Insect cell-derived human CD200-Fc increases zonula occludens-1 tight junction protein in urothelial carcinoma cells
Abstract
CD200 is a ligand that interacts with the CD200 receptor 1, with an anti-inflammatory effect. CD200 inhibits the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, attenuating the inflammatory response and maintaining barrier function. In this study, human CD200 was fused with the fragment crystallizable (Fc) region of human immunoglobulin G (IgG) to generate the recombinant CD200-Fc protein, produced in insect cells using the baculovirus expression vector system. The CD200-Fc gene was cloned under the control of the polyhedrin promoter in the pFastBac-1 vector of the baculovirus expression vector system. Immunoblot analysis confirmed the expression of CD200-Fc in insect cells. The CD200-Fc protein was successfully purified using protein A affinity chromatography. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay revealed that purified CD200-Fc protein inhibited the proliferation of TCCSUP cells, a bladder epithelial cancer cell expressing CD200 receptor 1, at concentrations of 1, 10 and 100 ng/mL. Quantitative real-time reverse transcription PCR (qRT-PCR) and immunoblot analyses confirmed that the mRNA and protein levels of zonula occludens-1, a tight junction protein for barrier protection in epithelial tissues, were increased in TCCSUP cells treated with insect cell-derived CD200-Fc. These results suggest that insect cell-derived CD200-Fc could alter zonula occludens-1 expression in bladder epithelial cancer cells, enhancing the function of the cell barrier protein, which could inhibit cancer metastasis. Taken together, the baculovirus expression vector system can be applied to express antitumor therapeutic CD200-Fc protein for the inhibition of bladder cancer.
Introduction
Recombinant proteins have been produced on multiple platforms, including mammalian, plant, insect, bacterial and yeast cells. Recombinant protein production is mostly based on mammalian cells. However, the baculovirus expression vector system (BEVS) can provide an alternative low-cost and scalable production platform for recombinant protein using eukaryotic insect cells, compared with the mammalian expression system (Hervas-Stubbs et al. 2007; Fernandes et al. 2022). Insect cell expression systems offer significant advantages. BEVS using insect cells includes post-translational modifications such as glycosylation, an essential process that allows recombinant proteins to function efficiently (Struble et al. 2022). Insect cells are easier to culture than mammalian cells and are less expensive for the production of recombinant proteins, as they do not require serum. In addition, insect cells with BEVS provide high clonal capacity, up to 1000 kb, and conversion efficiency (Du & Webb 2011). Insect cell BEVS mainly uses Spodoptera frugiperda cell lines such as Sf9 and Sf21. The S. frugiperda cell line accomplishes high protein yields with passaging stability. Licensed vaccines such as Nuvaxovid, Cervarix and Provenge have been produced in insect cells, indicating that insect cell BEVS serves as a useful platform to produce promising therapeutic proteins.
CD200 is a type-1 membrane glycoprotein of the immunoglobulin superfamily (IgSF) of cell surface proteins. CD200 exerts its effects by binding to a structurally related receptor (CD200R1), which is mainly expressed in bone marrow cells (e.g. macrophages, neutrophils, monocytes and microglia), and triggers an intracellular signaling cascade (Wright et al. 2003; Walker et al. 2009). CD200–CD200R1 signaling plays an important role in regulating the immune response to inflammatory stimuli by promoting the suppressive activity of the immune system (Snelgrove et al. 2008). Specifically, CD200 downregulates inflammation by inhibiting the nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) pathway. These molecules play a critical role in maintaining homeostasis and inhibiting inflammatory responses to external stimuli (allergens, pathogens, etc.) and internal stimuli (tissue damage, hypoxia, oxygen radicals, etc.) (Holmannová et al. 2012).
Fc-fusion proteins consist of an immunoglobulin Fc domain linked directly to another peptide. Binding of the Fc domain to the neonatal Fc receptor (FcRn) protects the protein from lysosomal degradation and promotes recycling, increasing its half-life (Rath et al. 2015). In addition, the attached Fc domain allows it to interact with Fc-receptors expressed on immune cells. Thus, Fc-fusion proteins have been introduced as promising biopharmaceuticals (Niu et al. 2022).
Tight junctions (TJs) play an important role in maintaining epithelial cells. The intracellular regions of TJs bind cytoskeletal elements and signaling molecules, and downstream signals regulate cell migration, proliferation and differentiation (Kyuno et al. 2021; Liu et al. 2022). In epithelial cells, TJs can function in an adhesive manner and prevent cell dissociation (Hollande et al. 2001). A critical step in the formation of cancer metastasis is the interaction and penetration of vascular endothelium by dissociated cancer cells. The regulation of tight junction protein (TJP) expression plays an important role in tumor invasion and metastatic progression in several cancers. Therefore, TJs have the potential to prevent cancer cell metastasis (Hoevel et al. 2002; Martin & Jiang 2009). Zonula occludens-1 (ZO-1) is an important TJP. It is essential for gene transcription, signal transduction, penetration between adjacent cells, cell proliferation and differentiation. Therefore, ZO-1 is used as an indicator and marker to observe the permeability and barrier function of various tissues (Liu et al. 2022; Brunner et al. 2023).
This study demonstrates that recombinant human CD200-Fc protein expressed in insect cells using BEVS inhibits the proliferation of urothelial carcinoma cells and increases ZO-1 gene expression in the cells.
Materials and methods
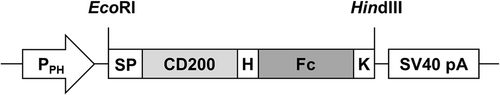
Construction of CD200-Fc gene expression vector
A synthetic DNA sequence encoding human CD200 extracellular region (Gln31-Gly232, National Center for Biotechnology Information, NCBI, accession no. NP_001352780.1) was fused to the fragment crystallizable (Fc) region of human IgG1 (Glu99-Lys330, UniProt accession no. P01857) to generate CD200-Fc. CD200-Fc was modified through the addition of an N-terminal extension containing a signal peptide and C-terminus extension with an ER retention signal (KDEL). The gene encoding CD200-Fc was cloned under the control of the polyhedrin promoter in insect cell baculovirus expression vector (pFastBac-1 vector; Invitrogen, Waltham, MA, USA) (Fig. 1). The recombinant expression vector was then transformed into DH5α Escherichia coli competent cells for amplification.
DH10Bac E. coli transformation for the generation of CD200-Fc bacmid
DH10Bac E. coli cells (ThermoFisher Scientific, Waltham, MA, USA) carrying the bacmid were precultured and then the amplified recombinant plasmids were extracted using a FavorPrep™ plasmid extraction mini kit (Favorgen, Taipei, Taiwan). The purified plasmid DNA was transferred into DH10Bac E. coli cells for subsequent transposition into the bacmids. Cells were spread on LB broth blue/white selection agar plates containing kanamycin (50 μg/mL), gentamicin (7 μg/mL), tetracycline (10 μg/mL), X-gal (100 μg/mL) and isopropyl β-d-1-thiogalactopyranoside (40 μg/mL) to select for DH10Bac transformants. Recombinant bacmids were isolated from transformed DH10Bac E. coli cells. Polymerase chain reaction (PCR) was conducted to confirm whether a recombinant bacmid contained the CD200-Fc gene. For PCR, 5′-CCCAGTCACGACGTTGTAAAACG-3′ was used as the forward primer and 5′-AGCGGATAACAATTTCACACAGG-3′ was used as the reverse primer.
Bacmid transfection into Sf9 insect cells
To generate the baculovirus, Sf9 cells (ThermoFisher Scientific) were cultured in SF-900 II serum-free medium (SFM; Gibco, Grand Island, NY, USA) supplemented with penicillin–streptomycin (100 U/mL) (Welgene, Gyeongsan, Republic of Korea) at 28°C with shaking at 140 rpm. In order to remove the antibiotic-containing medium, it was centrifuged at 500 × g for 4 min and discarded. Cells were suspended in antibiotic-free SF-900 II SFM. The Sf9 cells were seeded in six-well plates (SPL, Pocheon, Republic of Korea). Each well was seeded with 1 × 106 cells in 3 mL of antibiotic-free SF-900 II SFM and incubated at room temperature (about 25°C) for 1 h to allow cell attachment. Then, 10 μL of ExpiFectamine Sf Transfection Reagent (Gibco) was diluted in 250 μL Opti-MEM I Reduced Serum Medium (Gibco) to make the transfection mixture for one well. The mixture was incubated for 5 min at room temperature after inverting between five and ten times. After that, 1 μg of CD200-Fc bacmid DNA was added to the transfection mixture and incubated for 5 min at room temperature after inverting. The entire DNA-transfection mixture was then added dropwise to the cells. The plates were incubated at 28°C for 72 h to generate passage zero (P0) recombinant baculoviruses.
Generation of P2 recombinant baculovirus
At 72 h after transfection, the cells were scraped using a scraper, the medium containing the baculovirus was harvested, and then transferred to a 50 mL tube. The tube was centrifuged at 500 × g for 5 min, and the supernatant with P0 baculovirus was filtered with a 0.45 μm polyvinylidene fluoride syringe-based filter (PVDF; Millipore, Burlington, MA, USA). P0 viral stock was stored at 4°C and protected from light. To each well of a six-well plate, 2 mL of Sf9 insect cells (1 × 106 cells) was added to SF-900 II SFM with antibiotics for 1 h at 28°C. Then, 1 mL of P0 viral stock was added to each well and incubated for 72 h at 28°C. The baculovirus-containing medium was collected from each well by centrifugation and filtered to obtain the P1 baculovirus. P2 baculoviruses were generated following the same procedure described above.
Plaque assay for P2 recombinant baculovirus
Plaque assays were performed to determine the titer of the CD200-Fc P2 baculovirus stock. The plaquing medium was prepared before the experiment. Agarose gel (4%; Gibco) was dissolved using a microwave oven. Plaquing media were made by combining 30 mL of Sf-900 medium (1.3×) (Gibco) with 10 mL of dissolved 4% agarose gel with gentle mixing. The plaquing medium was then placed in a 40°C water bath until needed. Sf9 cells were seeded in six-well plates at 1 × 106 cells per well and kept at room temperature for 1 h. Then the medium was removed from each well. One milliliter of diluted virus (10−4, 10−5, 10−6, 10−7 and 10−8) was added to each well and incubated for 1 h at room temperature. For a negative control, virus-free medium was added. The inoculum was removed, and 2 mL of plaquing media was added. The agarose overlay was allowed to harden at room temperature for 1 h. The plate was incubated at 28°C for 4 days. After preparing a Neutral Red solution (Sigma-Aldrich, St. Louis, MO, USA) to 1 mg/mL in Sf-900 II SFM, it was filter-sterilized. Sixteen point five mL of Sf-900 II SFM was mixed with 1.5 mL of filter-sterilized Neutral Red solution (1 mg/mL) and placed in a 40°C water bath. Neutral Red overlay was prepared by adding 6 mL of 4% agarose gel dissolved in Neutral Red solution. One milliliter of Neutral Red overlay was added to each well. Once the agarose hardened, the plate was incubated for 3 days at 28°C. A total of 1 mg/mL of neutral red solution was prepared in cell-culture-grade distilled water. A total of 0.5 mL of Neutral Red solution was added to each well and then incubated at room temperature for 1 h. Plaques were counted after removing the solution. The virus titer was calculated based on the following formula: titer (pfu/mL) = number of plaques × dilution factor ÷ mL of inoculum/well. The obtained P2 baculovirus titer value was 2 × 107 pfu/mL.
Lysis of insect cells infected with baculovirus
HighFive insect cells 1 × 106 cells/mL (ThermoFisher Scientific) were inoculated into a sterile flask. A 2 mL volume of CD200-Fc P2 baculovirus (2 × 107 pfu/mL) was added. Cells were infected using a multiplicity of infection (MOI) of 1 pfu/cell. Cells were incubated at 28°C for 48 h. Then, the cells were collected by centrifugation (2544 × g for 10 min at 4°C) and dissolved by sonication (amplitude 20%, 1 min) in lysis buffer (1× phosphate-buffered saline [PBS], 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4). The lysed cells were centrifuged (14 650 × g for 30 min at 4°C) to obtain the cell lysate. The supernatant was harvested and passed through a 0.22 μm PVDF syringe-based filter.
Purification and dialysis of insect cell-derived CD200-Fc (CD200-FcI)
After adding protein A agarose resin (Amicogen, Jinju, Republic of Korea) to Poly-Prep Chromatography Columns (Bio-Rad, Hercules, CA, USA), the solution was washed with binding buffer (1× PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4). Filtered cell lysate samples were applied and the flow-through collected. This was washed five times using a binding buffer containing 0.5 M NaCl and washed five times with binding buffer. Elution buffer (0.1 M glycine–HCl, pH 3.0) was passed into a microcentrifuge tube containing neutralization buffer (1 M Tris–HCl, pH 8.5) for elution. The purified protein was dialyzed overnight at 4°C using 1× PBS. The purity and concentration of the purified protein were measured with a spectrometer (ThermoFisher Scientific) and compared with the band size of bovine serum albumin diluted to various concentrations using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Aliquots of purified protein were stored at −80°C for further studies.
SDS-PAGE and immunoblot analysis of purified CD200-FcI and cell lysate
Cell lysate sample or purified CD200-FcI protein were boiled for 5 min in 5× protein sample buffer (1.2 mL of 1 M Tris–HCl, 10 mL of 50% glycerol, 4 mL of 10% SDS, 2 mL of 5% bromophenol blue, 1 mL of 2-mercaptoethanol, 1.8 mL of distilled water) and subsequently allowed to cool. Samples were resolved on SDS-PAGE, and then either stained using protein gel staining solution (Dongin Biotech, Seoul, Republic of Korea) or transferred to nitrocellulose membrane (Cytiva, Marlborough, MA, USA). Transferred membrane was blocked with 5% skimmed milk (BD, Franklin Lakes, NJ, USA) for 1 h at room temperature. The blots were incubated for 2 h at room temperature with horseradish peroxidase-conjugated goat anti-human IgG Fc-specific antibody (Bethyl Laboratories, Montgomery, TX, USA) diluted in blocking buffer at 1:5000. The signal was detected using EzWestLumi plus chemiluminescence substrate (ATTO Corporation, Tokyo, Japan). Mammalian-derived CD200-Fc (CD200-FcM) was used as a positive control (recombinant human CD200-Fc chimera protein; R&D Systems, Minneapolis, MN, USA). HighFive insect cells not infected with virus and lysate of HighFive insect cells were infected with virus obtained by transfection with an empty vector used as negative controls.
Indirect enzyme-linked immunosorbent assay (ELISA) for binding activity assay
Purified CD200-FcI (60–3.75 ng/mL) was serially diluted by 1/2 in coating buffer (15 mM sodium carbonate, 35 mM sodium bicarbonate, pH 9.6), and 100 μL per well was coated on a Maxisorp 96-well plate (Nunc, Rochester, NY, USA) maintained at 4°C overnight. The plate was washed three times with 200 μL/well of 1× PBS-T (0.1% v/v Tween 20 in 1× PBS) and blocked with 200 μL/well 2% bovine serum albumin (BSA) at room temperature for 2 h. After washing, 100 μL of human anti-CD200 antibody (R&D Systems) diluted to 200 ng/mL in blocking buffer was added per well and incubated for 3 h at room temperature. After washing, horseradish peroxidase-conjugated goat anti-mouse IgG Fc-specific antibody (Bethyl Laboratories) diluted 1:10 000 in blocking buffer was added and incubated for 2 h at room temperature. Wells were washed and 100 μL of soluble 3,3′,5,5′-Tetramethylbenzidine (TMB) peroxidase substrate (SeraCare, Milford, MA, USA) was added to each well. After 5–10 min, this reaction was terminated by TMB stop solution (SeraCare). Finally, the measurement of absorbance at 450 nm was performed using a VICTOR Nivo multimode microplate reader (PerkinElmer, Waltham, MA, USA). CD200-FcM was used as a positive control, and insect cell-derived ACE2-Fc (ACE2-FcI) was used as a negative control.
Cell culture
The TCCSUP cell line (HTB-5™) was purchased from ATCC (Manassas, VA, USA) and cultured in minimum essential medium (MEM; Welgene) supplemented with 10% fetal bovine serum, 100 unit/mL of penicillin and 100 unit/mL of streptomycin. Cells were incubated in a humidified 5% CO2 incubator at 37°C.
Cell viability assay
To measure the effect of CD200-FcI on TCCSUP cell viability, the MTT assay (D-Plus™ CCK) (Dongin Biotech) was performed according to the manufacturer’s instructions. A density of 5.0 × 103 cells/well was seeded on a 96-well plate for 24 h. Ten microliters of CD200-FcI diluted to various concentrations was added to each well and reacted for 24 h. Ten microliters of D-Plus™ CCK was added to each well and incubated for 1 h. Absorbance was measured at 450 nm using a VICTOR Nivo multimode microplate reader.
Quantitative real-time reverse transcription PCR (qRT-PCR)
TCCSUP cells were plated in six-well plates at a density of 2.0 × 105 cells/well and incubated for 24 h. After treatment with CD200-FcI (0, 1, 10 and 100 ng/mL), the plates were cultured for 24 h. Total RNA was extracted from the cells using the FavorPrep™ Blood/Cultured Cell Total RNA Purification Mini Kit (Favorgen) according to the manufacturer’s instructions. Total RNA was reverse transcribed using the AMPIGENE® cDNA Synthesis Kit (Enzo Biochem, Farmingdale, NY, USA). Real-time quantitative PCR was performed with the AMPIGENE® qPCR Green Mix Lo-ROX (Enzo Biochem). Analysis was performed using the Rotor-Gene Q PCR machine (Qiagen, Hilden, Germany), with the two-step cycling protocol for denaturation, perfomed at 95°C for 5 s. The annealing/extension step was then performed at 60°C for 20 s and required 40 cycles. The cycle threshold (Ct) values were similar, to within a fraction of 0.1, among triplicates. The primers were designed for ZO-1 and 18S rRNA. 18S rRNA yielded a 2–ΔΔCt value that was used for normalization. In addition, this value was used as an internal marker. Primers were designed with the following oligonucleotides: 5′-GTCTGCCATTACACGGTCCT-3′ (forward) and 5′-CGTTGAATGCTTGCTGCTTA-3′ (reverse) for ZO-1; 5′-GTAACCCGTTGAACCCCATT-3′ (forward) and 5′-CCATCCAATCGGTAGTAGCG-3′ (reverse) for 18S rRNA. The experiments were performed in triplicate.
Western blot analysis
The TCCSUP cells were seeded in 60 mm cell culture plates at a density of 4.5 × 105 cells/plate and incubated for 24 h. The TCCSUP cells were treated with CD200-FcI (0, 1, 10 and 100 ng/mL) and incubated for 24 h. After the sample was washed with Dulbecco’s phosphate buffered saline (DPBS; Welgene), the TCCSUP cells were harvested. Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitor cocktail tablets (Roche, Basel, Switzerland) for 30 min, and centrifuged at 21 206 × g for 30 min at 4°C to collect the supernatant. Total protein concentration was determined by quantification using the Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific). Thirty micrograms of protein was separated by SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% skimmed milk at room temperature for 1 h, the membranes were incubated with the primary antibody at 4°C overnight. Primary antibodies against ZO-1 (1:2000) were purchased from Abcam (Cambridge, UK). Primary antibodies against β-actin (1:5000) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). After washing with 1× Tris-buffered saline with 0.1% Tween® 20 (TBS-T), membranes were incubated for 2 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000) or goat anti-mouse IgG (1:10 000) (Bethyl Laboratories). The signal was detected using EzWestLumi plus chemiluminescence substrate.
RESULTS
Expression and purification of CD200-FcI
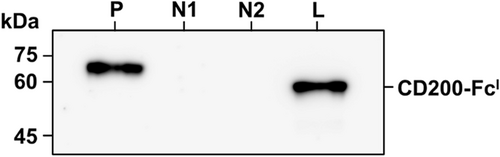
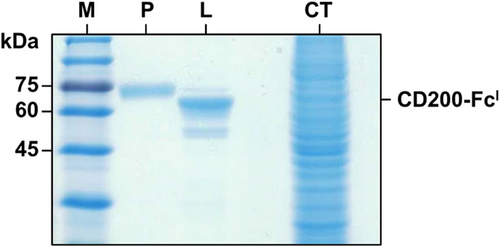
CD200-FcI expressed from HighFive cell lysate was visualized by immunoblot analysis (Fig. 2). In the immunoblot, the CD200-FcI protein band (approx. 52 kDa) was detected. CD200-FcM was used as a positive control and was detected at approximately 70 kDa. No band was detected in the non-infected and empty baculovirus infected HighFive cell lysates, as negative controls. The purification of CD200-FcI was performed using protein A affinity chromatography. Purified CD200-FcI (approx. 52 kDa) was confirmed by SDS-PAGE (Fig. 3).
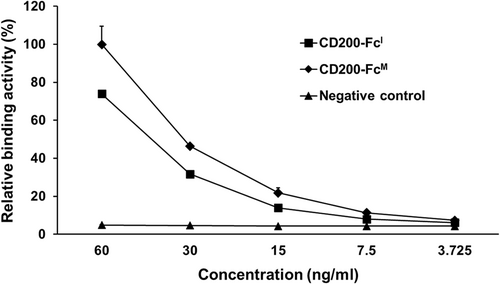
Binding activity between CD200-FcI and human anti-CD200 antibody
The interaction between CD200-FcI and human anti-CD200 antibodies was evaluated by ELISA (Fig. 4). It was confirmed that the antigen–antibody binding activity decreased by a factor of two in a concentration-dependent manner. CD200-FcM, used as a positive control, demonstrated a 20% higher binding activity than CD200-FcI. ACE2-FcI, used as a negative control, demonstrated no binding activity.
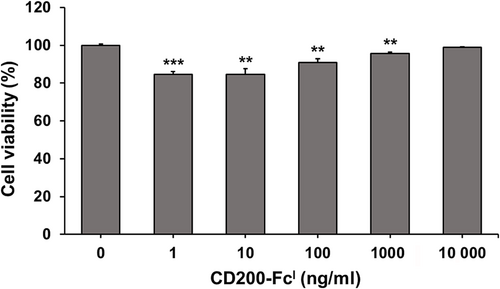
Effect of CD200-FcI on TCCSUP cell viability and proliferation
To investigate whether CD200-FcI influences cell viability, TCCSUP cells were treated with of CD200-FcI at various concentrations (0, 1, 10, 100, 1000 and 10 000 ng/mL) for 24 h (Fig. 5). After treatment with 1, 10 and 100 ng/mL of CD200-FcI, the cell viability decreased by about 10%–15%. From this, it was determined that CD200-FcI had no cytotoxicity. Thus, CD200-FcI did not have an effect on cell viability in any significant way. It was also shown that relatively low concentrations of CD200-FcI tended to inhibit the proliferation of TCCSUP cells.
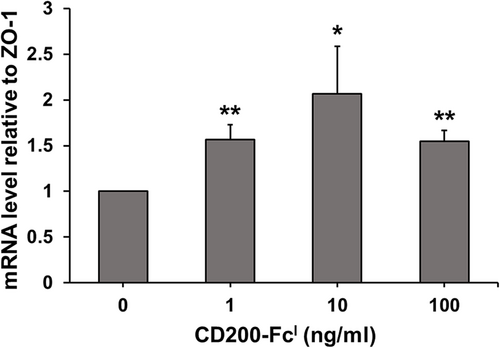
mRNA expression of ZO-1 gene in TCCSUP cells treated with CD200-FcI
The TCCSUP cells were treated with 0, 1, 10 and 100 ng/mL of CD200-FcI for 24 h. Then, total RNA was extracted, and real-time PCR was performed (Fig. 6). The primers were designed for ZO-1 and 18S rRNA. The mRNA level of ZO-1, a tight junction protein, was investigated and 18S rRNA was used for normalization. The results showed that CD200-FcI significantly increased the expression of the ZO-1 gene. After treatment with 10 ng/mL of CD200-FcI, expression of the ZO-1 gene was increased more than two-fold. Then, after treatment with 1 and 100 ng/mL of CD200-FcI, expression of the ZO-1 gene increased more than 1.5-fold. Therefore, the mRNA level of ZO-1 was increased in TCCSUP cells treated with a specific dose of CD200-FcI.
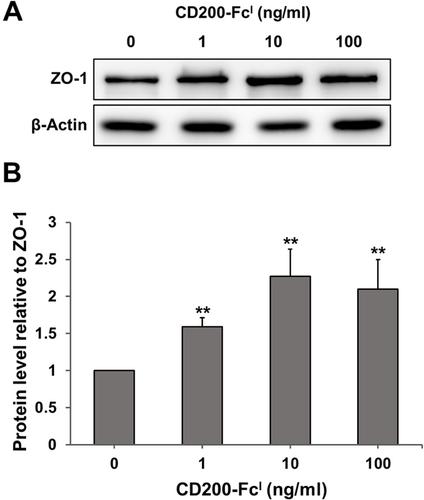
Expression of ZO-1 protein in urothelial carcinoma cells treated with CD200-FcI
The TCCSUP cells were treated with 0, 1, 10 and 100 ng/mL of CD200-FcI for 24 h. Subsequently, 30 μg of total soluble protein samples obtained from the treated cells was applied and ZO-1 protein was detected using an anti-ZO-1 antibody (Fig. 7A,B). CD200-FcI treatment (1, 10 and 100 ng/mL) significantly increased the ZO-1 protein band density. The density of the ZO-1 band in cells treated with 1 ng/mL of CD200-FcI increased by approximately 1.5-fold, compared with untreated cells. Treatment with 10 and 100 ng/mL CD200-FcI increased the density of the ZO-1 band significantly, by more than two-fold, compared with no treatment. Thus, CD200-FcI increased ZO-1 protein levels in TCCSUP cells.
DISCUSSION
Among eukaryotic expression systems with advantages such as the glycosylation of proteins and correct folding, BEVS using insect cells is easy to culture and has a low production cost, without human pathogen contaminants. With these advantages, the Novavax COVID-19 vaccine derived from insect cells is currently undergoing commercialization (Azali et al. 2022). In this study, the functional CD200-Fc recombinant protein was successfully expressed and purified in HighFive cells derived from Trichoplusia ni, which was useful as they could express between five and ten times more proteins than the S. frugiperda cell line. CD200-Fc gene expression was regulated by the Autographa californica multiple nucleopolyhedrovirus (AcMNPV) polyhedrin promoter (PPH) for high-level expression in pFastBac-1 vector (Fernandes et al. 2022; Silva et al. 2022). To improve the CD200-Fc protein expression yield, an endoplasmic reticulum retention peptide (KDEL) was tagged at the CD200-Fc C-terminus (Newstead & Barr 2020). CD200-FcI was confirmed by immunoblotting HighFive cell lysate infected with P2 baculovirus expressing CD200-Fc. Then, it was successfully purified using protein A affinity chromatography. The expected size of the CD200-FcI protein is about 52 kDa. However, a band was detected at approximately 60–75 kDa. The sizes of CD200-FcI (L) and CD200-FcM (P), used as positive controls, were different. This was expected as a result of the glycosylation of the proteins. Seven N-glycosylation sites were predicted to occur in CD200-FcI. This is presumably associated with the specific glycosylation pattern of each species being different (Lai et al. 2019). The glycosylation of proteins has important functional effects on protein activity, half-life, biological recognition of the epitope and antigenicity (Lee et al. 2015). In addition, it plays an important role in the folding of peptide chains, solubility and resistance to proteolysis during protein production (Pandey et al. 2022). The binding interaction between CD200-FcI and anti-CD200 antibody was verified using ELISA. As the concentration was serially diluted by half, the binding affinity was also reduced by half. It was confirmed that CD200-FcI had slightly lower binding activity with anti-CD200 antibody than commercial CD200-FcM. This is expected because of the different purities of the two proteins. CD200-FcI has a relatively lower purity than commercial CD200-FcM. Therefore, in the future, it is necessary to establish optimal protein purification conditions. Nevertheless, the success of the expression of interesting proteins indicates that BEVS serves as a useful platform to produce valuable therapeutic proteins.
CD200 triggers the inhibitory signal cascade in the cell. The binding of CD200 to CD200R1 activates the RAS p21 protein activator 1 (RasGAP), downstream of tyrosine kinase 2 (Dok2). This inhibits RAS activation and the downstream effect of phosphoinositide 3-kinase (PI3K) and extracellular signal-regulated kinase (Erk). As a result, various anti-inflammatory signals are generated because of the inhibition of NF-κB. Many cancers develop at sites of chronic irritation, infection and inflammation (Coussens & Werb 2002). Inflammation is one of the important factors in tumor progression. CD200 acts to inhibit inflammation by reacting with its receptor (CD200R1) expressed in a urothelial carcinoma cell line, called TCCSUP. This suggests that CD200-FcI, as an anti-inflammatory agent, inhibits the proliferation and reduces the aggression of bladder cancer cells. In this study, the expression of ZO-1 TJP was also investigated in urothelial carcinoma cells under CD200-FcI treatment. Both mRNA and protein levels of ZO-1 in CD200-FcI-treated cells significantly increased. These results suggest that the receptor-mediated interaction between CD200-FcI and CD200R1 increases the TJP of urothelial carcinoma cells. The mutual adhesion of cancer cells is significantly weaker than that of normal cells. Reduced cell–cell adhesion results in the dissociation and invasion of cancer cells, which have the potential to metastasize beyond where the tumor started, to other tissues and organs (Martin & Jiang 2009; Kyuno et al. 2021). Among various TJPs, there are proteins that inhibit the metastasis of bladder cancer cells, but some TJPs can increase metastasis. However, previous studies and the results of this study suggest that ZO-1 suppresses the metastasis of bladder cancer cells. In recent studies, Wang et al. (2021) reported that in bladder tumor tissue, ZO-1 downregulation leads to metastasis, whereas in adjacent non-tumor tissue, ZO-1 upregulation inhibits metastasis through the GSK3β/Snail/Par3/ZO-1 axis in bladder cancer cells and tissues. Therefore, CD200-FcI increases the TJP of urothelial cancer cells, suggesting the possibility of overcoming bladder cancer cell metastasis. Previous studies have shown that ZO-1 regulates cell proliferation, so that high levels of ZO-1 expression reduces cell proliferation (Kuo et al. 2021). It is suggested that CD200-FcI has the potential to reduce the proliferation and metastasis of bladder cancer cells. However, further studies are necessary to investigate the clinical efficacy of CD200-FcI.
Taken together, this study demonstrates the expression and purification of insect cell-derived CD200-Fc for use in diseases with barrier dysfunction as well as inhibiting the metastasis of bladder cancer cells, suggesting that BEVS is a promising platform for the production of valuable therapeutic recombinant proteins.
Acknowledgment
This research was supported by Chung-Ang University Research Scholarship Grants in 2022.




