Bombyx mori C-Type Lectin 4 Recognizes a Broad Range of Carbohydrates of Microbial Pathogens
Funding: This work was supported by Dong-A University Research Fund.
Min Ji Park and Bo Yeon Kim have equally contributed to this study.
ABSTRACT
C-type lectins (CTLs) are essential pattern-recognition receptors involved in insect innate immunity, mediating defense responses through carbohydrate binding. In this study, we aimed to clone and functionally characterize Bombyx mori CTL4 (BmCTL4), focusing on its immunity-related functions and binding specificity. To this end, the BmCTL4 cDNA was cloned and the recombinant BmCTL4 protein was expressed using a baculovirus expression system. The recombinant protein was then used to assess its binding specificity via carbohydrate-binding and microbial cell-binding assays. BmCTL4 contained a single carbohydrate recognition domain (CRD) with a QPD motif, suggesting specificity for galactose-type sugars. BmCTL4 was upregulated in the fat body following microbial inoculation. Recombinant BmCTL4 exhibited Ca2+-dependent binding to microbial cell wall constituents such as LPS, mannan, N-acetyl-D-glucosamine, and peptidoglycan, and directly bound to microbial cell surfaces as confirmed by confocal microscopy. Our findings demonstrate that BmCTL4 acts as a pattern-recognition receptor with broad carbohydrate-binding activity, contributing to the immune defense mechanisms of B. mori.
1 Introduction
Insects predominantly rely on innate immunity, rather than adaptive immunity, to combat pathogens such as viruses, bacteria, fungi, and parasites (Sadd and Schmid-Hempel 2006; Uvell and Engstrom 2007). Following the invasion of a pathogen in an insect, innate immunity is initiated with the recognition of pathogen-associated molecular patterns (PAMPs) on the pathogen's surface by pattern recognition receptors (PRRs) (Kanost et al. 2004; Lemaitre and Hoffmann 2007). PAMPs subsequently activate humoral and cellular immune responses, such as melanization, phagocytosis, encapsulation, blood clotting, and antimicrobial peptide synthesis (Lavine and Strand 2002; Lemaitre and Hoffmann 2007). C-type lectins (CTLs) possess at least one carbohydrate recognition domain (CRD), functioning as a PRR, and are classified based on their structural features. CTL-S possesses a single CRD, immunolectins possess two CRDs, and CTL-X possesses one CRD alongside other functional domains, such as a dipeptide domain, chitin-binding domain, epidermal growth factor (EGF), complement control protein (CCP), or an immunoglobulin (Ig) module (Rao, Cao, et al. 2015; Rao, Shahzad, et al. 2015; Yu et al. 2006; Yu and Kanost 2004; Zhan et al. 2016).
The domestic silkworm, Bombyx mori, has been used as a model insect in life sciences, in various fields including physiology, genetics, biochemistry, and molecular biology, among others (Jingade et al. 2011; Zhou et al. 2015). The Silkworm Genome Project (Xia and Yang 2004; Xia et al. 2009) has led to the development of bioinformatics on the silkworm and has accelerated its utilization as a model organism in research. To date, 16 CTL genes have been identified in B. mori, of which nine have been functionally characterized: a lipopolysaccharide (LPS)-binding protein referred to as BmLBP (BmCTL21) (Koizumi et al. 1997; Koizumi, Imai, et al. 1999; Koizumi, Imamura, et al. 1999; Park et al. 2024), a multiple-target-binding protein referred to as BmMBP (BmCTL10) (Watanabe et al. 2006), three low-expressed lectins referred to as BmLEL-1 (BmCTL17), BmLEL-2, and BmLEL-3 (BmCTL6) (Takase et al. 2009), an immunolectin referred to as BmIML (BmCTL11) (Kim and Jin 2017), CTL-S2 (Shahzad et al. 2017), CTL-S6 (Shen et al. 2021), and CTL5 (Sun et al. 2023). Despite extensive investigations into the bioinformatics of B. mori CTLs, limitations remain in predicting the functions of uncharacterized CTLs.
In this study, we report that B. mori CTL4 (BmCTL4) is upregulated in the fat body following microorganism inoculation and binds to carbohydrates as well as to the surfaces of microorganisms.
2 Materials and Methods
2.1 Silkworm Rearing
B. mori silkworms (F1 Baekok-Jam hybrid) were provided by the Department of Agricultural Biology, National Institute of Agricultural Science, Republic of Korea, and were reared on fresh mulberry leaves in accordance with the silkworm breeding standards outlined by the Rural Development Administration (Kim and Jin 2017; Lee et al. 2018; Park et al. 2024).
2.2 Cloning and Sequence Analysis of BmCTL4
To clone the BmCTL4 cDNA, total RNA was extracted from the entire body of B. mori larvae using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the AccuPower RT-PCR PreMix (BIONEER, Daejeon, Republic of Korea) and 10 pmol of each primer (Table S1) at 42°C for 60 min. PCR amplification was then conducted under the following cycling conditions: initial denaturation at 95°C for 5 min; 33 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min; followed by a final extension at 72°C for 5 min. The PCR products were then ligated into the pGEM-T vector (Promega, Madison, WI, USA), and the fidelity of the nucleotide sequences was verified using a 3730XL DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
For the analysis of the BmCTL4 amino acid sequence, the SignalP 5.0 program (http://www.cbs.dtu.dk/services/SignalP), MOTIF Search (https://www.genome.jp/tools/motif), known sugar-binding sites (Kolatkar and Weis 1996; Huang et al. 2013), and multiple sequence alignment using MacVector (Version 6.5, Oxford Molecular Ltd., Oxford, UK) were used. Phylogenetic analysis was conducted using MEGA 11.0 with the maximum likelihood method based on the Kimura 2-parameter model and 1000 bootstrap replicates. Lectin amino acid sequences from insects were aligned using ClustalW with default parameters prior to phylogenetic tree construction.
2.3 Microbial Injection and Reverse Transcription-Quantitative PCR (RT-qPCR)
For tissue-specific expression analysis, fat body, hemocytes, and epidermis were collected from 3-day-old fifth-instar larvae. To examine BmCTL4 expression induction by microorganisms, 3-day-old fifth-instar larvae were injected with 1.0 × 105 Escherichia coli in 50 μL per individual, while larvae injected with 50 μL of PBS served as the control (Park et al. 2024). Fat bodies were collected at 3, 6, 12, and 24 h post-injection. Total RNA was isolated using RNAiso Plus (TaKaRa Bio, Shiga, Japan) and quantified with a NanoDrop One spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized using the AccuPower RT PreMix (BIONEER, Daejeon, Korea) with 2 μg of total RNA for RT-qPCR. BmCTL4 expression levels were quantified using TB Green Premix Ex Taq (TaKaRa Bio) in a 25 μL reaction mixture containing 0.2 nmol/L of each primer (listed in Table S1) and 80 ng of cDNA. RT-qPCR was conducted using a CronoSTAR 96 real-time PCR system (Clontech, now TaKaRa Bio USA, San Jose, CA, USA). The thermal cycling conditions were 95°C for 5 min, followed by 40 cycles of 95°C for 15 s and 60°C for 30 s. BmCTL4 expression was normalized to the internal control gene actin A4 (Lee et al. 2018; Park et al. 2024). Relative gene expression was calculated using the 2−ΔΔCT method (Livak and Schmittgen 2001) on three biological replicates for each treatment.
2.4 Purification of Recombinant BmCTL4 and Antibody Production
Recombinant BmCTL4 protein was produced using a baculovirus expression system with Autographa californica multiple nucleopolyhedrovirus (AcMNPV) and Sf9 insect cells (Je et al. 2001). Primers (Table S1) used to amplify BmCTL4 cDNA from fat body RNA carried restriction sites for cloning into the pBacPAK8 vector and a 6×His tag for purification. Sf9 cells were co-transfected with AcMNPV DNA and pBacPAK8-BmCTL4 using Lipofectin (Gibco BRL, Gaithersburg, MD, USA). The recombinant virus was propagated in TC100 medium at 27°C. The protein was purified with the MagneHis system (Promega, Madison, WI, USA) and concentrated using Vivaspin 500 (Sartorius Stedim Biotech, Göttingen, Germany). Its molecular weight was estimated using a standard calibration curve (Dunker and Rueckert 1969; Shapiro et al. 1967; Shapiro et al. 1969; Weber and Osborn 1969).
Anti-BmCTL4 antibodies were produced as described in previous studies (Lee et al. 2015; Zou et al. 2015; Kim and Jin 2017; Lee et al. 2018; Park et al. 2024). BALB/c mice (Samtako Bio Korea Co., Osan, Republic of Korea) were injected with ~5 μg of recombinant BmCTL4 protein once a week for 4 weeks. Freund's complete adjuvant was included in the initial dose, Freund's incomplete adjuvant was used in the subsequent two doses (Sigma-Aldrich, St. Louis, MI, USA), and the final dose consisted solely of protein. Blood was collected 3 days after the last injection. Antibodies were obtained by centrifugation (9600 ×g, 10 min, 4°C) and stored at −70°C for western blot analysis.
2.5 SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot Analysis
Proteins from fat bodies were extracted for western blot analysis using BioMasher II tubes (NIPPI, Tokyo, Japan), followed by centrifugation to obtain the supernatant. Protein concentrations were determined using a protein assay reagent (Bio-Rad, Hercules, CA, USA).
Following protocols reported in previous studies (Lee et al. 2015; Zou et al. 2015; Kim and Jin 2017; Lee et al. 2018; Park et al. 2024), protein samples (~10 μg) were separated on a 12% SDS-PAGE. The separated proteins were transferred and analyzed through western blotting using anti-BmCTL4 or anti-His antibodies (BETHYL Laboratories, Montgomery, TX, USA), and detected with an enhanced chemiluminescence (ECL) system (Amersham Biosciences, Piscataway, NJ, USA), following the manufacturer's instructions. A horseradish peroxidase-conjugated anti-mouse IgG (1:5000 dilution, v/v; Enzo Life Sciences, Farmingdale, NY, USA) was used as the secondary antibody. Band intensities were quantified using an Alpha Imager 1220 v5.5 (Alpha Innotech Co., San Leandro, CA, USA).
2.6 Binding Properties of Recombinant BmCTL4
The binding properties of recombinant BmCTL4 as a PRR were evaluated using a binding assay as described by Kim and Jin (2017) and Park et al. (2024). Microbial cell wall components—LPS, mannan, N-acetyl-D-glucosamine, and peptidoglycan (PGN) (all from Sigma-Aldrich)—were coated onto a 96-well plate. The plate was subsequently blocked with 0.1 mg/mL bovine serum albumin (BSA) solution in Tris buffer (50 mmol/L Tris–HCl, 50 mmol/L NaCl, pH 8.0). After washing four times with Tris buffer, the plate was incubated with 0, 20, 40, 60, 80, or 100 nmol/L of recombinant BmCTL4, with or without 5 mmol/L CaCl2, for 3 h at room temperature. After washing with Tris buffer, the plate was then incubated with polyclonal anti-His antibodies (1:1000, v/v) for 1 h at room temperature. Subsequently, it was washed again and incubated with HRP-conjugated goat anti-mouse IgG (1:5000, v/v; Enzo Life Sciences) for an additional hour. After a final wash, 100 μL of TMB substrate was added to each well and was allowed to incubate for 10 min. The reaction was terminated with 100 μL of 2 mol/L H2SO4, and absorbance was measured at 450 nm using an Infinite F50 microplate reader (Tecan, Grödig, Austria).
Next, to verify the binding of recombinant BmCTL4 to the surface of living microorganisms, cultures of E. coli, B. thuringiensis, and B. bassiana were prepared for the microbial binding assay following a protocol of a previous study (Park et al. 2024). Upon reaching an optical density of 0.4 at 600 nm (OD600), 4 mL of each culture was harvested, washed twice with PBS, and resuspended in 40 μL of PBS containing 1 μg of recombinant BmCTL4. The mixtures were incubated at room temperature for 10 min and then centrifuged at 1500 ×g for 5 min to collect the microbial pellet. After a PBS wash, the pellet was resuspended in 40 μL of PBS. To assess binding, both pellet and supernatant fractions were subjected to western blot analysis using anti-His antibodies (BETHYL Laboratories; 1:10,000, v/v), following previously described methods. Microbial immunofluorescence staining (Park et al. 2024) was subsequently carried out by fixing the microorganism pellets onto slides with cold methanol (−20°C) for 2 min. Microorganisms were subsequently incubated with anti-BmCTL4 antibodies, followed by fluorescein-conjugated goat anti-mouse secondary antibodies (Santa Cruz Biotech. Inc., Santa Cruz, CA, USA). The samples were visualized using a laser scanning confocal microscope (Carl Zeiss LSM 510, Zeiss, Jena, Germany).
2.7 Statistics
Statistical analysis was carried out using SPSS Statistics 22.0 (IBM, Armonk, NY, USA). Each treatment comprised three biological replicates and is presented as the mean ± standard deviation. Comparisons between two groups were performed using the Wilcoxon rank sum test, followed by an independent unpaired two-tailed Student's t-test. Statistical significance was defined as *p < 0.05 and **p < 0.001.
3 Results
3.1 Structural Feature of BmCTL4
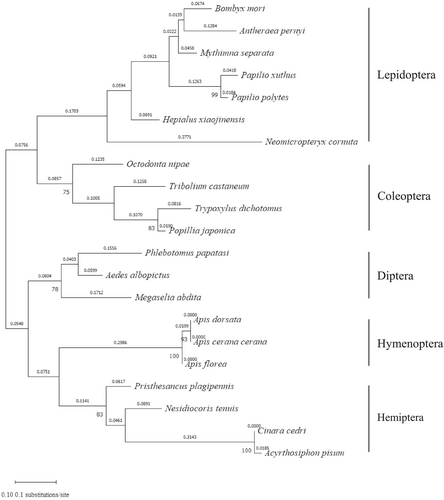
B. mori CTL4 (BmCTL4) cDNA encodes a protein of 221 amino acids, including a 19-amino-acid signal peptide and a single carbohydrate recognition domain (CRD) of 172 amino acids. The predicted molecular weight and isoelectric point of the mature BmCTL4 were 23.1 kDa and 8.2, respectively. The amino acid sequence of the cloned BmCTL4 contains eight conserved cysteine residues and a Gln-Pro-Asp (QPD) motif, which is associated with potential galactose-binding specificity (Figure S1). The phylogenetic tree was divided into two groups: one containing lepidopteran and coleopteran CTLs, and the other including dipteran, hymenopteran, and hemipteran CTLs. BmCTL4 showed the highest homology with Antheraea pernyi CTL4 (Figure 1).
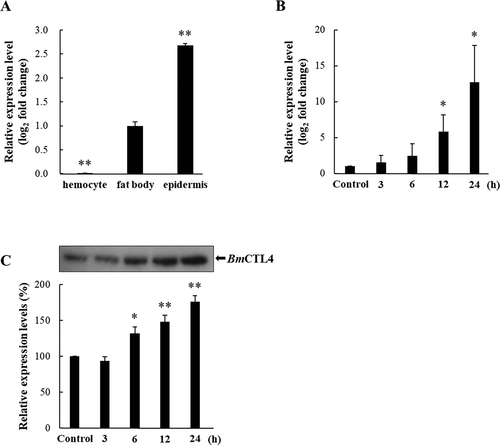
3.2 Tissue Expression Profiling and Induction Analysis
The tissue-specific expression of BmCTL4 in hemocytes, fat body, and epidermis of 3-day-old fifth-instar larvae was assessed with RT-qPCR. BmCTL4 expression was predominantly observed in the fat body and epidermis, whereas its expression in hemocytes was negligible (Figure 2A). To investigate whether BmCTL4 functions as a PRR responsive to E. coli, its expression in the fat body was further evaluated at multiple time points following E. coli injection. BmCTL4 expression was gradually upregulated starting at 3 h, with higher expression levels observed at 12 and 24 h following E. coli injection (Figure 2B). Next, western blot analysis was performed to confirm BmCTL4 upregulation at the protein level. Consistent with the RT-qPCR results, BmCTL4 protein expression was induced in the fat body, exhibiting a time-dependent increase in band intensity (Figure 2C).
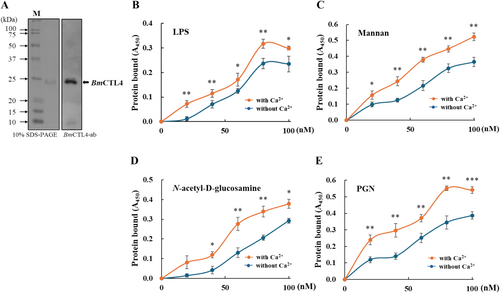
3.3 BmCTL4 Binding Affinity to Carbohydrates and Microorganisms
To investigate the functional role of BmCTL4, a baculovirus expression system was employed for the production of the recombinant BmCTL4 protein. The 24.5-kDa recombinant protein was purified from total cellular lysates and used to immunize mice for the generation of polyclonal antibodies (Figure 3A). Binding assays demonstrated that the recombinant BmCTL4 interacted with LPS (Figure 3B), mannan (Figure 3C), N-acetyl-D-glucosamine (Figure 3D), and PGN (Figure 3E) in a dose- and a Ca2+-dependent manner.
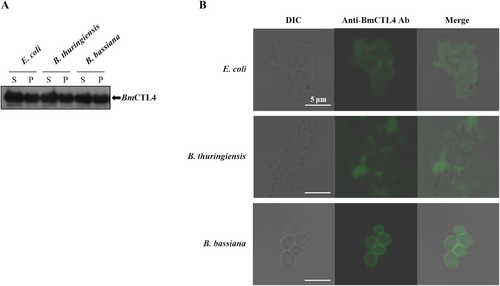
To further assess its binding target recognition capabilities, we tested whether recombinant BmCTL4 could bind to the surfaces of live microorganisms, including B. thuringiensis, E. coli, and B. bassiana. Western blot-based microbial binding assays confirmed its affinity and binding to all three organisms (Figure 4A). Furthermore, BmCTL4 was localized to the cell walls of bacteria, fungi, and yeast, as evidenced by immunofluorescence staining (Figure 4B). Collectively, these findings indicate that BmCTL4 is a CTL capable of recognizing both Gram-positive and Gram-negative bacteria, as well as fungi.
4 Discussion
Insect CTLs constitute a family of carbohydrate-binding proteins that recognize and bind to carbohydrates located on the surfaces of pathogens (Zhang et al. 2015). CTLs are categorized into three groups according to their domain architecture: CTL-S (single carbohydrate recognition domain CRD), immunolectins (dual CRDs), and CTL-X (single CRD and complex domains). In B. mori, there are at least 12 CTL-S, 6 immunolectins, and 5 CTL-X proteins. In B. mori, 16 CTL genes have been reported, yet several have not been functionally characterized. In our previous work, we reported the dual-CRD proteins, BmIML (Kim et al. 2003) and BmCTL21 (Park et al. 2024) in the silkworm B. mori. In this study, we investigated the ligand and functional roles of BmCTL4.
CTLs exhibit high specificity in binding carbohydrates, which is determined by hydrogen-bond patterns in conserved CRD motifs (Wang et al. 2012). The WND motif, which is essential for carbohydrate binding, is frequently retained in vertebrate lectins (Iwanaga and Lee 2005), while substitutions such as FRD or VND are observed in invertebrates (Xiu et al. 2016). In particular, the EPN motif mediates mannose binding, and the QPD motif targets galactose and similar sugars (Kolatkar and Weis 1996; Huang et al. 2013). Sequence analysis indicated that BmCTL4 possessed a canonical CRD with a QPD motif, implying its functions in polysaccharide binding and the recognition of pathogen surface carbohydrates.
It has been reported that B. mori CTLs are expressed in various organs involved in immune responses. In this study, we found that BmCTL4 was expressed in the fat body and epidermis, with its expression upregulated in the fat body after E. coli injection. Our recent research has also demonstrated that the fat body was the only organ in which BmCTL21 was expressed (Park et al. 2024), although the BmCTL21 protein was detected and purified from hemocytes following microorganism inoculation (Koizumi et al. 1997; Koizumi, Imai, et al. 1999; Koizumi, Imamura, et al. 1999; Takase et al. 2009). BmMBP was expressed in the fat body and hemocytes, as demonstrated by immunoblotting (Watanabe et al. 2006), whereas BmIML (Kim et al. 2003) and BmCTL5 (Sun et al. 2023) expressions were detected specifically in the fat body. BmLEL-1, -2, and -3 were shown to be expressed in the testis and ovary, with BmLEL-2 being upregulated following bacterial infection (Takase et al. 2009). BmCTL-S6 was induced in the fat body and hemocytes upon microbial challenge; however, its highest expression was observed in the midgut even in the absence of such stimulation (Shen et al. 2021).
Agglutination is a fundamental defense mechanism through which organisms combat invading pathogens, with binding serving as the crucial first step (Xu et al. 2010). BmCTL4 could bind to LPS, mannan, N-acetyl-D-glucosamine, and PGN in a dose-dependent manner, resembling the behavior of QPD-motif-containing CTLs in the silkworm that interact with diverse microbial cell wall components (Zhan et al. 2016). Similarly, five CTLs from the mosquito Armigeres subalbatus have been shown to recognize diverse polysaccharides, including LPS, lipid A, PGN, lipoteichoic acid, zymosan, and laminarin (Shi et al. 2014), highlighting the broad microbial recognition potential of CTLs. In this study, recombinant BmCTL4 exhibited strong, Ca2+-dependent binding to microbial cell wall components. This broad binding profile resembles the activities of MsLec from Manduca sexta and BmLEL-1 and -2 from B. mori (Yu et al. 1999; Yu and Kanost 2000; Takase et al. 2009; Wei et al. 2018), all of which exhibit extensive interactions with diverse microorganisms.
In addition, BmCTL4 was shown to directly bind to the cell walls of Gram-negative E. coli, Gram-positive B. thuringiensis, and the entomopathogenic fungus B. bassiana. This pattern is consistent with the binding affinities and functions of lepidopteran immune lectins, which are typically characterized by tandem dual CRDs and are secreted into the hemolymph to mediate pathogen recognition and opsonization (Yu and Kanost 2001). For example, BmMBP is expressed and produced in multiple tissues and binds various microorganisms (Watanabe et al. 2006). In contrast, lectins containing only a single CRD typically exhibit narrower binding spectra. For instance, the Drosophila melanogaster galactose-specific CTL binds and agglutinates E. coli but not E. chrysanthemi (Tanji et al. 2006), while Anopheles gambiae CTLs (AgCTL4 and AgCTLMA2) form a disulfide-linked heterodimer that exclusively targets E. coli (Schnitger et al. 2009). Collectively, these findings suggest that, despite BmCTL4 possessing a single CRD, it demonstrates broad binding capabilities and is more similar to lepidopteran immune lectins.
5 Conclusion
In summary, we cloned and characterized BmCTL4 from B. mori and demonstrated its crucial functions in pathogen detection in immune defense. BmCTL4, containing a single CRD with a QPD motif, was upregulated in the fat body following microbial challenge. Recombinant BmCTL4 exhibited Ca2+-dependent binding to diverse microbial cell wall constituents and directly bound to microbial surfaces, suggesting broad recognition capacity. These findings underscore BmCTL4 as a major pattern-recognition receptor involved in the innate immune responses of B. mori.
Acknowledgments
This work was supported by the Dong-A University Research Fund.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data will be made available upon request to the corresponding authors.




