Dietary tannins alter growth, behavior, and the gut microbiome of larval amphibians
Abstract
Research has shown that leached plant toxins negatively impact the growth and development of larval amphibians. However, tadpoles may encounter these same toxins in food material, and differential exposure routes and distribution of toxic chemicals can yield variable downstream effects on animals. To date, most research understanding the interactions between dietary plant toxins and herbivores has been conducted in terrestrial systems. Despite the abundance of plant toxins in food and water sources, the effects of dietary plant toxins on larval amphibians have not been studied, and tannins could negatively affect these species. Here, green frog tadpoles (Lithobates clamitans) were fed diets with or without 2% tannic acid to test how their growth, development, behavior, and gut microbiome respond to dietary tannins. At the end of the trial, we conducted a behavioral assay to measure tadpole activity and boldness and inventoried the gut microbiome using 16S rRNA sequencing. Dietary tannins significantly decreased body mass by 66% and length by 28%, without influencing tadpole developmental stage. We found significant differences in exploratory behavior and boldness during the first minute of our behavioral assay, demonstrating that tannins have the potential to influence behavior during novel or stressful events. Finally, tannins significantly sculpted the gut microbiome, with an increase in the measurement of Shannon entropy. We observed 7 microbial phyla and 153 microbial genera that exhibited significantly differential abundances differences between control and tannic acid-fed tadpoles. Collectively, our results demonstrate that dietary tannins have the potential to alter amphibian growth, behavior, and microbiome.
INTRODUCTION
Herbivorous animals must overcome many challenges that accompany their plant-based diet, which is primarily composed of indigestible fiber and often contains toxic compounds. These toxins can cause numerous effects on herbivores, such as acting as neurotoxins, limiting digestibility, decreasing growth, and altering the gut microbiome (Dearing et al. 2005). These interactions have been best studied in mammalian herbivores, which have numerous strategies to overcome these challenges, including enhanced detoxification physiology, behavioral changes, and assistance from gut microbial communities (Freeland & Janzen 1974; Kohl et al. 2014; McArthur et al. 2014). However, our understanding of plant–herbivore interactions in aquatic systems is more limited, except for a handful of studies on fish being raised in aquaculture (Francis et al. 2001). While the natural diets of larval amphibians, such as tadpoles, are not entirely clear (Montaña et al. 2019), they are thought to be herbivorous or detrivorous and often ingest plant material, some of which can contain high levels of plant toxins (Stoler et al. 2016). Therefore, tadpoles could face adverse consequences from consuming plant secondary compounds. However, the potential interactions between larval amphibians and natural dietary toxins are poorly understood.
One common class of plant defense chemicals are polyphenolic compounds called tannins (Hassanpour et al. 2011). Hydrolysable tannins, in particular, are composed of a polyol group (most commonly glucose) esterified with either gallic acid or hexahydroxydiphenic acid (Macáková et al. 2014). These secondary plant compounds often stay in deciduous leaves, and studies in terrestrial herbivores have shown that when ingested, tannins can decrease protein and dry matter digestibility (Robbins et al. 1987a,b), sometimes leading to decreased growth and even mortality (Mehansho et al. 1987). However, deciduous leaves frequently enter aquatic systems, where tannins can be leached into aquatic environments with harmful effects on animal species (Cameron & LaPoint 1978; Larcher 2003).
Previous research has shown that tannins can negatively impact the survival of larval amphibians (Watling et al. 2011; Dodd 2018). However, these studies have all used dissolved tannins present in the water, meant to mimic the leaching of plant tannins. For example, research has shown that toad tadpoles had increased rates of mortality when raised in water containing invasive plant extracts compared to those raised in native plant extracts and control water (Watling et al. 2011). Additional research found that two species of frog tadpoles had increased rates of mortality and were unable to metamorphose when raised in water containing tannin-rich leaf litter leachate (Earl et al. 2012). Similarly, gray treefrog tadpoles (Hyla chrysoscelis) had increased rates of mortality when raised in water with commercial tannic acid (Dodd 2018). While tannins seem to consistently decrease survival, the effects of tannins on body size and development are not as clear. Tannin-rich leachate has been found to increase snout–vent length, body mass, and developmental rate in some amphibian species (Watling et al. 2011; Earl & Semlitsch 2015) or have no effect on snout–vent length and body mass in others (Watling et al. 2011; Dodd 2018). However, given the use of leachate in these studies, it cannot be confirmed that these effects resulted from the tannins rather than other plant compounds. Additionally, these previous studies all used an aquatic form of tannins, such as leachates from leaves or dissolved tannic acid. A major aspect of toxicology is to more accurately consider the temporal and spatial impacts of an interaction with a compound, namely a compound's absorption, distribution, metabolism, and excretion (ADME). These toxicological concepts can also be applied to natural toxin systems (Sorensen et al. 2006). Although tannins are likely ingested when aquatic organisms consume water, it is possible that dissolved tannin leachates and ingested dietary tannins interact differently with larval amphibians. However, the effects of dietary sources of tannin on tadpoles have not been tested, though they are likely to be consumed in their natural diet (Skelly & Golon 2003; Stoler et al. 2016).
Dietary tannins may also interact with the behavior of herbivores (Mlambo et al. 2015). Behavioral adaptations to these toxic food sources include regulated intake (Torregrossa & Dearing 2009) and having bold personality traits to forage for better food sources across the landscape. For example, dietary toxins may compromise the energy budget of animals, which may push them to move to different environments in search of food material with fewer toxins. This movement may make animals more susceptible to predation, and so there is a “food-fear” tradeoff that has been established in terrestrial herbivores (McArthur et al. 2014). While this idea has not been tested in aquatic herbivores, it is reasonable to assume that dietary tannins could influence aspects of tadpole behavior. Amphibian behavior has primarily focused on activity, boldness–shyness, and exploration–avoidance (Kelleher et al. 2018). There is increasing evidence that tadpoles display exploratory boldness (Wilson & Krause 2012; Carlson & Langkilde 2013; Urszán et al. 2015a), which could aid in dispersal to find new food resources. However, if tannins hinder tadpole nutrition, tadpoles may exhibit alterations to overall activity levels and cannot act that boldly. Similarly, we hypothesized that tadpoles could display differences in exploration of a novel environment because of the “food-fear” tradeoff. Therefore, we aimed to test how dietary tannins affected these aspects of tadpole behavior.
Finally, tannins may interact with their hosts through alterations to their gut microbiome. Tannins and other plant compounds are known to be antimicrobial, and thus may alter the microbiome through these activities (Scalbert 1991). Research has shown that dietary tannins significantly alter the community structure of the gut microbiome of lab rats (Kohl et al. 2016), lab mice (Fang et al. 2022), and pigs (Lee et al. 2010), though there also are some tannin-degrading bacteria which are important for diet detoxification in wild mammalian herbivores (Dai et al. 2014). In the same lab rat study (Kohl et al. 2016), lab rats inoculated with tannin-degrading bacteria from wild, herbivorous rodents (Neotoma spp.) exhibited increased ability to feed on tannins while exhibiting lower indicators of liver damage (Kohl et al. 2016). We currently have no understanding of how the amphibian microbiome responds to dietary tannins, and microbiome studies in general are largely biased toward mammalian species (Colston & Jackson 2016; Pascoe et al. 2017). Additionally, scientists are recently appreciating the interactions and relationships between the gut microbiome and animal behavior in nonmodel systems (Cryan et al. 2019). This study offers an initial view into how dietary tannins alter the gut microbiome of larval amphibians.
In this study, green frog tadpoles (Lithobates clamitans) were fed a diet with or without 2% tannic acid to test how their growth, development, behavior, and gut microbiome respond to dietary tannins. At the end of the trial, we conducted a behavioral assay to measure tadpole activity and boldness. We then measured tadpole body length and mass before dissecting to remove the whole gut for microbiome analysis. Overall, we predicted that tadpoles that consumed tannic acid would be less developed and have a smaller body mass. We also predicted that tadpoles would exhibit differential behavior due to tannic acid. Tadpoles may increase exploration, boldness, or activity with the goal of searching for new food resources, or these aspects may decrease if animals’ energy budgets are compromised by the presence of tannic acid. Last, we predicted that the gut microbiomes of the tadpoles would differ significantly in community structure and richness as a result of tannic acid, as has been seen in mammalian herbivores. Understanding how the tadpole gut microbiome interacts with tannins is ecologically relevant because tadpoles commonly encounter tannins in their food and water sources.
MATERIALS AND METHODS
Animal husbandry
All procedures were approved under University of Pittsburgh IACUC protocol #21049000 and conform to the provisions of the Declaration of Helsinki. In July 2019, a single green frog egg mass was collected from an ephemeral pond in Kisatche National Forest (Louisiana, USA) and shipped overnight to the University of Pittsburgh. Although there could be limitations in using a single egg mass, we believe this would allow for less variation in microbial analyses. Nonetheless, we acknowledge that our results could be limited to a specific hereditary lineage. Individuals were housed at an initial density of 20 individuals/tank (6 tanks/dietary treatment) inside a walk-in, climate-controlled environmental chamber, with replacement of mortalities for the first week (mean 10.4% ± 2.3% mortality in week 1 across the 12 tanks), after which there was no more replacement, and mortality was tracked weekly. The chamber is maintained at 24°C on a 12-h light: 12-h dark cycle. Tanks were 15.24 cm wide, 25.4 cm long, and 12.7 cm high and were cleaned and filled weekly with 3 L of laboratory filtered water.
Blocks of tadpole food were prepared by grinding commercial, alfalfa-based rabbit chow and mixing it with agar (25 g agar, 250 g rabbit chow, 1 L water). For the tannic acid treatment, commercial tannic acid powder was mixed into the ground rabbit chow to prepare a 2% tannic acid diet (based on dry mass). We believe that this concentration is ecologically relevant because green frog tadpoles readily consume newly provided leaves of red maple (Acer rubrum), containing over 3% phenolic compounds under captive conditions (Stoler et al. 2016).The ecological relevance of this particular tannin concentration is further considered below in the Discussion. These solutions were boiled briefly to dissolve the agar, poured onto stainless steel trays to cool, and cut into blocks of roughly 0.5g, and frozen at −20°C. Boiling should not impact tannic acid, as depolymerization/hydrolysis does not occur until 190°C (Ahmad et al. 2018). Each tank received one block of agar-suspended food each week. Animals were fed ad libitum, as food was always in excess at the end of the week when tanks were changed, and food blocks were replaced. The tadpoles were on their respective diets for a period of 14–17 weeks, from late July to mid November 2019. During weeks 14–17, tadpoles underwent behavioral recording and dissection. The treatment groups were alternated between dissections to ensure a random and balanced design.
Behavioral assays
Open field trials are a standard behavioral assay used to measure animal behavior in a novel environment (Walsh & Cummins 1976; Crusio 2001; Burns 2008; Carlson & Langkilde 2013). Here, tadpoles were placed individually in a new, clear tank over a marked gridline system of 0.75-inch squares. A Go-Pro camera was used to record the tadpole's activity for 10 min immediately after being placed in the novel environment. The behavioral assay was assessed manually. Boldness was determined by the proportion of center gridlines that the tadpole crosses in the middle of the open field. Tadpoles have been observed to reside in the periphery of ponds in the wild, so time spent in the middle is considered a measurement of boldness (Carlson & Langkilde 2013). Activity was calculated by the total number of gridlines that the tadpole crossed during each minute, with activity during the first minute representing exploration. Prior work has calculated exploration as the total number of gridlines crossed during the assay or the number of rectangles visited at least once divided by the number of available rectangles (Carlson & Langkilde 2013; Urszán et al. 2015b). While activity and boldness were a priori hypotheses of our study design, we observed temporal effects within our data, and thus, in a post hoc manner, we decided to distinguish initial activity (exploration) from later behavior. However, we recognize that it is difficult to accurately characterize these behaviors and to determine the true ecological and evolutionary relevance of these labels. Thus, we echo previous calls for validation of these metrics in relation to fitness consequences and under natural conditions (Carlson & Langkilde 2013).
Sample collection
After the behavioral assay, tadpoles were euthanized by immersion in buffered MS-222 (10 g L−1). We then recorded each tadpole's body length (snout to beginning of tail, mm), body width (mm), mass (g), and Gosner stage (Gosner 1960). We dissected each individual to remove its entire gastrointestinal tract. Gut samples were immediately frozen and stored at −80°C.
Statistical analyses
For morphological and behavioral measurements, our final sample sizes were 25 and 24 tadpoles for the control and tannic acid groups, respectively. We first compared tadpole development (Gosner stage) between groups using a linear mixed model to test for the effect of diet treatment while controlling for tank and date of collection as random effects. Next, we investigated tadpole body mass using an ANCOVA, with diet treatment as the main treatment variable and Gosner stage as a covariate, and tank and date of collection as random effects.
Behavioral analyses were conducted on the final sample sizes in each group. For activity, the total number of gridlines crossed during each minute and in total was counted. We calculated boldness (at each minute and average) by calculating the ratio of center gridlines crossed to the total number of lines crossed. Activity and boldness over the course of the behavioral trial were compared using a repeated-measures ANOVA, while average boldness and normalized total activity were compared with a linear mixed model to test for the effect of diet treatment while controlling for tank and date of collection as random effects. All analyses were performed in JMP (version 14.1.0) using α = 0.05.
Microbiome analysis
For a smaller subset of tadpoles chosen at random (n = 12 per treatment group), we inventoried the composition of the gut microbiome. Total DNA was extracted from all whole gut samples using the QIAamp PowerFecal DNA Isolation Kit (Qiagen) following the manufacturer's protocol. Blank extractions were performed using the same kit to control for potential contaminants found in the kit (Salter et al. 2014). Extracted DNA was sent to the DNA Services Facility at the University of Illinois at Chicago, where the V4 region of 16S rRNA gene was amplified using primers 515F and 806R. The resulting amplicons were then sequenced on the Illumina MiSeq platform using 500-cycle v2 chemistry (Caporaso et al. 2012).
Bioinformatics analysis was conducted using the CLC Genomics Workbench version 22.0.2 (QIAGEN Aarhus A/S). Paired sequence reads were imported into the program in fastq format, and we quality filtered using the “NCBI/Sanger or Illumina Pipeline 1.8 and later” option for quality scores. With the CLC Microbial Genomics Module version 22.1.1 plugin, we performed data quality control, amplicon sequence variant (ASV) clustering, and calculated indices of alpha and beta diversities.
The quality control (QC) workflow applied to the paired reads was trimming with the following settings: quality limit = 0.05, maximum ambiguity = 2, and filtering samples based on a minimum number of 100 reads and the minimum percent from the median = 50. The remaining high-quality reads were clustered into ASVs using the following clustering parameters: finding best match = true, chimera crossover cost = 3, Kmer size = 6, mismatch score = 1, minimum score = 40, and gap cost = 4. The chimera tables were separated from the rest of the sequences. Taxonomic identity of ASVs was determined based on 80% similarity to reference sequences in the SILVA database (Quast et al. 2013). Sequences identified as mitochondria and chloroplasts were removed from downstream analyses.
The merged ASV table was rarefied to 7146 reads and was used to measure the number of observed taxa (akin to microbial richness) and Shannon entropy, which takes into account the number of microbial ASVs (richness) and their relative abundance (evenness) (Shannon 1948). These values were compared using a linear mixed model to test for the effect of diet treatment while controlling for tank as a random effect. We also compared microbial community structure by calculating Bray–Curtis distances (Bray & Curtis 1957), visualized these differences by conducting principal coordinates analysis (PCoA), and finally conducted statistical comparisons by conducting a PERMANOVA using CLC Microbial Genomics Module 23.0.1, CLC-Genomic Work Bench version 23.0.3 (QIAGEN, Aarhus, Denmark). We also compared the abundances of microbial genera using the differential abundance analysis tool within the CLC Genomics Workbench, which performs TMM normalization (Robinson & Oshlack 2010), performs generalized linear models using a negative binomial distribution, and FDR-correction for multiple comparisons. To ensure the statistical robustness of the taxa we present as differentially abundant, we performed a secondary differential abundance analysis using DESeq2 and report results of these tests in the Supporting Information (Love et al. 2014; Nearing et al. 2022).
Sequences were deposited to the NCBI Sequence Read Archive under BioProject PRJNA926636.
RESULTS
Growth, development, and survival
There was no significant effect of dietary tannins on tadpole developmental stage, as determined by Gosner stage (F1,47 = 0.02; P = 0.91). Similarly, there were no differences in mortality rates as measured in tanks of each treatment group. Though we observed a subset of tadpole deaths in both the control (16.6% ± 4.0% of tadpoles, n = 6 tanks), and tannic acid groups (15.0% ± 7.2%) during weeks 2–14, these rates were not significantly different across treatment groups (t-test: t(10) = 0.20; P = 0.84). The majority of these mortalities (82%; 31 of 38 total deaths, out of 240 total tadpoles) occurred within weeks 2–4, with very little mortality occurring afterward.
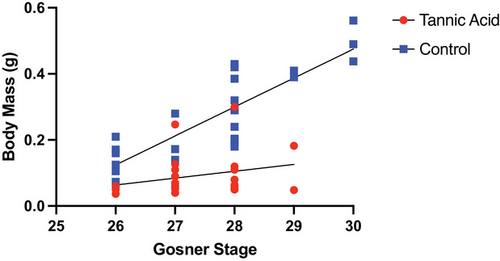
Dietary tannins significantly reduced the growth of tadpoles such that control tadpoles had higher body masses (M = 0.091 g for tannic acid fed tadpoles, M = 0.275 g for control tadpoles). As expected, developmental Gosner stage had a significant positive relationship with tadpole body mass, but dietary tannins significantly impacted this relationship, such that tadpoles fed tannic acid exhibited significantly smaller body mass (Fig. 1; ANCOVA of body mass: dietary tannins: F1,45 = 79.25; P < 0.0001; Gosner stage: F1,45 = 30.07; P < 0.0001; dietary tannins × Gosner stage: F1,45 = 11.27; P = 0.0016). While not shown, tannic acid had similar effects of decreasing measures of tadpole body length and width (P < 0.001).
Behavior
There was a significant interaction of time (minute of assay) and tannin treatment group across overall activity during the assay (Fig. 2a). Control tadpoles exhibited significantly more activity in the first minute of the assay when compared to tadpoles fed diets containing tannic acid (Fig. 2a; F1,47 = 39.63; P < 0.0001). Despite being less active during this period, the tadpoles fed tannic acid focused their movement during minute 1 more toward the middle of the tanks, indicative of greater boldness, when compared to control tadpoles (Fig. 2b; F1,47 = 4.94; P = 0.031).
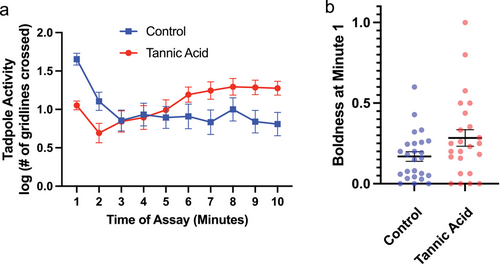
These differences in behavior were only exhibited during the beginning of the experimental assay. Comparisons of per-minute activity toward the end of the assay are not statistically significant. Further, when comparing data accumulated over the full 10-min observation, there were no group differences in total activity (F1,47 = 1.20; P = 0.28) or boldness (F1,47 = 0.30; P = 0.58) as a result of tannic acid.
Gut microbiome
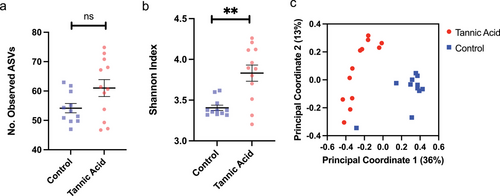
Dietary tannins had significant effects on the tadpole gut microbiome. Microbiome sequencing resulted in a total of 718 710 high-quality microbial 16S rDNA sequences (average of 31 248 ± 2947 SEM sequences per sample). While dietary tannic acid had no significant effect on microbial richness (the number of ASVs; (Fig. 3a; F1,10 = 2.94; P = 0.12)), the inclusion of this toxin resulted in a gut community with greater Shannon entropy when compared to control tadpoles (Fig. 3b; F1,10 = 11.80; P = 0.006). Additionally, there was a significant effect of this treatment on gut community structure, such that tadpoles fed control diets or those laden with tannic acid harbored distinct microbial communities (Fig. 3c; PERMANOVA; pseudo-F = 8.89; P = 0.00002).
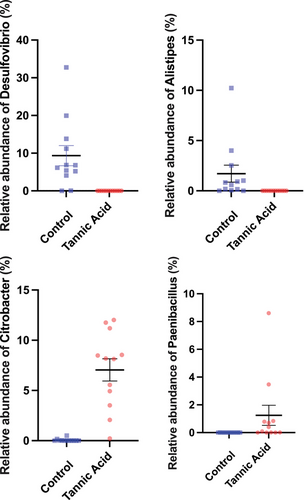
When comparing the abundances of microbial taxa, we observed 7 microbial phyla and 153 microbial genera that exhibited significantly differential abundances differences between control and tannic acid-fed tadpoles. We present four genera that were relatively prevalent and abundant, but with differential responses to tannic acid; adding tannins extirpates the genera Desulfovibrio and Alistipes, while enriching the abundances of Citrobacter and Paenibacillus (Fig. 4). The differential abundances of these taxa were supported by both the CLC Genomics Workbench differential abundance analysis and DESeq2. All relative abundances and statistical results for other genera can be found in Supporting Information.
Finally, we investigated for connections between various measures of tadpole behavior and aspects of the microbiome (diversity, values of principal coordinates, and abundances of taxa). However, we did not detect any significant effects from these analyses.
DISCUSSION
Here, we examined the effects of dietary tannic acid on tadpole growth, development, behavior, and the gut microbiome. Most studies investigating the effects of these compounds on larval amphibians have used exposure through leached or dissolved compounds. We found that dietary tannic acid significantly stunted growth such that experimental tadpoles had lower body masses at a given Gosner stage. The effects of tannic acid on behavior were subtle and context specific. We found a significant effect of tannic acid on exploration and boldness of the first minute of the assay, but no significant effects of treatment on total activity during the second half of the assay. Last, tannic acid significantly altered the diversity and community composition of the gut microbiome.
This study is the first of our knowledge to demonstrate the negative effects of dietary tannins on amphibian growth and development. We observed that dietary tannic acid decreased tadpole body mass, length, and width. These negative effects are consistent with another study that demonstrated that metamorphs raised in water containing leaf litters with high polyphenolic concentrations (such as red maple) were smaller and survived poorly (Stephens et al. 2013). However, tannin-rich leachate has been found to increase body mass and Gosner stage in some amphibian species (Watling et al. 2011; Earl & Semlitsch 2015) or have no effect on body mass in others (Watling et al. 2011; Dodd 2018). The equivocal results across studies may be the result of several factors that remain to be disentangled. First, many toxicological agents exhibit hormetic dose response curves, or those that are biphasic, U-shaped, or inverted U-shaped (Calabrese & Blain 2005). Such response curves have been demonstrated between tannins and the model nematode Caenorhabditis elegans (Saul et al. 2013). Tannin concentrations are not standardized across different studies. Thus, equivocal results could stem from varying tannin concentrations causing effects at different points on the dose response curves. Additionally, the absorption and distribution of plant toxins may differ between dietary and leachate exposure (Sorensen et al. 2006). For example, leached tannins have been shown to damage the epithelial gill tissues of fish (Temmink et al. 1989), and it has been argued that effects on respiration could underlie the negative effects of leached plant toxins in tadpoles (Maerz et al. 2005; Earl & Semlitsch 2015). Conversely, our understanding of dietary tannins, primarily through studying terrestrial herbivores, is that these compounds generally decrease nutrient assimilation through the precipitation of dietary proteins and digestive enzymes (Ferreira et al. 2008). Future work may compare differential exposure routes to tannic acid and other plant secondary compounds in aquatic herbivores and the downstream physiological effects.
Results of the impacts of tannins on tadpoles may also vary across studies due to ecological and evolutionary contexts. Previous work has demonstrated differences across anuran species exposed to the same concentrations of leached tannins (Earl & Semlitsch 2015), suggesting the potential for evolved responses. It could be that species or populations that regularly interact with tannins have higher tolerance to these compounds, similar to how amphibian populations have been shown to evolve to anthropogenic xenobiotics (pesticides) over relatively short time scales (Hua et al. 2015). This notion is also supported by a study demonstrating that invasive leachates are more detrimental to tadpoles than leachates from native plants (Stephens et al. 2013), though does not fully exclude the confounding effects of differential concentrations and structures of tannin and phenolic compounds across plant taxonomy (Stoler & Relyea 2013; Earl & Semlitsch 2015). Other research has suggested that plant traits have greater impact on larval amphibians than plant origin (Cohen et al. 2012).
Another component that may vary experimentally and ecologically is the concentration of tannins. Species might encounter different levels of tannins naturally and thus be more adapted to grow in those conditions (Earl & Semlitsch 2015). While tannin and phenolic content varies across plant species, our 2% tannic acid treatment is ecologically relevant for maple and oak leaves that tadpoles have been shown to consume (Skelly & Golon 2003; Stoler et al. 2016). Under captive conditions, green frog tadpoles readily consume newly provided leaves of red maple (Acer rubrum), containing over 3% phenolic compounds (Stoler et al. 2016). However, given that tannins leach into the water from the leaves, it would be worth investigating the propensity for tadpoles to consume tannin-rich plants under natural conditions (Montaña et al. 2019) and measuring the concentrations of tannins of material consumed under these scenarios.
We found significant effects of dietary tannic acid on tadpole exploration and boldness during minute one of the assay. While prior behavioral research has allowed a grace period before measuring exploration in a new environment, we believed minute one of the assay could capture the essence of exploration before the tadpoles adjusted to the new environment. Although the tannic acid-fed tadpoles displayed less exploration, they acted more boldly. This finding might be counter-intuitive, but it could imply that the tannic acid-fed tadpoles conserved their energy to reside in the center of the new environment. The result might suggest that the tadpoles with poor diets would initially avoid exploring a new environment but still leave the periphery of their environment to seek better food sources. Dietary changes might influence general activity patterns, as seen in our interaction term of time and group, but more research is needed to understand how tannins influence amphibian behavior.
In the second half of the assay, tadpoles treated with dietary tannic acid tended to exhibit more activity than the control tadpoles. These activity patterns and the increased boldness in experimental tadpoles during minute one generally support the idea of the “food-fear” tradeoff (McArthur et al. 2014). The initial lack of exploration seen in the experimental tadpoles might capture a fear hesitancy that the tadpoles eventually overcame during the latter half of the assay. It is possible that our assay design did not fully capture the boldness behavior, as it was limited to 10 min, and similarly may not truly capture dispersal behavior in tadpoles. Other studies that measure boldness and exploration used assays that lasted 20 and 25 min (Urszán et al. 2015b; Mühlenhaupt et al. 2022). Boldness can also change as tadpoles age (Mühlenhaupt et al. 2022), so it is possible that dietary effects on boldness are more distinguished in adulthood. Ultimately, addressing this fear-food tradeoff in aquatic herbivores will require more studies.
Our work also shows that the presence of dietary tannins alters the diversity and composition of the tadpole gut microbiome. Tannins may resculpt these communities through antimicrobial activities, such as through the inhibition of nutrient acquisition through binding to microbial enzymes and micronutrients (Scalbert 1991). In general, antibiotic compounds decrease metrics of alpha diversity (McDonnell et al. 2021), though there are examples where antimicrobial compounds increase microbial diversity (Schokker et al. 2014; Nielsen et al. 2021), perhaps through reductions in competitive dominance (Cray et al. 2013). Additionally, some bacteria are able to utilize plant toxins, including tannins, as carbon and nutrient sources, and thus, these bacteria may benefit from the addition of these toxins. A diversity of mammalian herbivores harbor tannin-degrading microbes in their guts which ameliorate the negative impacts of these compounds (Kohl et al. 2016). It could be that such tannin-degrading bacteria also have the capacity to improve tadpole growth in the face of these chemicals. Future studies may use more microbial manipulation (Fontaine et al. 2022) and metagenomic sequencing to better understand the functional interactions occurring in this system.
Research shows that microbiome can interact with aspects of host behavior in many animal taxa, including exploratory behavior and social interactions (Ezenwa et al. 2012). For example, antibiotic treatment alters the boldness of zebrafish (Almeida et al. 2019), and female guppies with more diverse skin microbiomes exhibit lower activity (Kramp et al. 2022). Our current study was not designed to parse apart the relationship between the microbiome and behavior. We did not observe any significant correlations between measures of behavior and microbial diversity, though our small sample size could cause limitations. However, it could still be the case that under more natural conditions or alternative experimental designs, dietary tannins could indirectly alter these connections. Looking specifically at amphibians, greater tadpole body size and boldness have previously been found to increase social acquisition of a bacterial symbiont (Keiser et al. 2019). Thus, changes in body size, activity, and boldness could yield differences in microbial communities or acquisitions of symbionts under more natural scenarios. However, more research on amphibian microbiome and behavior is needed to elucidate the relationship.
Our work demonstrates that dietary plant toxins have the capacity to influence amphibian growth, much akin to what has already been demonstrated for leached plant toxins. More work is required to understand the different potencies and physiological effects of leached versus dietary compounds. Collectively, understanding the interactions between plant compounds, aquatic herbivores, and their growth could have implications for decomposition and carbon transfer in these aquatic ecosystems (Stoler et al. 2016; Stoler & Relyea 2020).
ACKNOWLEDGMENTS
This project was supported by start-up funds awarded to K.D.K. We thank K. Kohler, S. Reilly, and S. Hartill for assistance with animal husbandry. We also thank S. Fontaine for collecting the egg mass. Finally, we would like to acknowledge that Biorender was used to create the graphical abstract.




