Comparative phylogeography reveals dissimilar genetic differentiation patterns in two sympatric amphibian species
Abstract
Global climate change is expected to have a profound effect on species distribution. Due to the temperature constraints, some narrow niche species could shift their narrow range to higher altitudes or latitudes. In this study, we explored the correlation between species traits, genetic structure, and geographical range size. More specifically, we analyzed how these variables are affected by differences in fundamental niche breadth or dispersal ability in the members of two sympatrically distributed stream-dwelling amphibian species (frog, Quasipaa yei; salamander, Pachyhynobius shangchengensis), in Dabie Mountains, East China. Both species showed relatively high genetic diversity in most geographical populations and similar genetic diversity patterns (JTX, low; BYM, high) correlation with habitat changes and population demography. Multiple clustering analyses were used to disclose differentiation among the geographical populations of these two amphibian species. Q. yei disclosed the relatively shallow genetic differentiation, while P. shangchengensis showed an opposite pattern. Under different historical climatic conditions, all ecological niche modeling disclosed a larger suitable habitat area for Q. yei than for P. shangchengensis; these results indicated a wider environment tolerance or wider niche width of Q. yei than P. shangchengensis. Our findings suggest that the synergistic effects of environmental niche variation and dispersal ability may help shape genetic structure across geographical topology, particularly for species with extremely narrow distribution.
INTRODUCTION
A key focus of biogeography is exploring how and why environmental gradients, such as elevation and latitude, affect global patterns of biodiversity (Addo-Bediako et al. 2000; Gaston 2000). The evolutionary models disclosed by phylogeographical studies best illustrate the population demography among related lineages in geographic landscapes (Avise 2000; Buckley 2009) based on multiple datasets (e.g. environmental, genetic, and morphological information) (Hickerson et al. 2010). Due to the specificity of species traits, patterns disclosed by single species may be incomplete or misleading (Leaché et al. 2020; Wan et al. 2021). Simultaneous analysis of multiple species can clarify how the environment and heterogeneous landscapes shape the phylogeographic pattern across their range or the population demographic patterns over time (Carstens & Richards 2007), and how the ecological niche affects the genetic differentiation and distribution among populations or species, which is of particular interest under biodiversity conservation. Topographical or climatic barriers (e.g. a region's climatic history, hydrology, and geodynamics) act as the physiological barriers for species dispersal, which is directly related to species distribution patterns and range (Janzen 1967; Ghalambor et al. 2006).
Global climate change is expected to negatively affect the biodiversity or species distribution (Purvis et al. 2000; Parmesan & Yohe 2003; Thomas et al. 2004; Parmesan 2006; Barnosky et al. 2011; Dawson et al. 2011). Due to global warming and increased temperatures, some cold-adapted species may shift their ranges to higher altitudes or latitudes due to their sensitivity to high temperatures. However, species with slow migration rates or the limited availability of new habitats may experience a decrease or even extinction under the synergistic effects of narrow range size and niche (Ohlemüller et al. 2008; Chen et al. 2011; Lenoir & Svenning 2015). Furthermore, the fragmentation and contraction of the species’ geographical distribution directly cause biotic impoverishment, thus increasing the threat to these species (Thuiller et al. 2005; Devictor et al. 2008; Saupe et al. 2019). For the mechanism of species distribution contraction, the associated changes in climate conditions may not meet species’ climatic niche requirements, leading to local and/or global extinction (Walther et al. 2002; Thomas et al. 2004; Jiguet et al. 2010; Wiens 2016). For those species, the climate change features, such as speed and intensity, and biological variables, like physiological tolerance, morpho-functional traits, and life history, may also determine the responses to climate change (Araújo et al. 2013).
Climate stability during the geological period provides favorable conditions for the survival and evolution of more species (Rosenzweig 1995; Jansson 2003; Graham et al. 2006), thus promoting the formation of endemic species (Jansson 2003). These species form corresponding phylogenetic relationships along spatial and environmental gradients and branching changes related to phylogeny (Rosauer & Jetz 2015). In contrast, species with strong dispersal capabilities may be favored by dramatic climate changes, thus strongly affecting biodiversity patterns and resulting in the persistence of generalist species with large range sizes (Sandel et al. 2011). Also, under the dramatic climate change, species readjust their range and track suitable habitats due to the tendency of species to retain ancestral ecological features over time (i.e. niche conservatism) (Peterson et al. 1999; Parmesan & Yohe 2003; Thomas 2010), thereby leaving a marked imprint on future biogeographic patterns (Thuiller et al. 2011). To test niche conservatism hypotheses, populations or species’ niche measurements (e.g. similarity, identity, and overlap) were identified and compared (Broennimann et al. 2012). Under different climatic regimes, niche conservatism species often have relatively stable geographical distribution because they cannot disperse into others adjacent to (or potentially reachable from) their original and current distribution. In contrast, species with strong dispersal ability are generally unlikely to have relatively conserved niches. Therefore, although geographical or environmental barriers can limit the dispersal of the species, its range may also be affected by the species' dispersal ability (Wiens & Donoghue 2004). Widely distributed species tend to prefer a heterogeneous environment, while species with restricted distributions like more homogeneous environments (Slatyer et al. 2013).
The intrinsic factors for the coexistence of related species or similar niche species are the core of ecological and evolutionary research (Schoener 1974). For those coexistence species, the impact of abiotic/extrinsic factors (biogeographic barriers, geological events, or past environmental change) leaves prints on the genetic variation, which is of essential importance in forming phylogeographic concordance (Avise 2000; Chase 2003). According to the classical ecological theory, two species with similar niches cannot be sympatric due to the interspecific competition caused by limited resources (Schoener 1974; Finstad et al. 2011; Violle et al. 2011). On the other hand, for some species with similar niches, differences in the use of habitats and their associated environmental variables (e.g. through character displacement) make sympatric distribution possible (Grant & Grant 2006). Also, habitat structure and resource availability may be key in the sympatric distribution.
In nature, some closely related species have two completely different distribution ranges, that is, high restriction and wide distribution. This has attracted the attention of many researchers (Gaston 1996), as this type of study allows us to distinguish the degree of similarity or difference among these climatic conditions in different regions of species (Quintero & Wiens 2013). To disclose the intrinsic factors affecting variation in geographical ranges between species, several hypotheses have been proposed, such as niche breadth (Boulangeat et al. 2012; Botts et al. 2013; Slatyer et al. 2013; Vela Díaz et al. 2020), climatic variability (Boucher-Lalonde et al. 2016), climate tolerance (Pither 2003; Sheth & Angert 2014), evolution (Gaston et al. 1998), complex interactions (Brown et al. 1996), energy availability (Morin & Chuine 2006), glacial history (Jansson 2003), and the integration of habitat area and climate stability (Morueta-Holme et al. 2013). Considering the link between range sizes and global extinction risk (Purvis et al. 2000; Botts et al. 2013), the determinants of range sizes are more likely to influence their ability to alter ranges in response to global climate change (Thomas et al. 2001; McCauley et al. 2014). Generally, narrowly distributed species are more susceptible to climate changes than widely distributed species (Thuiller et al. 2005; Ohlemüller et al. 2008; Slatyer et al. 2013) as they tend to have a lower level of adaptability to climate change (Thuiller et al. 2005; Loarie et al. 2009). Additionally, if species with a narrower distribution are more susceptible to climate change than those with a wider distribution, the outcome of global biodiversity is unfavorable (Thomas et al. 2004), especially given the narrow geographical distribution of most species (Gaston 1996). Compared with narrow distribution, species with wider geographical distribution are statistically more likely to cover a wider range of climatic conditions, indicating broader climatic tolerances and lower relative vulnerability for widely distributed species relative to narrowly distributed species (Birand et al. 2012). Consequently, addressing species range determinants (e.g. the environmental factors) is of crucial importance to elucidate which processes underlie species range at broad or narrow geographical distribution, and provide valuable perspectives for evolutionary and conservation biology in the context of expected climate change (Brown et al. 1996; Böhm et al. 2017; Faurby & Antonelli 2018; Newsome et al. 2020).
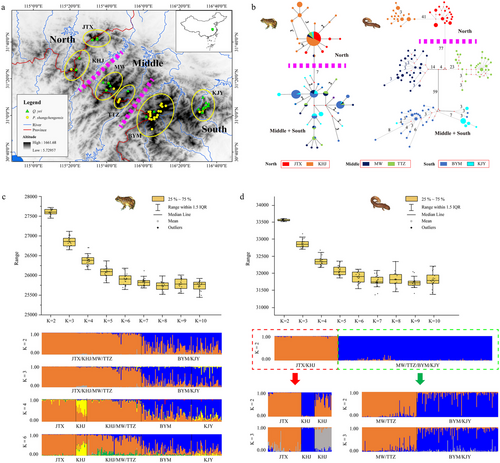
The Dabie Mountain is an ancient isolated mountain system with low and middle altitudes in East Asia, which has been listed as a priority area of biodiversity conservation in China (www.mee.gov.cn). The climate of this region is characterized by a transition from subtropical to temperate (Zheng et al. 2012). Ye's spiny-vented frog, Quasipaa yei (Anura, Dicroglossidae), is the unique anuran stream-dwelling species endemic to Dabie Mountain (Jiang et al. 2006), which is normally restricted to montane rocky stream habitats at altitudes of approximately 500 to 1000 m (Fei et al. 2012). On the other hand, the Shangcheng stout salamander (Pachyhynobius shangchengensis; Caudate, Hynobiidae) is a cool and oxygen-rich stream salamander narrowly distributed above 500 m in the Dabie Mountains, slightly higher than that of Q. yei (Fei et al. 2012). Based on the survey data, only six isolated populations of these two co-distributed species were found, corresponding to Jiaoyuan–Tanghui–Xiaolongtan (JTX), Kangwangzhai–Huangbaishan–Jiufengjian (KHJ), Mazongling–Wochuan (MW), Tiantangzhai (TTZ), Baimajian–Yaoluoping–Mingtangshan (BYM), and Kujingyuan (KJY) (Fig. 1). Also sky island distribution and deep genetic divergence were found among the P. shangchengensis populations (Pan et al. 2019a,b). Moreover, the spiny-vented frog has a slightly larger distribution area than P. shangchengensis. Considering the distribution characteristics of the two species, together with an existing phylogeographic pattern for P. shangchengensis, we predicted that these co-distributed species were ideal model systems that could elucidate how climate change and species traits (niche breadth and dispersal ability) can impact phylogeographic structure in mid-latitude mountain systems in China.
Herein, we used the two types of amphibians (frog and salamander) as a model to disclose the combined effects of climatic niche breadth (environmental factors) and dispersal ability on genetic structure and species geographical range sizes across different species in Dabie Mountains, East China. At the finer geographical scale, the divergence processes of different species can complement the relevant knowledge gaps, that is, they can be used to assess whether there is consistency or inconsistency in the spatial structure of different species, whether there is a synchronous or asynchronous divergence of species, whether there is an intrinsic link between the species traits (niche breadth and dispersal ability) and the heterogeneity of spatial structure (habitat preference), and whether a combined climatic niche breadth and dispersal ability influence the change of geographical range sizes. Therefore, based on these two ideal model species, we aimed to disclose the above scientific question within the mid-latitude mountain systems based on genetic and ecological data. to supplement the relevant knowledge system.
MATERIALS AND METHODS
Sampling collection
For Q. yei, we collected 262 samples from six populations (JTX, KHJ, MW, TTZ, BYM, and KJY) during 2012–2015 (Fig. 1). The toe tip of capturing adults (about 0.5 cm) was cut as samping. Absolute ethanol was used to preserve the samples, which were stored in the refrigerator (−80°C) immediately upon collection. For each population, no fewer than 16 individuals were sampled. In addition, all the genetic data and geographic information of the Shangcheng salamander were collected as previously described (Pan et al. 2019b).
DNA extraction, sequencing, and genotyping
Standard proteinase K/phenol–chloroform protocol was used to extract whole-genomic DNA (Sambrook et al. 1989). Samples were purified using an EasyPure PCR Purification Kit to purify the genome (TransGene Biotech, Beijing, China). Based on the mitogenome of Q. boulengeri (NC021937) and Nanorana pleskei (NC016119), Primer v.5 was used to design specific primer sets of NADH dehydrogenase 2 (ND2) gene (Table S1, Supporting Information) (Clarke & Gorley 2001). The total volume of the PCR amplification system was 30 μL, which contained 1 μL of genomic DNA (concentration 5–25 ng μL−1), 1.2 U Taq polymerase (TransGene Biotech), 2.4 μL of 1.2 mM dNTPs, 1.2 μL of 1.5 mM MgSO4, 3 μL 10× buffer, 0.5 mM of each primer, and sufficient pure molecular biology grade water. The PCR amplification program included an initial denaturation step at 95°C for 5 min, followed by 32 cycles of denaturation at 95°C for 30 s, primer annealing at 55°C for 30 s, extension at 72°C for 80 s, and finally extension at 72°C for 10 s. The PCR products were purified by an EasyPure PCR Purification Kit (TransGene) and sequenced on an ABI Prism 3730 automated sequencer by the BigDye Terminator v3.0 Ready Reaction Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). For the nuclear genes, we selected 11 microsatellite loci (Table S1, Supporting Information) from previous studies (Ding et al. 2013). The PCR amplification system and conditions were consistent with the above-mentioned ND2 gene's system and conditions, except for the DNA chain elongation time and temperature (30 s at 50–65°C; Table S1, Supporting Information). The fluorescent dye (FAM, HEX, or TAMRA) was added to the forward primers. ABI 3730 semiautomated sequencer (Applied Biosystems) with GS500 marker was applied for the genotype of the PCR products. GeneMarker v.1.85 (SoftGenetics LLC, State College, PA, USA) was used to analyze data (Holland & Parson 2011).
Genetic diversity
SeqMan, embedded in DNAStar, was used to assemble ND2 sequences (Burland 1999). DNA sequences were aligned and trimmed by Clustal X v.2.0 (Larkin et al. 2007). All the sequences were deposited into GenBank (OR094584–OR094631). DnaSP v.5.10 (Librado & Rozas 2009) was used to estimate population genetic indices, including nucleotide diversity (π) and haplotype diversity (Hd). For the microsatellite data, we checked the deviation of each locus and all loci for each population from Hardy–Weinberg equilibrium (HWE) by the exact probability tests in GenePop v.4.2.1 (Rousset 2008). The presence of null alleles and genotyping errors in microsatellite data was checked by Micro-Checker (Van Oosterhout et al. 2004). Several population genetic diversity parameters were estimated using GenAlEx v.6.5 (Peakall & Smouse 2006) and GENETIX v.4.05 (Belkhir et al. 2001), including a mean number of alleles (Na), observed heterozygosities (Ho), expected heterozygosities (He), and fixation index (F).
Phylogeography and population structure
Pairwise genetic differentiation (FST) among populations was calculated with 10 000 permutations using GenAlEx v.6.5 (Peakall & Smouse 2006) and Arlequin v.3.5.1.3 (Excoffier et al. 2005). For visualizing the mtDNA haplotype relationships among localities, a minimum-spanning network was constructed by Network v.4.6.1 (Fluxus Technology, Suffolk, UK) based on the full median-joining algorithm (Bandelt et al. 1999). For probing the proportion of total genetic variation within and among populations or groups, analysis of molecular variance (AMOVA) was calculated by Arlequin.
The BEAST v.1.8 (Drummond et al. 2012) was used to estimate the divergence time of Q. yei. The homologous sequences of eight Dicroglossinae species were selected as outgroups. The best-fit model of evolution was selected by JMODELTEST.0.1 under the Bayesian information criterion (Darriba et al. 2012). Two calibration points were employed, 22.9 Mya (with a 95% highest posterior density [HPD] between 15.60 and 30.20 Mya) Nanorana and Quasipaa and 18.4 Mya (with a 95% HPD between 12.09 and 24.71 Mya) from Quasipaa (Che et al. 2010). The other parameters were set as follows: strict clock, 10 million MCMC generations, sampling every 1000th iteration, the initial 25% burn-in, and an uncorrelated lognormal model of lineage variation with an expansion growth tree prior. TRACER v.1.6 (Rambaut et al. 2014) was used to create a model comparison and check the convergence of model parameter values (effective sample size [ESS]) to ensure ESS values >200. The tree and posterior distribution were summarized with TREEANNOTATOR v.1.8 (Rambaut & Drummond 2016) and visualized with FIGTREE v.1.4.3 (Rambaut 2016).
Bayesian analysis was used for the microsatellite data to infer the population structure by STRUCTURE, including the number of potential clusters and individuals to inferred clusters (Pritchard et al. 2000). Inferred cluster number (K) was set from 1 to 8, using no prior information about individual location, and assuming admixture and correlated allele frequencies. Ten independent runs were conducted for each K value. The MCMC parameters were set as 1 million generations and the first 100 000 as burn-in. Based on the criterion that the highest log likelihood [Ln Pr(X/K)] of the posterior probability (Pritchard et al. 2000) and the ΔK statistic (Evanno et al. 2005), we determined the most likely K value to explain the variation in the data.
To further explore the genetic structure of the two species, two other Bayesian clustering algorithms were calculated in TESS v.2.3 (Chen et al. 2007) and the Geneland package (Guillot et al. 2005) in R 3.3.1. For the TESS analysis, 20 replicates of each K (K, 2–8) were run based on 50 000 sweeps with a burn-in period of 10 000. The deviance information criterion (DIC) of each K was calculated and plotted to probe the cluster number. For Geneland analyses, we set the parameters as K number (1–10), 100 000 MCMC repetitions, 100 thinning, 100 burn-in period, and 20 different runs. The multilocus genotypes and spatial coordinates of each individual were also included. For further probing the genetic structure of Q. yei, the factorial correspondence analysis (FCA) was also calculated by GENETIX (Belkhir et al. 2001).
The spatial autocorrelation analyses were calculated as a mantel correlogram for mtDNA and microsatellite data to test the genetic similarity of individuals within a distance class against the similarity of all other individuals in GenAlEx. Distance classes for our analysis were based on the nearest sampling distance (Q. yei, 10 km; P. shangchengensis, 5 km). Significance values across distance classes were adjusted using 999 random permutations. Significant positive r indicated that individuals within a particular distance class were genetically more similar than expected by random. In addition, we conducted a Mantel test with 1000 random permutations on matrices of FST values and geographical distances in GenAlEx based on mtDNA and microsatellite data to assess the significance of isolation by the distance between populations.
Population demography
Mismatch distributions embedded in Arlequin were calculated to test the recent population growth. Rag (Harpending's raggedness index) and sum of squares deviation (SSD) were used to test the goodness-of-fit: the smoothness of the observed mismatch distribution and the degree of fit between the observed and simulated data (Harpending 1994). The neutrality tests, including Tajima's D (Tajima 1989) and Fu's Fs (Fu 1997), were used to calculate the population demography by 10 000 coalescent simulations in Arlequin. For determining the past population dynamics of Q. yei, the extended Bayesian skyline plot (EBSP) analysis of mtDNA data was conducted by BEAST. The estimated divergence time mentioned above was used to estimate the clock rate. The other parameters for the extended Bayesian skyline process were set as follows: 10 million MCMC generations, sampling every 1000th iteration, and the initial 25% burn-in. The convergence of the MCMC analyses was checked by the criteria (ESS values >200) by Tracer.
The Msvar v.1.3 (Storz & Beaumont 2002), assuming that a current population (of size N0) passed through a demographic change (a bottleneck or an expansion) to the history population size (N1) at time T in the past, calculated the recent demographic history for each population of Q. yei by microsatellite data. The various prior and hyperpriors used to do the coalescent simulations in Msvar are shown in Table S4, Supporting Information. Four prior distribution models were conducted based on stable (i.e. N0 and N1 had the same prior upper bound), expansion (i.e. N0 had a wider prior than N1), and bottleneck models (i.e. N1 had a wider prior than N0). For the microsatellite mutation rate, the average vertebrate microsatellite mutation rate of 10−4 (range from 10−2 to 10−6, Table S4, Supporting Information) was set (Bulut et al. 2009). The other parameters were set as follows: generation time of Q. yei (2 years), 200 million MCMC generations, and the initial 10% burn-in. The convergence of the MCMC analyses was checked by the criteria (ESS values >200) by Tracer. The convergence of the chains was checked based on the four runs of different priors for each population by Gelman and Rubin's diagnostic (Brooks, Gerlman 1998) using the CODA library (Plummer et al. 2006) implemented in R.
Niche reconstruction
Ecological niche modeling (ENM) was used to simulate the distribution pattern of the two species under different climate backgrounds based on climatic data and GPS coordinates of distribution localities by the maximum entropy algorithm in MAXENT v. 3.3.3k (Phillips et al. 2006). To ensure one species distribution point in each 1×1 km grid, all the selection criteria of distribution localities were expressed as the distance between all species distribution data > 1.42 km calculated using ArcGis10.2 software. After the screening, 22 and 34 occurrence records were selected for Q. yei and P. shangchengensis, respectively.
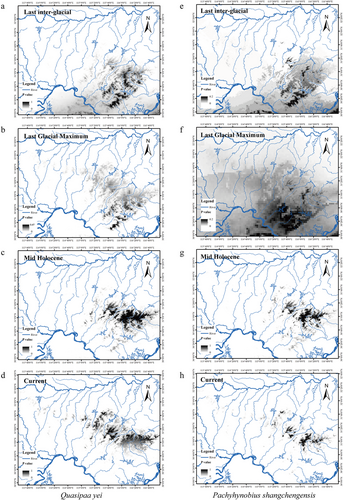
In this study, we collected 22 environmental variables, which can be roughly divided into two categories: bioclimate (Bio1–Bio19) and topography (elevation, slope, and aspect) at 30 arc-seconds resolution (∼1 km2) (data sources are shown in Table S6, Supporting Information). The entire Dabie Mountain range (30–32°N, 114–117°E) was clipped from these climatic and topography coverages (Hijmans et al. 2005). To avoid model overfitting, all collected variables were processed by the band collection statistics function in ArcGIS10.2, and the environment variables with high correlation (r>0.75) were removed (Table S6, Supporting Information). After screening, six variables were selected, including Bio8 (mean temperature of wettest quarter), Bio9 (mean temperature of driest quarter), Bio13 (precipitation of wettest month), Bio15 (precipitation seasonality), Bio17 (precipitation of driest quarter), and Elev (elevation). The correlation coefficient of the selected environmental variables is shown in Table S7, Supporting Information. Assuming niche conservatism of species (Wiens & Graham 2005), four climate data of different periods (current environmental conditions; the mid-Holocene, 6000 years ago; the last glacial maximum [LGM] about 22 000 years ago; the last inter-glacial [LIG], 0.13–0.12 Mya) (Ottobliesner et al. 2006) were used to obtain the predicted species distribution across time based on the species distribution model. The LIG, LGM, and mid-Holocene climate data (maximum resolution, 2.5 arc minutes) were downloaded from the WorldClim database. The paleodistribution was reconstructed by the community climate system model (Collins et al. 2003).
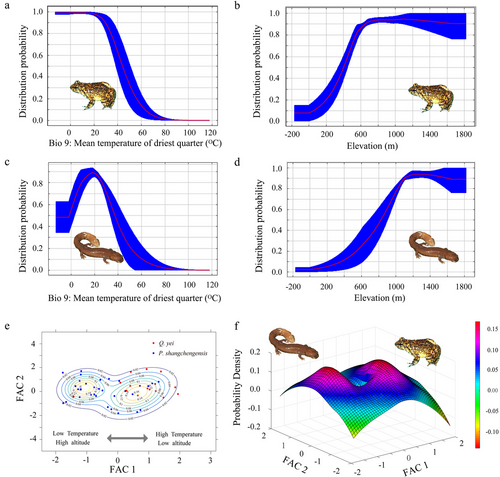
The jackknife method was selected to evaluate the contribution of environmental variables. Next, the distribution data of these two sympatric species were randomly divided into two groups: 25% were randomly selected as the test set, and the remaining 75% as the training set; the analysis was then performed using MaxEnt.3.4.1 with 10 bootstrap replicates. Analyses were run using the default program parameter cumulative outputs, a 0.00 001 convergence threshold, and a maximum of 5000 iterations. Other parameters were used with the default settings. Finally, for model evaluation, the predictive performance of the model was validated based on the area under the receiver-operating characteristic (ROC) curve (AUC) and the mean omission error (Phillips et al. 2006); the higher the AUC value, the more accurate the model performance. Usually, an AUC value of > 0.9 indicates excellent prediction performance of the model. The ensemble threshold of the model was calculated according to the maximizing sensitivity and specificity (MSS) by using the dismo package in R; the MSS method is commonly used in presence-only occurrence data (Liu et al. 2013; Hijmans et al. 2017). All models were post-processed and visualized in ArcGIS 10.0 (Esri, Redlands, CA, USA). To further assess niche differences between the two species, Statistics and Machine Learning Toolbox in Matlab 2021 (Mathworks Inc., USA) was used to fit the two-dimensional Gaussian mixture model based on the FAC1 and FAC2 for describing the probabilities of niche parameters of the two species. The FAC1 and FAC2 were collected from dimension reduction analysis of extracted climatic and ecological factors.
Ethics approval and consent to participate
In the present study, our experimental procedures and sample collection complied with the current laws on animal welfare and research in China and were specifically approved by the Animal Research Ethics Committee of Anhui Normal University.
RESULTS
Genetic diversity
We sequenced 999 bp of the ND2 gene from 180 individuals. Total Hd and π across all populations were 0.93 and 0.0073, respectively (Table 1). The MW population exhibited the highest haplotype diversity (Hd, 0.94) while the JTX population had the lowest value (0.64). The TTZ population exhibited the highest nucleotide diversity (π, 0.0056) while JTX had the lowest diversity (0.0009). For microsatellite data, 262 individuals were successfully genotyped at 12 loci (Table 1). No consistent departure from HWE or linkage disequilibrium was observed across the six populations. The nuclear genetic diversity varied between populations and a summary statistic used with Na ranging from 9.73 (MW) to 18.18 (BYM), Ho ranging from 0.75 (JTX) to 0.82 (MW), while He varied from 0.85 (MW) to 0.90 (BYM, KJY). BYM revealed higher genetic diversity than the other five populations, while the JTX population had a relatively lower genetic diversity (Table 1). This pattern of genetic diversity was generally consistent with the results of P. shangchengensis (Pan et al. 2019b).
| Mitochondrial gene (ND2) | Microsatellites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | N | Hd ± SD | π ± SD | Tajima's D | Fu's Fs | N | Na ± SE | Ne ± SE | He ± SE | Ho ± SE | F ± SE |
| JTX | 20 | 0.64 ± 0.12 | 0.0009 ± 0.0002 | −1.22 | −2.78 | 50 | 13.45 ± 0.87 | 8.85 ± 0.55 | 0.88 ± 0.01 | 0.75 ± 0.06 | 0.15 ± 0.07 |
| KHJ | 45 | 0.78 ± 0.05 | 0.0025 ± 0.0002 | −0.82 | −3.65 | 51 | 13.81 ± 1.08 | 8.82 ± 0.72 | 0.88 ± 0.01 | 0.79 ± 0.05 | 0.10 ± 0.05 |
| MW | 19 | 0.94 ± 0.04 | 0.0055 ± 0.0009 | −0.18 | −2.6 | 16 | 9.73 ± 0.38 | 7.00 ± 0.46 | 0.85 ± 0.01 | 0.82 ± 0.04 | 0.03 ± 0.05 |
| TTZ | 18 | 0.88 ± 0.05 | 0.0056 ± 0.0009 | 0.29 | 0.8 | 28 | 13.27 ± 0.83 | 8.79 ± 0.62 | 0.88 ± 0.01 | 0.78 ± 0.06 | 0.11 ± 0.06 |
| BYM | 48 | 0.83 ± 0.03 | 0.0027 ± 0.0002 | −1.06 | −2.26* | 73 | 18.18 ± 1.19 | 10.69 ± 0.71 | 0.90 ± 0.01 | 0.79 ± 0.05 | 0.13 ± 0.05 |
| KJY | 30 | 0.91 ± 0.03 | 0.0039 ± 0.0007 | −1.35 | −3.76 | 44 | 16.27 ± 1.32 | 10.36 ± 0.96 | 0.90 ± 0.01 | 0.80 ± 0.05 | 0.13 ± 0.05 |
| Total | 180 | 0.93 ± 0.01 | 0.0073 ± 0.0002 | −0.87 | −15.98** | 262 | 14.12 ± 0.50 | 9.09 ± 0.31 | 0.88 ± 0.01 | 0.79 ± 0.02 | 0.11 ± 0.02 |
- P values of Tajima's D test and Fu's Fs test were calculated with 10 000 simulations; significant tests are indicated with an asterisk (* P < 0.05, **P < 0.02). N, sample size; Hd, haplotype diversity; π, nucleotide diversity; Na, number of alleles; Ne, number of effective alleles; Ho, observed heterozygosity; He, expected heterozygosity; F, fixation index. BYM, Baimajian–Yaoluoping–Mingtangshan; JTX, Jiaoyuan–Tanghui–Xiaolongtan; KHJ, Kangwangzhai–Huangbaishan–Jiufengjian; KJY, Kujingyuan; MW, Mazongling–Wochuan; TTZ, Tiantangzhai.
Population structure
To compare the genetic response of different types of amphibians under similar climatic and geographic backgrounds, we used multiple methods to calculate the genetic structure of these two species within Dabie Mountain. For Q. yei, the network analysis showed two haplogroups corresponding to the North populations (JTX and KHJ) and Middle-South populations (MW, TTZ, BYM, and KJY), which indicated relatively shallow genetic differentiation (Fig. 1). For P. shangchengensis, there were five haplogroups with no shared haplotypes among different populations, especially between the North and South populations, which disclosed deep genetic differentiation among populations (Fig. 1).
TESS analysis for Q. yei showed that the appropriate K value was K = 2, 3 or K = 4 due to the stabilization or slow variation of DIC values against Kmax (Fig. 1). When K = 2, 3, the JTX–KHJ–MW–TTZ and BYM–KJY populations formed into a distinct genetic cluster, respectively; when K = 4, one population of KHJ formed an independent genetic cluster from JTX–KHJ–MW–TTZ (Fig. 1). For P. shangchengensis, the relative distinct genetic structure within populations was disclosed (Pan et al. 2019b). When K = 2, the JTX–KHJ and MW–TTZ–BYM–KJY populations formed distinct genetic clusters, respectively. The results for northern populations (JTX and KHJ) showed a relatively large genetic differentiation among populations (Fig. 1). For southern populations, a similar genetic pattern was observed among populations (MW–TTZ, BYM–KJY). For the phylogenetic analyses, the chronogram of Q. yei did not show a distinct genetic cluster corresponding to the geographical population (Fig. S1, Supporting Information), which was distinct from the phylogenetic structure of salamander (five distinct genetic clusters corresponding to geographical structure). As to the structure results, significant genetic introgression was observed among populations within Q. yei (Fig. S2, Supporting Information), while there were relatively distinct three genetic clusters corresponding to the North (JTX, KHJ), Middle (MW, TTZ), and South (BYM, KJY). Geneland spatial model showed that the geographical population of salamanders disclosed six corresponding genetic groups. However, the genetic structure of the frog displayed more complex results (Table S2, Figs S3,S4, Supporting Information). Additionally, the degree of genetic differentiation among genetic clusters showed different patterns (Q. yei, low; P. shangchengensis, high). Nevertheless, the FCA results of Q. yei (Fig. S5, Supporting Information) showed consistent results with Geneland.
For Q. yei, the FST values among populations ranged from −0.017 (MW and TTZ) to 0.813 (JTX and BYM) for mtDNA data and from 0.009 (BYM and KJY) to 0.024 (MW and KJY) for microsatellite data, which indicated genetic differentiation among these Q. yei populations (Fig. 2). For P. shangchengensis, the FST values among populations ranged from 0.494 (KJY and BYM) to 0.962 (MW and JTX) for mtDNA data and ranged from 0.087 (TTZ and MW) to 0.59 (KJY and JTX) for microsatellite data, which indicated the larger genetic differentiation among populations than that of Q. yei. The spatial autocorrelation analysis also showed that Q. yei had a more significant correlation when separated by >10 km apart (Fig. S6a,b, Supporting Information) while P. shangchengensis showed greater geographical genetic structure at closer distances (>5 km apart; more significant distance classes, ≥20 km based on Fig. S6c, Supporting Information, or ≥25 km based on Fig. S6d, Supporting Information). This indicated that Q. yei showed relatively shallow isolation by distance (IBD) affection than P. shangchengensis. The AMOVA results revealed different patterns within Q. yei depending on the marker type (mtDNA, [JTX, KHJ][MW, TTZ, BYM, KJY]; microsatellite, [JTX, KHJ, MW, TTZ][BYM, KJY]) based on the maximum value of among groups (68.46; 0.87) within the source of variance and fixation index (0.723; 0.023) (Table S3, Supporting Information), which indicated the relative shallow genetic differentiation comparing with that of P. shangchengensis (Pan et al. 2019b). In addition, the Mantel test revealed a weak but significant positive correlation between genetic distance and geographical distance based on mtDNA and microsatellite data (Fig. S7, Supporting Information).
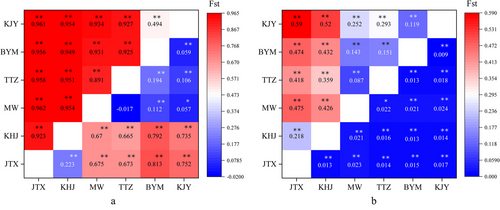
Overall, there was a relatively shallow genetic differentiation among the Q. yei populations compared to that among the P. shangchengensis populations. In addition, the IBD phenomenon was also disclosed among these two co-distributed species, and the isolation of Q. yei was lower than that of P. shangchengensis.
Historical demography
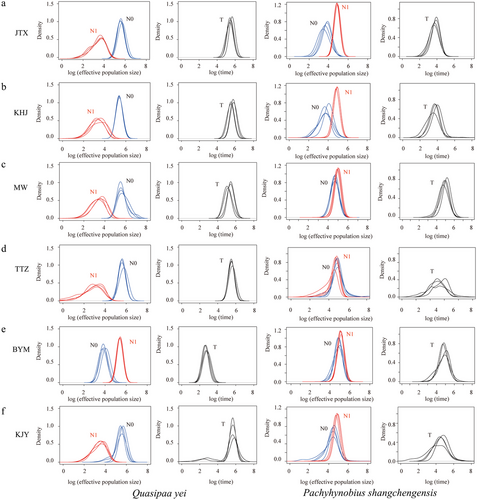
The neutrality test (Tajima's D and Fu's Fs), mismatch distribution analysis, and BSPs were conducted on the mtDNA sequence data. Fu's Fs and Tajima's D were negative in all populations except the TTZ population; however, only BYM presented significant Fs (Table 1). Additionally, the Fs of the total population showed a significant negative value (Table 1), which indicated the statistically significant population expansion of Q. yei. For the two Q. yei mtDNA genetic clusters, we examined departures of the sequence mismatch distributions from those simulated under a population expansion model. Insignificant departures were detected regardless of whether Rag or SSD measures were used (Fig. S8, Supporting Information), which indicated fit to population expansions. For the Msvar analysis, the convergence of the MCMC was checked by the Gelman and Rubin statistic (i.e. smaller than the upper threshold of 1.2). Compared with the results of P. shangchengensis (i.e. stable demography), Msvar's results of Q. yei showed distinct population expansions of most geographical populations, except the BYM population (Fig. 3 and Table 2; Table S5, Supporting Information). In addition, the EBSP results revealed that Q. yei experienced an expansion of effective population size since 0.015 Mya after the LGM (Fig. 4). This result was consistent with those from other mtDNA- or microsatellite-based demographic analyses, together pointing to expansions of the frog populations.
| Pop | log10(N0) ± SD | N0 | log10(N1) ± SD | N1 | N1/N0 | N0/N1 | log10(T) ± SD | T |
|---|---|---|---|---|---|---|---|---|
| JTX | 5.575 ± 0.13 | 388 451 ± 120 923 | 2.9625 ± 0.79 | 3472.75 ± 6080 | – | 102.56 | 5.625 ± 0.10 | 429 663 ± 98 486 |
| KHJ | 5.375 ± 0.05 | 238 364 ± 28 983 | 3.31 ± 0.72 | 5517 ± 8269 | – | 47.62 | 5.71 ± 0.13 | 521 926 ± 162 253 |
| TTZ | 5.65 ± 0.082 | 452 631 ± 85 001 | 3.1375 ± 0.335 | 1675 ± 1121 | – | 250 | 5.55 ± 0.082 | 359 537 ± 67 519 |
| MW | 5.5125 ± 0.09 | 331 647 ± 78 386 | 3.4625 ± 0.59 | 5138 ± 5415 | – | 58.82 | 5.25 ± 0.24 | 198 514 ± 98 540 |
| BYM | 3.9 ± 0.1 | 8114 ± 2071 | 5.4 ± 0.06 | 252 855 ± 33 467 | 32.80 | – | 2.675 ± 0.13 | 489.25 ± 152.15 |
| KJY | 5.6125 ± 0.06 | 414 070 ± 56 489 | 3.6875 ± 0.11 | 5 008 ± 1 066 | – | 76.92 | 5.675 ± 0.043 | 475 598 ± 50 081 |
- Posterior probabilities and 95% highest posterior intervals for the parameters are inferred in Msvar. N0, current effective population size; N1, historical effective population size; T, time of the bottleneck. BYM, Baimajian–Yaoluoping–Mingtangshan; JTX, Jiaoyuan–Tanghui–Xiaolongtan; KHJ, Kangwangzhai–Huangbaishan–Jiufengjian; KJY, Kujingyuan; MW, Mazongling–Wochuan; TTZ, Tiantangzhai.
Ecological niche modeling
Based on ENM, the predicted distribution of these two co-distributed species (Q. yei and P. shangchengensis) is generally consistent with their actual distribution (Fig. 5), differing significantly from random (Q. yei: AUC = 0.992; P. shangchengensis: AUC = 0.994; Fig. S9, Supporting Information). Under different climatic conditions (LIG, LGM, Mid Holocene, and current), habitats suitable for these two co-distributed species have been maintained and kept isolated from each other by an unsuitable environment at low altitude in the Dabie Mountains, although the distribution area has changed (Fig. 5). Additionally, the predicted potential distribution area of Q. yei was relatively larger than that of P. shangchengensis under different climatic conditions (Fig. 5 and Table 3).
| Area (km2) | Percentage (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Time | US | PS | MS | HS | US | PS | MS | HS |
| Q. yei | Current | 130 150 | 2150 | 1272 | 570 | 97.02 | 1.60 | 0.95 | 0.42 |
| MID | 130 438 | 1440 | 883 | 1381 | 97.24 | 1.07 | 0.66 | 1.03 | |
| LGM | 134 014 | 127 | 1 | 0 | 99.90 | 0.09 | 0.00 | 0.00 | |
| LIG | 131 059 | 2494 | 545 | 44 | 97.70 | 1.86 | 0.41 | 0.03 | |
| P. shangchengensis | Current | 132 847 | 573 | 316 | 406 | 99.03 | 0.43 | 0.24 | 0.30 |
| MID | 132 447 | 786 | 433 | 476 | 98.74 | 0.59 | 0.32 | 0.35 | |
| LGM | 134 142 | 0 | 0 | 0 | 100 | 0.00 | 0.00 | 0.00 | |
| LIG | 134 142 | 0 | 0 | 0 | 100 | 0.00 | 0.00 | 0.00 | |
- HS, highly suitable habitat (0.75–1); MS, medium-suitable habitat (0.5–0.75); PS, poorly suitable habitat (0.24–0.5); US, unsuitable habitat (<0.24). LGM, Last Glacial Maximum; LIG, Last Inter-glacial.
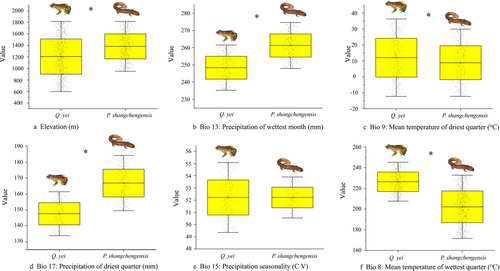
The Jackknife analyses results for Q. yei showed that the variables with the first three high contributions to the model were Elevation (81.4%), Bio13 (7.6%), and Bio 9 (5.5%), with a total contribution rate of 94.5%. For P. shangchengensis, the variables were Elevation (93.5%), Bio9 (3.2%), and Bio8 (1.3%), with a total contribution rate of 98% (Table S8, Supporting Information). Next, we compared four climatic (Bio13, Bio 9, Bio 17, Bio 15, and Bio 8) and one topographic variables (Elevation) between Q. yei and P. shangchengensis, which indicated the relatively significant ecological factor differences between these two species, except the Bio15 (Fig. 6). Overall, Q. yei had higher temperature adaptability and lower altitude distribution, while P. shangchengensis had relatively lower temperature adaptability and higher altitude distribution (Figs 6, 7).
DISCUSSION
The inner causes of species range size difference
Range size is a fundamental unit of biogeography, reflecting differences in interspecies dispersal capacity, evolutionary history, and ecological tolerance (Thompson et al. 1999; Lester et al. 2007; Olalla-Tárraga et al. 2011). The principal goal of biogeography and biodiversity conservation is to identify the mechanisms governing the distributions of species, such as species trait, dispersal ability, and ecological tolerance (Brown et al. 1996; Gaston 1996), which are directly related to the global extinction risk (Purvis et al. 2000; Botts et al. 2013; Böhm et al. 2017) due to the dynamic changes between species distribution areas and climate change (Thomas et al. 2001; McCauley et al. 2014). To explain the differences in the geographic range sizes between species, various hypotheses have been proposed, such as organisms' attributes (e.g. ecological, morphological, and life-history) (Brown et al. 1996), climatic variability (Stevens 1989), niche breadth (Gaston et al. 1997; Botts et al. 2013; Slatyer et al. 2013), environmental tolerance (Pither 2003; Sheth & Angert 2014), adaptive capabilities (Brown 1984), population size(Gaston & Blackburn 1996), dispersal ability (Thompson et al. 1999; Lester et al. 2007; McCauley et al. 2014), and a combination of habitat area and climate stability (Morueta-Holme et al. 2013). Among these hypotheses, the niche breadth and environmental tolerance hypotheses have recently gained more support, suggesting a positive correlation between niche breadth and geographical range size (Pither 2003; Boulangeat et al. 2012; Botts et al. 2013). Species with broad niches can persist in a variety of different habitat types, while species with narrow niches are confined to places that meet the requirements of their particular niche (Brown 1984). Additionally, species with narrower environmental tolerance are expected to have narrower distributions than broad species, which means that larger geographic range sizes are directly correlated to the larger environmental tolerances and habitat breadths (Slatyer et al. 2013).
Q. yei and P. shangchengensis are sympatric species. Sympatric species evolve into co-existence in an ecosystem by dividing habitat resources (Juarez & Marinho-Filho 2002; Soininen et al. 2015). Q. yei has a larger distribution area and lower altitude restrictions than P. shangchengensis. These results were also proved by the ENM results under different historical climate backgrounds (LIG, LGM, Mid Holocene, current), which indicated that Q. yei has a relatively wider potential distribution area, lower altitude distribution, and higher temperature tolerance than P. shangchengensis (Figs 5-7 and Table 3). The differences in the degree of genetic differentiation, the degree of isolation by distance, and the population demography of these two species also led to the same conclusion (Figs 1,2). The niche breadth–range size hypothesis states that the greater the breadth along one or more niche axes, the species can survive under a wider range of local conditions and thus spread over a larger geographic area (Botts et al. 2013; Slatyer et al. 2013; Varzinczak et al. 2020). Therefore, we can infer that Q. yei has a broader niche breadth and environmental tolerances than P. shangchengensis, which may lead to a larger distribution of Q. yei. On the other hand, dispersal ability is another frequently cited potential key factor of the species distribution range size involving various taxa within terrestrial and aquatic systems (Thompson et al. 1999; Gaston 2003; Lester et al. 2007; Rundle et al. 2007; McCauley et al. 2014; Waters et al. 2020). Species with high dispersal capability are expected to disperse more easily over long distances to colonize new areas and maintain viable populations (Gaston 2003; Böhning-Gaese et al. 2006; Lester et al. 2007). Reduced dispersal may increase the sensitivity of lineages to landscape/environmental changes through reduced gene flow between populations, thereby driving the range size change and the phylogeographical structure formation (Gaston 2003), affecting the spatial scale of speciation (Kisel & Barraclough 2010). Q. yei is more likely to inhabit woodlands, grasses, rocks, and other places along streams, while P. shangchengensis shows strict aquatic attributes, reflecting that the frog has a stronger dispersal ability than the salamander, which is also considered one of the main factors in shaping the range size of these two species.
The inner causes of phylogeographic pattern difference
For ecological processes that influence population viability, population connectivity is of critical importance (Curtis & Bevers 2002; Hayden 2006; Saastamoinen et al. 2018). It can be affected by dispersal behavior and the presence of natural barriers (e.g. rivers and mountains), influencing genetic variation and structure (Duellman & Trueb 1994; Clobert et al. 2001; Bowler & Benton 2005; Olah et al. 2017), especially for amphibians (Pan et al. 2019a,b; Lucati et al. 2020; Trevisan et al. 2020; Niwa et al. 2021; Svinin et al. 2021; Tominaga et al. 2021). For those species that inhabit streams, stream network structure and other natural landscape features may be effective dispersion barriers, providing opportunities for genetic differentiation (Sunny et al. 2014; Pan et al. 2019a,b; Niwa et al. 2021). On the other hand, sky islands are mountainous regions isolated from each other by valleys with different environmental conditions (McCormack et al. 2009). Species confined to montane sky island habitats, especially aquatic taxa, generally exhibit high levels of interpopulation genetic variation and unique genetic structure patterns (Shepard & Burbrink 2011; Atkins et al. 2020; de Oliveira et al. 2021), especially for species in mid to high latitude mountain ranges (Beniston 2016; Herman & Champagnac 2016). In such high-altitude habitats, populations are often separated by a valley with unsuitable habitats due to niche conservatism (McCormack et al. 2009; Shepard & Burbrink 2011; He & Jiang 2014; Pan et al. 2019a,b). This segregation is usually exacerbated by the restriction of gene flow, habitat fragmentation, etc., caused by climate contrast between different elevation gradients or elevation differentiation at lower altitudes brought about by orogeny (McCormack et al. 2009). As a result, mountain systems tend to have more hidden clades or species, as the highly subdivided habitats and rugged terrain form sky island habitats (McCormack et al. 2009; He & Jiang 2014). Abiotic factors (e.g. climatic and tectonic events) and biological factors (e.g. interspecific or intraspecific interactions, competition, and predation) may be the main driving forces of biological diversification and evolution temporally and geographically (Benton 2009). Generally, mountainous areas exhibit a variety of microhabitats with different ecological conditions from the surrounding landscape due to the interaction of multiple biological and abiotic factors, which urged the formation of unique and endemic species with relatively small populations and well-defined geographic boundaries (Shepard & Burbrink 2011; Huang et al. 2017).
Numerous scattered mountain ranges (e.g. Dabie Mountains, Qinling Mountains, and Hengduan Mountains) form potential sky islands in China's vast subtropical regions, showing spatial isolation within confined areas and are considered ideal natural laboratories for studying endemic plant and animal species formation (Che et al. 2010; Ye et al. 2016). During the Quaternary, violent climatic oscillations drastically affected the population demography of mid-latitude species (contractions or expansions) (Hewitt 2004). During the LGM, RegCM2 simulation showed that annual surface temperature decreased by 2–4°C over most land areas, and the strongest cooling was up to 8°C over coastal areas in East Asia (Ju et al. 2007), which may indicate that the annual temperature has an impact on many species distributions (Cheng et al. 2021; Luo et al. 2021; Wei et al. 2022). The stream-dwelling frog (Q. yei) and salamander (P. shangchengensis) are typical stream-inhabited species, endemic to the Dabie Mountains, which live in or along the cool and oxygen-rich streams above 500 m in elevation (Fei et al. 2012). Based on the ENM prediction results, although the distribution area of these two co-distributed species (Q. yei and P. shangchengensis) changed over different climatic conditions (LIG, LGM, Mid Holocene, and current), suitable habitats for these two co-distributed species have been maintained and kept isolated from each other by the unsuitable environment at low altitude in the Dabie Mountains (Fig. 5), even during the most drastic climate oscillations in Pleistocene (LGM) (Senczuk et al. 2017). Additionally, the unsuitable habitats at low altitudes act as a strict and effective isolation barrier for the two species due to the strict niche conservatism, although these two species were found to have different limitation degrees (Fig. 5). This limits the dispersal among the population and facilitates allopatric divergence, which is directly correlated to the high levels of inter-population genetic divergence and unique patterns of genetic structure. Such phylogeographic pattern caused by the sky island effect is also reflected in some other amphibians in mid-latitudes, including three Plethodon species (P. ouachitae, P. fourchensis, and P. caddoensis) within the Ouachita Mountains in western Arkansas (USA) (Shepard & Burbrink 2011) and the stream salamander (Batrachuperus tibetanus) in the Qinling Mountains, East China (Huang et al. 2017).
The width of a species’ climatic niche can be defined as a series of all the climatic conditions under which the species is distributed (Futuyma & Moreno 1988; Gaston 2003). Niche breadth has been used in various studies, for example, to build species' response models to global changes (e.g. species distribution models) (Guisan & Thuiller 2005; Slatyer et al. 2013; Rolland & Salamin 2016; Varzinczak et al. 2020; Vela Díaz et al. 2020). As specialist species have fewer dispersal capacities than generalist species, the connection among populations with narrow niches is less likely to thrive (Kisdi 2002; Birand et al. 2012; Waters et al. 2020). Over time, higher spatial or ecological isolation may lead to reproductive barriers, that is, the formation of new species (Kisel & Barraclough 2010; Birand et al. 2012; Ye et al. 2016; Pan et al. 2019a).
In contrast, the generalist species' range size is larger than specialist species (Colwell & Rangel 2009; Slatyer et al. 2013; Boucher-Lalonde et al. 2016), and the dispersal rates are probably higher (Gaston 2003). The increased connectivity among generalist species populations prevents speciation. In this study, the population genetics analyses of Q. yei disclosed the existing but relatively small genetic differentiation across the entire range, especially between north (JTX and KHJ) and south populations (BYM and KJY). Conversely, the deep genetic divergences (the MRCA of Pachyhynobius dates back to ∼7.84 Ma) were disclosed among P. shangchengensis populations. Additionally, the different population demography status (P. shangchengensis, stable or decline; Q. yei, expansion) has an important contribution to the formation of biogeographical structure (Figs 3, 4). Based on ENM results, Q. yei has higher temperature adaptability, lower altitude distribution, and larger distribution area, while P. shangchengensis have relatively lower temperature adaptability, higher altitude distribution, and smaller distribution area (Figs 5-7 and Table 3), which indicates the relatively wider ecological niche breadths and environmental tolerances of Q. yei than that of P. shangchengensis. Differences in niche breadths and environmental tolerances are presumed to drive the differences in range size among species (Pither 2003). Range size is strongly correlated with population connectivity for these species affected by the sky island effect, while the connection level between populations directly affects the formation of genetic differentiation (Shepard & Burbrink 2011; Beniston 2016; Herman & Champagnac 2016; Atkins et al. 2020; de Oliveira et al. 2021). Therefore, based on the correlation of niche breadths, environmental tolerances, and range size, we could infer that the population connectivity within Q. yei would be more frequent than that within P. shangchengensis, which has a key role in the formation of different genetic differentiation and structure of these two co-distributed species. Additionally, dispersal ability is another key factor constraining the geographical range size and genetic variation (Gaston 2003) and thus influencing the spatial scale of speciation (Lester et al. 2007; McCauley et al. 2014; Saastamoinen et al. 2018; Lucati et al. 2020; Waters et al. 2020). As mentioned in the previous paragraph, Q. yei has a stronger dispersal ability than P. shangchengensis, which has also been proposed as one of the main factors shaping the genetic differentiation of these two species.
ACKNOWLEDGMENTS
We thank Tianma National Nature Reserve and Yaoluoping Nature Reserve for their help in the Biodiversity Survey and sample collection. This work was financially supported by the National Natural Science Foundation of China (No. 32070417), the Anhui Natural Science Foundation (Youth, 1908085QC127), the Biodiversity Survey, Monitoring and Assessment Project (2019–2023) of Ministry of Ecology and Environment, Special Investigation of Basic Science and Technology Resources (2019 FY101803), and Anhui Province Academic and Technical Leader and Backup Candidate Academic Research Activities Fund (2017H130).
CONFLICT OF INTEREST
The authors declare no conflict of interest.




