarely infected and have fewer complications during wound healing (Weller et al. 1997; Giménez et al. 2011; Zasloff 2011), which means that cetaceans have a very marked ability to protect themselves from bacterial and virus infections in the marine environment.
Wound healing in mammals requires a large number of immune-related genes and proteins to finish diverse physiological activities. The regulation of these events is multifactorial, and the process is controlled by a range of physical, chemical, and biological signals. These processes triggered by tissue injury involve four overlapping but well-defined phases of coagulation, inflammation, proliferation, remodeling, and scar maturation (Stuart & John 2008).
The first step during wound healing—coagulation—generally occurs right after the body is harmed and blood vessels are damaged (Opneja et al. 2019). More than 20 proteins in the blood are involved in blood coagulation, which is a complicated and regular process (Doolittle 2009); a large proportion of these proteins are serine proteases, which are present in the blood as zymogens when these proteases are inactive (Eming et al. 2014). Among them, the core fibrinogen-related proteins consist of 11 coagulation factors, including FG, FII, FIII, FV, FVII, FVIII, FIX, FX, FXI, FXII, and FXIII (Kawthalkar 2013). Among them, FG is encoded by the α, β, and γ genes of fibrinogen (FG) (FGA, FGB, and FGG); and FXIII is produced from two genes—coagulation factor XIII A chain (F13A1) and coagulation factor XIII B chain (F13B).
In the currently accepted model of the coagulation cascade (Fig. 1), coagulation is mainly divided into two stages: the extrinsic coagulation pathway caused by tissue damage and the intrinsic coagulation pathway, which is self-activated in vivo. The extrinsic coagulation pathway includes FIII, FVII, and FX (Norris 2003). FIII and FVII could self-activate and combine in ruptured blood vessels to form the active tissue factor/coagulation factor VIIa (TF/FVIIa) complex after injury, which initiates the coagulation pathway.
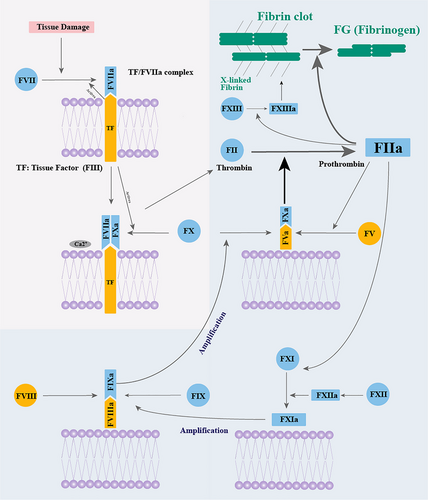
As an initiation factor in the intrinsic coagulation pathway, FXII self-activate upon encountering an intravascular foreign body to further amplify the coagulation cascade. When cetaceans ascend too quickly from deep dives, the nitrogen dissolved in their blood can form bubbles due to the decrease in pressure. These bubbles, which are considered intravascular foreign bodies, could initiate FXII to form thrombus. Previous studies have shown that the knockout of F12 could decrease the formation of the thrombus in mouse and did not influence the hemostasis after the vascular damage. That is to say, the lack of FXII and the pseudogenization could reduce the chance of blood clots.
During the intrinsic coagulation pathway, the activated FVa cofactor forms a FVa/FXa complex with activated FX, which is essential for thrombin formation. After a series of reactions, the soluble fibrinogen forms a stable fibrin clot by interacting with coagulation factor XIII A chain (FXIIIa). Then, plasminogen dissolves the fibrin of blood clots to maintain blood circulation.
In blood circulatory system, the coagulation and anti-coagulation systems intricately interact and mutually condition each other, which is essential for appropriate hemostasis after injury and for keeping blood circulation by preventing excessive blood clotting.
The transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF) gene families play comparable immunological roles, such as inflammatory response, angiogenesis, granulation tissue development, and epithelium regeneration (Yoshimura & Muto 2011). The PDGF is encoded by four genes—platelet-derived growth factor subunit A (PDGFA), platelet-derived growth factor subunit B (PDGFB), platelet-derived growth factor C (PDGFC), and platelet-derived growth factor D (PDGFD)—each of which plays a different role in all stages of wound healing (Heldin & Westermark 1999).
Along with physiological experiments and morphological observations, evolutionary analyses have been done on the wound healing of cetaceans. Research on the cetacean genome found that the cetacean coagulation-related genes F12 and kallikrein B1 (KLKB1) were terminated prematurely during the evolution process; researchers believe that it may help relieve the pressure on the blood vessels of cetaceans when they dive into the deep ocean (Huelsmann et al. 2019).
In addition, strong signals of positive selection were detected in F7, F8, and FGA of cetaceans compared to non-mammals such as fish (Mariz & Nery 2020), which means that the wound healing-related genes of cetaceans and non-mammals are under different selective pressures in the same habitat. The studies above suggest that the evolution of several wound healing-related genes in cetaceans might be associated with the adaptation to aquatic habitats. However, the mechanism of strong wound-healing ability under the condition of partial loss of genes and dramatic changes in habitat is still unclear.
Therefore, positive selection and specific AA substitutions in cetaceans were performed using 37 wound healing-related gene sequences from 47 mammals to explore the molecular evolution of wound healing-related genes in marine mammals (especially cetaceans) and other mammals. The results provide new insights into the efficient wound-healing ability of marine mammals, especially of cetaceans. Meanwhile, the specific AA substitutions of wound healing-related proteins in marine mammals were also analyzed to illustrate the shared features among marine mammals. This study also provides a reference for treating human (Homo sapiens) coagulation disorders, atherosclerosis, and other diseases.
MATERIALS AND METHODS
Sequence retrieval and alignment
Coagulation, inflammation, proliferation, remodeling, and scar maturation are the well-defined phases during the wound-healing process. Wound healing-related genes used in this study were collected from the previous studies (Table S1, Supporting Information). We selected 37 wounding healing-related genes from 47 (22 marine and 25 terrestrial) mammals (Table S2, Supporting Information). The orthologous coding sequences were retrieved mainly from online databases, including National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/), Ensembl (http://uswest.ensembl.org/index.html), and the Orthologous Mammalian v10 database (OrthoMaM, https://orthomam.mbb.cnrs.fr/) (Table S2, Supporting Information) (Berta et al. 2005).
For the sequences that were not available from the online database, we used the function BLAST GUI Wrapper–Several Sequences to a Big File in TB tools (Chen et al. 2020) to extract each exon of the wound healing-related genes from the genome downloaded by NCBI using the well-annotated gene sequences of cow (Bos taurus) as the query. Then, we connected the exons into the entire coding sequence according to the known sequences with the highest sequence similarity. All taxonomy and sequence information are in Table S2, Supporting Information.
To analyze the molecular evolution of pseudogene F12, the sequences of F12 of 96 species in 20 orders were retrieved by the basic local alignment search tool (BLAST) program online (http://www.ncbi.nlm.nih.gov/BLAST) and local BLAST database (Table S3, Supporting Information). All nucleotide sequences were aligned using the MUSCLE algorithm (Edgar 2004) in MEGA6 (Tamura et al. 2013) and trimmed manually, and the phylogenetic trees were retrieved using TIMETREE (http://www.timetree.org/) (Figs 2, 4,5).
Analysis of selective pressure and identification of pseudogenes
The measure of natural selection acting on the genes was determined by estimating the ratio of nonsynonymous (dN) and synonymous (dS) substitution rates (ω = dN/dS) implemented in the CodeML program of the PAML software package v4.9 (Yang 2007). Briefly, ω < 1, ω = 1, and ω > 1 indicate purifying selection, neutral evolution, and positive selection, respectively.
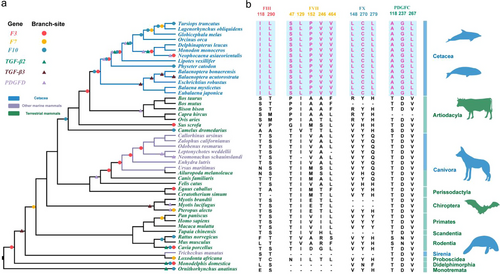
In this study, we used the widely recognized phylogenetic tree in the evolution of mammals (Fig. 2a). The branch-site model was used to detect positive selection in wound healing-related genes. We chose all terminal branches as foreground branches and ancestral branches as foreground branches to run the PAML script each time; we analyzed the positive selection sites until all points in the phylogenetic tree had been set as foreground branches. The sites on different branches have different ω values. We compared the null hypothesis, Ma0 (neutral model: 0 < ω0 < 1, ω1 = 1, and ω2 = 1), with the alternative hypothesis, Ma (positive selection model: 0 < ω0 < 1, ω1 = 1, and ω2 ≥ 1), to detect any positive selection.
Pseudogenes are those sequences in the genome that bear similarity to specific protein-coding genes, but are unable to produce functional proteins due to the existence of frameshifts, premature stop codons, or other deleterious mutations (Mighell et al. 2000). Depending upon the functionality, pseudogenes have been described as either “Dead” pseudogene that is non-transcribing or as “Ghost” with intermediate functionality (Zheng & Gerstein 2007).
In this study, the pseudogenes were analyzed in terms of their evolutionary history, especially F12. Previous studies have found that F12 is terminated prematurely in cetaceans (Lewis et al. 1969; Huelsmann et al. 2019). The selective pressure on pseudogene F12 was analyzed using a previously developed approach (Zhao et al. 2010). We estimated the rate ratio (ω) of nonsynonymous to synonymous substitutions using PAML (Yang 2007), aligned the F12 sequences from the 96 species in 20 orders, and removed the codons where indels are found. We then compared a series of evolutionary models in the likelihood framework, using the phylogenetic tree shown in Fig. 2.
We also used RELAX, from the internet service Datamonkey (http://www.datamonkey.org/), to identify selective pressure relaxation in cetaceans. To further evaluate the results of the five models above, the method uses the branch-site model to calculate the value and determine if the gene is under relaxed selective pressure based on the range of the ω value.
Labeling positively selected sites on the three-dimensional (3D) structure of proteins
To highlight the importance of positively selective sites in terms of protein function, we mapped the sites onto the 3D structure of proteins. First, the AA sequences of FIII, FVII, and FX from humans were used to predict the 3D structure by the I-TASSER-MTD web tool according to the given quality criteria (https://zhanggroup.org/I-TASSER-MTD/) (Zhou et al. 2019, 2022). Then, we mapped the positively selected sites onto three-dimensional protein structures by PyMOL (The PyMOL Molecular Graphics System, Version 1.4 Schrödinger, LLC). Finally, we used the Uniport (https://www.uniprot.org/) (Rolf et al. 2004) and the Pfam database (http://pfam.xfam.org/) to view the important functional domains of each gene.
Convergent AA substitutions analysis
Convergent phenotypic characteristics are attributed to specific AA substitutions that evolve in different species independently (Natarajan et al. 2015). To explore how changes in single AA sites contribute to the evolution of wound healing-related genes, we detected the molecular basis of convergent evolution among marine and terrestrial mammals. AA sites which are the same in at least two lineages of mammals are considered group-specific convergent AA changes in this study (Huang et al. 2021). The specific AA substitutions in marine mammals were detected using the function Segregating in FasParser (Sun 2017), which could provide evidence for the convergent evolution of wound healing-related genes in marine mammals. Forty-seven mammal species were classified into marine mammals and terrestrial mammals in this part (Fig. 4). When using this program, we designated the coding sequences (CDS) of marine mammals as Group 1, and those of terrestrial mammals as Group 2. We defined the frequency cutoff for both Gropu 1 and Group 2 as 0.8. This means that if a specific amino acid locus in the CDS is consistent in over 80% of soecies in Group 1 and similary in Group 2, then that locus is recognized as a convergent AA substitution in Group 1. That is to say, the AA sites that were shared among marine mammals but differed from most species of terrestrial mammals were defined as specific AA substitutions in marine mammals. Human canonical sequences in UniProt (https://www.uniprot.org/) (Rolf et al. 2004) were taken as references for the locations of amino acids.
Functional effects estimating of convergent AA substitutions
We tested the potential effects of these convergent AA substitutions on the corresponding proteins using PolyPhen-2 and the sorting intolerant from tolerant (SIFT). The output of the PolyPhen-2 prediction pipeline is a prediction of benign, possibly damaging (with low confidence), and probably damaging (with high confidence), along with a score ranging from 0 (benign) to 1 (damaging) (Adzhubei et al. 2013). Meanwhile, SIFT was also used to test the effect; the SITF score ranges from 0 to 1. When AA substitution is predicted damaging, it is less than or equal to 0.05; otherwise, the substitution is tolerated (equal to benign or possibly damaging in PolyPhen-2) (Sim et al. 2012).
RESULTS
Positively selected wound healing-related genes in cetaceans
Using the branch-site model in PAML, the signals of positive selection were found in the three coagulation-related genes (F3, F7, and F10), and three members of TGF-β and PDGF gene family—TGF-β2, TGF-β3, PDGFD genes (Fig. 2a; Table S4, Supporting Information). The results suggest that there were more positively selected branches detected in the cetaceans than in other mammals. The ratio of the number of positively selected branches of cetaceans and the number of cetacean species is 2, and for other mammals, the ratio is approximately equal to 1, which means that the cetaceans have more positively selected branches (Table S5, Supporting Information). Studies above show that compared with other mammals, the cetaceans have experienced stronger positive selection in genes related to wound healing.
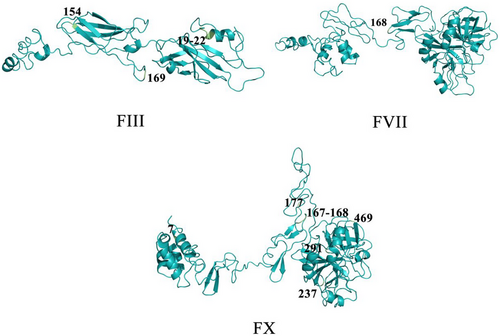
To highlight the importance of positively selective sites in terms of protein function, we mapped the sites onto the protein 3D structure of humans. The protein structures showed the positively selected sites of FIII, FVII, and FX are located within or near important functional domains. For example, residues 19–22 of FIII and residue 7 of FX are located in the alpha-helix. Residue 130 of FIII is located in the beta-strand. Residues 130 and 169 of FIII, residue 168 of FVII, and residues 167, 168, 177, 237, 291, and 469 of FX are located in random coils. Moreover, residues 19–22 of FIII are located in the tissue factor domain, and residues 154 and 169 are located in the fibronectin type III domain, which could promote the efficiency of the formation of TF/FVIIa complex (Muller et al. 1994). In addition, residues 237 and 291 of FX are located in the trypsin domain, which could activate prothrombin transformation process (Hertzberg 1994) (Fig. 3; Tables S4,S6, Supporting Information).
Cetacean-specific AA mutations and indels were involved in functional domains
The cetacean-specific AA substitutions were detected to identify any evolutionary differences in wound healing-related genes between cetaceans and other mammals. Thirteen cetacean-specific AA substitutions were detected in four proteins: FIII (118I, 290L), FVII (47S, 129L, 152P, 246V, 464V), FX (148L, 270C, 279L), and PDGFC (118A, 237G, 267L) (Fig. 2b). Of these, FVII 129L was located in the EGF-like domain, FVII 152P was located in the EGF-like 2 domain, and FVII 246V was located in the trypsin domain; the 148L sites of FX are located at the coagulation factor Xa inhibitory site and 270C and 279L were found in the trypsin domain; the 118A site of PDGFC was located in the complement C1r/C1s, Uegf, Bmp1 domain (CUB domain), while residue 267L is located in the PDGF/VEGF domain (Table S6, Supporting Information). All the AA residues above take the human protein sequence as a reference.
Relaxation of selective pressure of F12 in cetaceans
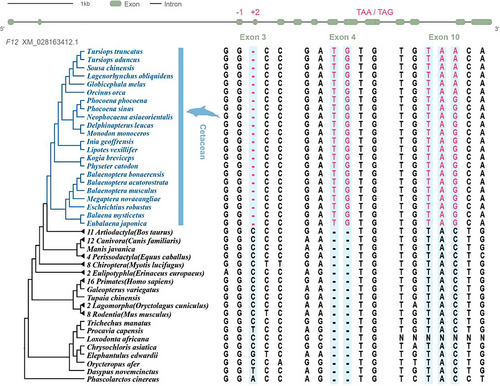
We found that F12 in cetaceans has multiple inactive mutations in exon 3 (a deletion of a base C), exon 4 (the insertion of two bases T and G), and exon 10 (a mutation of TAC in exon 10 to stop codon TAA or TAG) of cetaceans (Fig. 4).
To analyze the selective pressure on F12, we estimated the rate ratio (ω) of nonsynonymous to synonymous substitutions using PAML. We found that the average ω across the tree is 0.20, which is significantly lower than 1 (P = 0; Table 1), indicative of a generally strong purifying selection on F12 in marine mammal evolution, consistent with the fact that F12 became pseudogenized in the cetacean branch. The two-ratio model that allows a difference in ω between the cetacean branch (ω = 0.42) and all other branches (ω = 0.18) fits the data significantly better than a one-ratio model that assumes a single ω for all branches (P = 2.17 × 10−6; Table 1). Interestingly, a more general model that allows the variation of ω across all branches does not fit significantly better than the above two-ratio model (P = 0.34; Table 1), suggesting that ω does not vary significantly among the non-cetacean branches. We further found that the ω for the cetacean branch in the two-ratio model is significantly lower than 1 (P = 1.37 × 10−6; Table 1), suggesting that the relaxation of the functional constraint on F12 did not happen immediately after the cetaceans diverged from other marine mammals but rather more recently.
| Models | ω | lnL | Models compared | 2Δ (lnL) | P-value |
|---|---|---|---|---|---|
| A: One ratio ω | 0.20 | −8865.9 | |||
| B: One ratio ω = 1 | 1 | −9297.56 | B vs. A | 863.32 | 0 |
| C: The cetacean branch with pseudogenized F12 has ω2; others have ω1 |
ω1 = 0.18 ω2 = 0.42 |
−8854.68 | A vs. C | 22.44 | 2.17 × 10−6 |
| D: The cetacean branch with pseudogenized F12 has ω2 = 1; others have ω1 |
ω1 = 0.18 ω2 = 1 |
−8866.34 | D vs. C | 23.32 | 1.37 × 10−6 |
| E: Each branch has its own ω | Variable ω by branch | −8787.7 | C vs. E | 133.96 | 0.34 |
- 2Δ(lnL): twice the differences in lnL (likelihood) between the two models compared.
In addition, the test by RELAX also showed that when the P-value is significant (P = 0), F12 in cetaceans (k = 0.58) experienced relaxed selective pressure (Fig. S1, Supporting Information), which is consistent with results from the five models above.
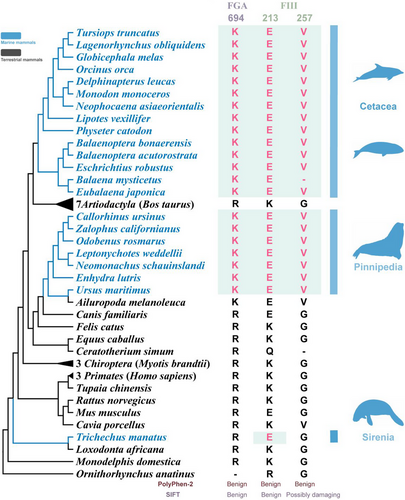
Convergent AA substitutions among marine mammals
FGA 694K, FIII 213E, and FIII 257V were identified as shared substitutions in marine mammals (Fig. 4). All the residues above take the human protein sequence as a reference. The AA residue at location 694 of FGA in marine mammals was Lys, and the AA residues at locations 213E and 257V of FIII in marine mammals were Glu and Val. Hereinto, FGA 694K was located at the fibrinogen beta and gamma chains/C-terminal globular domain. FIII 213E was located at the interferon-alpha/beta receptor (fibronectin type III) domain (Table S6, Supporting Information). These domains all play crucial roles in FGA and FIII (Mosesson 2005; Ivashkiv & Donlin 2014; McGlasson et al. 2015). Upon detailed examination of these three simultaneous amino acid alterations, it's evident that the shifts—R694K in FGA, K213E in F3, and G257K in FIII—are considered benign according to PolyPhen-2 results. Meanwhile, we also detected that two AA changes (R694K in FGA and K213E in FIII) are benign, the G257K in FIII is possibly damaging based on the results from SIFT, and all three AA changes in this study are tolerated. Taking these two methods into consideration, we speculate that these three AA changes yield a low proportion of affected phenotypes.
DISCUSSION
Adaptive evolution of wound healing-related genes in cetaceans
A series of evolutionary analyses of wound healing-related genes reveal the distinct evolutionary trajectories between cetaceans and other mammals, providing insight into the molecular mechanisms underlying strong wound-healing abilities of cetaceans in the harsh marine environment.
First, we found the positively selected wound healing-related genes—F3, F7, and F10—in cetaceans, which indicates that wound healing-related genes of cetaceans are under strong selective pressure compared to other mammals. Meanwhile, residues 154 and 169 of FIII are located in the fibronectin type III domain, through which FIII could combine with FVII to form the TF/FVIIa complex more effectively and initiate the coagulation cascade according to the previous study (Muller et al. 1994). Residues 237 and 291 of FX are located in the trypsin domain, which could activate the ser-his-asp (S-H-D) AA residues (Hertzberg 1994a) and dominate the binding of FX and FV during coagulation (Hertzberg et al. 1992); these processes may contribute to the transformation of prothrombin.
The immune system evolves continuously to protect against ever-changing pathogens. Marine pathogens and bacteria frequently infect the body through wound infection and blood circulation, and the coagulation response is an important line of defense against pathogens (Sun 2006). At the same time, pathogens can use the host's coagulation system to promote infection (Sutherland et al. 2012), which implies that the interaction between the body and pathogens may also be related to the positive selection in coagulation factors; the positive selection of the F7 in both cetaceans and fishes also supports this view (Mariz & Nery 2020). As a result, the positive selection of these coagulation factors of cetaceans may help them initiate the extrinsic coagulation cascade and form fibrin to engage in hemostasis more efficiently.
Meanwhile, the signals of positive selection were found in three members of the TGF-β and PDGF gene family—TGF-β2, TGF-β3, PDGFD genes, which are generated by pro-inflammatory cells like macrophages and fibroblasts, and they perform similar immune functions during wound healing, such as inflammatory response, angiogenesis, and remodeling. These genes encoding the proteins that are in direct contact with the external environment are likely to evolve more readily than other genes. Therefore, we speculate that genes involved in host–pathogen interactions are more likely to be under positive selection.
The signals of positive selection in immune-related genes were found in vertebrates and arthropods according to genomic analysis of different species (Nielsen et al. 2005; Studer et al. 2008; Roux et al. 2014). Thus, we hypothesize that, due to the evolution of inflammatory response-related genes, cetaceans may have enhanced inflammatory response and tissue debridement, which could accelerate wound healing and reduce the risk of infection and complication (Zasloff 2011).
Previous studies have primarily focused on understanding the heterogeneity of wound healing in terrestrial mammals, leaving significant gaps in our knowledge regarding the mechanisms, timing, and sequences of wound healing in marine mammals. Cetaceans, as secondary aquatic mammals, have garnered attention for their remarkable wound-healing abilities (Elwen & Leeney 2010; Bossley & Woolfall 2014; Zasloff 2011). However, to the best of our knowledge, there have been no comparative studies between cetaceans and fish in this field, warranting further investigation.
The extraordinary wound-healing capacity of cetaceans has been documented for several years (Zasloff 2011). Researchers have also identified histological features that contribute to their robust cutaneous wound-healing abilities (Bruce-Allen & Geraci 1985; Geraci & Bruce-Allen 1987). It is worth noting that fish, which inhabit a fluid environment, face unique challenges that demand rapid re-epithelialization of wounds. Fish epidermal cells demonstrate swift responses to in vivo wounds, and their immune systems exhibit differences compared to other mammals (Griffeth et al. 2014; George & Martin 2022).
Our focus on the formidable wound-healing capacities of cetaceans and other marine mammals aims to provide valuable insights into the comparison between cetaceans and their marine counterparts. This research seeks to shed new light on the intriguing field of wound healing in marine mammals.
Finally, specific AA substitutions were identified in four proteins (FIII, FVII, FX, and PDGFC) in 13 cetacean species. Most of these AA substitutions were located within specific functional domains of the genes. For instance, FVII 129L is situated within the EGF domain, known to enhance the formation of the TF/FVIIa complex through binding to Ca2+ (Eigenbrot 2002). The evolution of these residues may improve FVII's ability to bind Ca2+ effectively.
Cetacean-specific AA substitutions were also found in the trypsin domains of FVII and FX. These domains play a crucial role in activating downstream target genes and facilitating the coagulation cascade. Intriguingly, all four of these genes were among those identified as positively selected by the branch-site model. This suggests that adaptive evolution and specific mutations may have assisted cetaceans in adapting to the challenging marine environment.
The early termination of F12 in cetaceans might be beneficial to reduce the risk of vascular blockage during deep diving
In this study, we conducted a selective pressure analysis on the pseudogene F12. Functional assays from a previous study confirmed the loss of F12 in short-finned pilot whales (Globicephala macrorhynchus) (Semba et al. 1998). Genome analysis also revealed premature termination of F12 in baleen whales (Warren et al. 2017). However, previous studies primarily focused on specific cetacean species. Here, we discovered that premature termination of F12 is a common feature across all cetaceans. Additionally, the results of model comparison and RELAX analysis indicate that F12 has undergone relaxed selection in cetaceans.
Cetaceans, as secondary aquatic mammals, experience a decrease in heart rate, vasoconstriction, and decreased peripheral blood flow during diving (Kooyman & Ponganis 1998). At the same time, nitrogen microbubbles easily form in the blood after diving, which may activate F12 as a vascular foreign body and lead to thrombus formation (Radziwon et al. 2015). Functional assays have shown that F12 knockout can effectively reduce thrombosis in mammals and does not affect hemostasis after vascular injury (Kokoye et al. 2016). Interestingly, turtles in the same habitat also have deep diving habits and have been reported to lack F12 (Kokoye et al. 2016). Therefore, we hypothesize that the early termination of F12 may provide benefits to these species by reducing the risk of vascular blockage during deep diving.
Overall, the lack of F12 may slow down the amplification of the coagulation cascade, such as the intrinsic coagulation pathway. Meanwhile, positive selection signals were detected in genes related to the extrinsic coagulation pathway (F3, F7, F10 etc.). These signals suggest that the evolution of certain genes might enhance the adaptation of cetaceans to the physiological changes resulting from the partial absence of F12.
Functional adaptation of wound healing-related proteins of marine mammals
The variability in wound healing among various terrestrial mammals has been explored in previous research, while there are relatively few articles about the wound-healing abilities of marine mammals, apart from cetaceans. According to Stéphane Lair, a veterinarian and wildlife health expert (Whales Online 2023), seals enjoy substantial protection thanks to their thick layer of blubber. The State of Victoria Department of Environment, Land, Water & Planning also found that seals possess remarkable recuperative abilities. For instance, they frequently experience bites or scratches from other seals, which are typically superficial wounds that can heal on their own. It is worth noting that seal products can also benefit wound healing (The State of Victoria Department of Environment, Land, Water & Planning 2023). Furthermore, marine mammals have a remarkable capacity for recovering from injuries, and the saltwater environment plays a vital role in aiding the healing of their wounds (Whales Online 2021). Therefore, the analysis of convergent amino acid sites offers valuable insights into the study of these extraordinary healing abilities across different marine mammal species.
Three specific AA substitutions were found in coagulation-related proteins FGA and FIII among marine mammals. These proteins are involved in blood clot formation and coagulation initiation, respectively. Among them, FGA 695K is located in the fibrin C-terminal globular domain, which could catalyze the cross-linking of fibrin to form dimers through FXIII (Mosesson 2005). Cross-linked fibrin clots have greater stretching ability than non-crosslinked fibrin, which is helpful for hemostasis (Roska & Ferry 1982). Additionally, a previous study found parallel AA substitutions in the anticoagulation pathway-related gene SERPINC1 among marine mammals, such as walruses and cetaceans. Therefore, we speculate that marine mammals maintain homeostasis through the co-evolution of coagulation and anticoagulation mechanisms. Although the phenotypic effects of these AA substitutions are still unclear, the specific substitutions detected in coagulation pathway-related genes of various marine mammal species suggest that marine mammals might have developed similar evolutionary mechanisms to maintain strong wound-healing abilities in the same habitat conditions.
CONCLUSIONS
In summary, our study suggests that the adaptive evolution of wound healing-related genes (F3, F7, F10, TGF-β2, TGF-β3, and PDGFD) benefits the survival of cetaceans in the harsh marine environment. In addition, we speculated the early termination of F12, which is subjected to relaxed selection in cetaceans, is beneficial to reduce the risk of vascular blockage during deep diving and to blood circulation. Moreover, several convergent AA substitutions were detected in coagulation factors, which means that similar evolutionary mechanisms might have developed to maintain the normal wound-healing capabilities of marine mammals. Overall, this study provides novel insights into the study on the molecular mechanism of efficient wound-healing ability in cetaceans and also provides wound healing-related genes' target locus for coagulation disorder and atherosclerosis treatments in humans.
ACKNOWLEDGMENTS
This research was financially supported by the National Key Research and Development Program of China (grant no. 2022YFF1301602), the National Natural Science Foundation of China (grant nos. 32270442, 31872219, 31370401, 32030011, 31630071, and 31772448), the National Key Programme of Research and Development, Ministry of Science and Technology (grant no. 2016YFC0503200), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).




