Baculovirus titration method based on MOI values for optimizing recombinant protein expression of the anti-cancer vaccine candidate GA733-Fc using Sf9 insect cells
Abstract
The baculovirus expression system has been considered as a highly efficient tool for the production of recombinant biopharmaceutical proteins. The recombinant antigenic glycoprotein GA733 is a cell surface protein that is strongly expressed in human colorectal cancer. Efficient virus titration should be established to achieve optimal multiplicity of infection (MOI) conditions, which are in turn essential for strong expression of the recombinant GA733 fused to the human immunoglobulin IgG Fc fragment (GA733-Fc) in the baculovirus-insect system. In the present study, the Sf9 cell line was transfected with plasmid DNA containing the GA733-Fc expression cassette under the control of the baculovirus polyhedron promoter. MOI values (0.05, 0.1, 0.5, 1, and 3) were calculated based on both microscope observations and results of titration assay and then used to determine the optimum recombinant expression and harvested sample [cell culture media (CM) or cell lysate (CL)]. The pFastBac dual vector carrying the GA733-Fc gene was constructed to express GA733-Fc and used to generate recombinant baculoviruses. Western blotting results showed that recombinant protein expression was dependent on the MOI. In addition, CM and CL showed significant differences in protein synthesis and protein secretion capacities. Our findings suggested that our proposed titration method can be used for reliable calculation of MOI values, which significantly influence recombinant GA733-Fc protein expression in the baculovirus-insect cell system.
Introduction
The baculovirus expression vector system (BEVS) has been proven useful for the production of recombinant proteins in eukaryotic insect cells (Deparis et al. 2003; Song et al. 2010; Park et al. 2011, 2014, 2015; Lee & Ko 2014; Kim et al. 2015; Qiao et al. 2015; Lim et al. 2015a, 2015b; Lee et al. 2016). BEVS has many advantages, including scalability using high-density suspension cultures, capacity for expressing multiple genes at high expression levels, and the possibility of post-translational modifications (Deparis et al. 2003; Malde & Hunt 2004; Kost et al., 2005). In the present study, we used the BEVS technology to express diverse recombinant proteins, including anti-colorectal cancer monoclonal antibody CO17-1A (Song et al. 2010; Park et al. 2011, 2014; Lee & Ko 2014 and anti-cancer vaccine candidate GA733-Fc (Kim et al. 2015; Park et al. 2015; Qiao et al. 2015; Lim et al. 2015a, 2015b; Lee et al. 2016).
Optimization of infection conditions influences the expression of recombinant proteins, such as the monoclonal antibody CO17-1A (Park et al. 2011), which recognizes the colorectal antigen GA733–2 (gastrointestinal tumor-associated antigen 2) (Strassburg et al. 1992; Zaloudik et al. 2002; Lu et al. 2012; Kim et al. 2015; Park et al. 2015; Qiao et al. 2015; Lim et al. 2015a, 2015b; Lee et al. 2016). Accurate viral stock titration is crucial for determining the multiplicity of infection (MOI) value, which represents the total number of virions added per cell during infection (Neutra et al. 1992; Mulvania et al. 2004; Janakiraman et al. 2006) and ultimately determines recombinant protein expression (Radford et al., 1997).
Thus, in this study, the Sf9 cell line, which was derived from the parental Spodoptera frugiperda cell line, was used for virus titration (Lee & Ko 2014; Park et al. 2014; Qiao et al. 2015). We then investigated the effect of the KDEL sequence on the expression of the colorectal cancer vaccine candidate GA733-Fc [a human epithelial cell adhesion molecule (EpCAM)] fused to the human IgG Fc region. Titration assay can return variable results, leading to inaccurate calculated MOI values. Thus, we also employed microscopy methods to confirm the accuracy of the MOI calculations. Sf9 cells were infected with baculovirus according to MOI values determined based on our described study titration method. Our findings suggested that various MOI infection conditions should be optimized to improve recombinant GA733-Fc protein yields using the baculovirus-insect cell system.
Materials and methods
Construction of spGA733-Fc and GA733-Fc gene expression cassettes
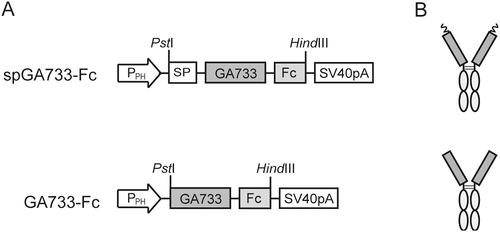
A synthetic DNA sequence encoding GA733 (Gln38-Lys279, Gen Bank accession no. AY189981) was fused to the Fc fragment of human IgG1 (Val97-Gly328, GenBank accession no. AY172957) to generate GA733-Fc. GA733-Fc was modified via the addition of an N-terminal extension containing a signal peptide (SP) to generate the recombinant proteins GA733-Fc and spGA733-Fc. The genes encoding GA733-Fc and spGA733-Fc were cloned under the control of the polyhedrin promoter (PPH) in a baculovirus-insect cell expression vector (pFastBac Dual vector; Invitrogen, Carlsbad, CA) (Fig. 1). The two resulting vectors were then transformed into DH5α Escherichia coli-competent cells for amplification.
DH10Bac E. coli transformation
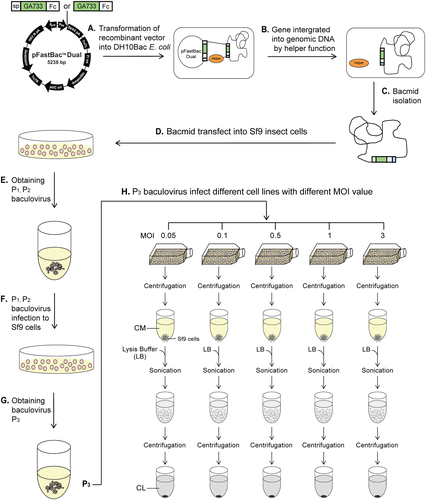
DH10Bac E. coli cells carrying the recombinant vectors were pre-cultured, and the amplified plasmids were extracted using a Plus Plasmid Mini Kit (NucleoGen, Daejeon, Korea). Purified plasmid DNA was transformed into DH10Bac E. coli cells for subsequent transposition into the bacmids. Cells were streaked on lysogeny broth (LB) blue/white selection agar plates containing 50 μg/mL kanamycin, 7 μg/mL gentamicin, 10 μg/mL tetracycline, 100 μg/mL Bluo-gal, and 40 μg/mL isopropyl β-d-1-thiogalactopyranoside (GTK) to select for DH10Bac transformants. Ten white colonies were selected and restreaked on fresh LB agar plates (GTK), and a single confirmed colony was pre-cultured in LB broth (GTK). Bacmids containing the spGA733-Fc and GA733-Fc gene expression cassettes were isolated from transformed DH10Bac E. coli cells according to the manufacturer's recommendations (Invitrogen).
Bacmid transfection into Sf9 insect cells
Spodoptera frugiperda (Sf9) cells (Invitrogen) were used as transfection carriers to produce baculoviruses. Sf9 insect cells were cultured in 15 mL of Sf900II SFM (Gibco, Gaithersburg, MD, USA) containing 10% penicillin (HyClone, South Logan, UT, USA) in T-75 flasks (Nunc, Roskilde, Denmark) at 105 rpm in a constant temperature incubator at 28°C. Next, Sf9 cells were seeded in six-well tissue culture plates (Nunc). Each well was seeded with 9 × 105 cells (4.5 × 105/mL) and incubated at 28°C for 1 h to allow cell attachment. spGA733-Fc and GA733-Fc bacmids (1 μg each) were mixed with 8 μL of Cellfectin II Reagent (Invitrogen) in 200 μL of antibiotic-free SF900II SFM and incubated for 30 min at room temperature. The culture media were removed, and the wells were washed with 2 mL of antibiotic-free medium. Each well was added with 2 mL of antibiotic-free medium and 200 μL of the bacmid mixture, and the plates were incubated at 28°C for 5 h. After 5 h of transfection, the medium containing the transfection agent was removed and replaced with 2 mL of fresh SF900II SFM supplemented with penicillin. The plates were placed in a constant temperature incubator at 28°C for 72 h to generate passage (P)1 recombinant baculoviruses.
Generation of P3 recombinant baculoviruses
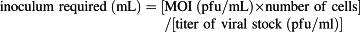 (1)
(1)Titer assay of spGA733-Fc and GA733-Fc P3 baculovirus
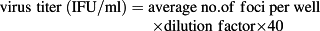 (2)
(2)Optimization of MOI and infection periods for spGA733-Fc and GA733-Fc expression in Sf9 insect cells
For recombinant gene expression, insect cells were infected at MOI values of 0.05, 0.1, 0.5, 1, or 3. The MOI is defined as the number of virus particles used to infect each cell. The viral stock required to obtain a specific MOI was calculated based on formula (1) and the results of the titer assay. After confirming the volume of the P3 viral stock, 20 ml of Sf9 insect cells (1 × 106 cells/ml) was infected with the P3 viral stock at varying MOI values and cultured in an incubator for 48, 72, or 96 h at 28°C at 105 rpm. Thereafter, cells were collected by centrifugation (3000 rpm, 30 min, 4°C), and the supernatants were filtered through a syringe-driven filter unit (0.45 μm, PVDF; Millipore). The resulting pellets were washed with 1× PBS and then lysed in cell lysis buffer (50 mM Tris and 500 mM NaCl, pH 8.0) containing protease inhibitor cocktail tablets (Roche, Mannheim, Germany) via two rounds of sonication at 150 W for 60 s. Lysed cells were centrifuged at 13,000 rpm for 30 min at 4°C to obtain the CL.
Western blotting of spGA733-Fc and GA733-Fc
The CM and CL samples were boiled for 5 min in 5× protein sample buffer (0.06 mL of 1 M Tris–HCl, pH 8.8, 25% glycerol, 2% SDS, 5% 2-mercaptoethanol, 0.1% bromophenol blue) and subsequently allowed to cool for 3 min. Proteins were separated by 8% SDS-PAGE and transferred onto a PVDF membrane (Millipore). The membrane was then incubated with blocking buffer (5% skim milk in Tris-buffered saline) overnight at 4°C and then incubated for 90 min at room temperature with horseradish peroxidase-conjugated goat anti-human IgG Fc-specific antibody (Jackson ImmunoResearch, West Grove, PA) diluted at 1:5000 in blocking buffer. After three washes, protein bands were detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA).
Results
Generation and expression of spGA733-Fc and GA733-Fc P3 recombinant baculoviruses
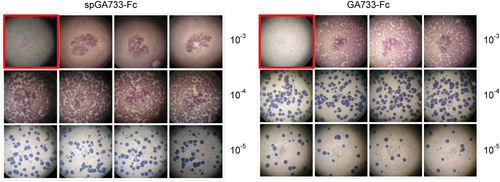
Recombinant pFastBac Dual vectors carrying the spGA733-Fc and GA733-Fc expression cassettes were constructed (Fig. 2). Recombinant bacmids obtained from DH10Bac Escherichia coli transformed with the recombinant vectors were transfected into Sf9 cells to obtain P1 recombinant baculoviruses. Small amounts of the P1 viral stock were obtained at low viral titers. Sf9 cells were infected with the P1 viral stock to generate the P2 stock (Fig. 2FG). The P3 viral stock was obtained from Sf9 cells infected with the P2 stock (Fig. 2H). In general, the titers of the P1 stocks ranged from 1 × 106 to 1 × 107 pfu/mL, while the titers of the P2 stocks ranged from 1 × 107 to 1 × 108 pfu/mL. The titers of the spGA733-Fc and GA733-Fc P3 stocks were 2.76 × 108 pfu/mL and 9.9 × 107 pfu/ml, respectively. The calculated IFU/mL values were 2.76 × 108 IFU/mL spGA733-Fc and 1 × 108 pfu/mL for GA733-Fc. The required volume of P3 viral stock for infecting the Sf9 cells at different MOI values was estimated using formula (1) (Table 1). For the virus plaque assay, all plaques were photographed and counted. The numbers denote the dilution factors. Plaques were expressed as plaque-forming units per mL (pfu/mL).
| Vectors | Average no. of FPC | Virus titer | MOI | Required inoculum (mL) | |
|---|---|---|---|---|---|
| spGA733-Fc | 69.5 × (10−5) | 2.76 × 108 | 0.05 | 0.004 | |
| 0.1 | 0.008 | ||||
| 0.5 | 0.037 | ||||
| 1 | 0.073 | ||||
| 3 | 0.218 | ||||
| GA733-Fc | 26 × (10−5) | 1 × 108 | 0.05 | 1.75 | |
| 0.1 | 3.5 | ||||
| 0.5 | 17.5 | ||||
| 1 | 35 | ||||
| 3 | 105 |
- z Mean PFU values of four samples at the dilution factors 10−5 (spGA733-Fc) and 10−4 (GA733-Fc), respectively.
- y Average number of foci per well × dilution factor × 40
- x [MOI (pfu/ml) × number of cells] / [Titer of viral stock (pfu/mL) MOI, multiplicity of infection
In the spGA733-Fc baculovirus, the plaques appeared red at the dilution factors 10−3 and 10−4 (Fig. 3A). On the other hand, the plaques were blue and countable at the dilution factor 10−5. For the GA744-Fc baculovirus, the plaques were colored red at dilution factor 10−3 (Fig. 3B). At both the dilution factors 10−4 and 10−5, the plaques were blue and countable.
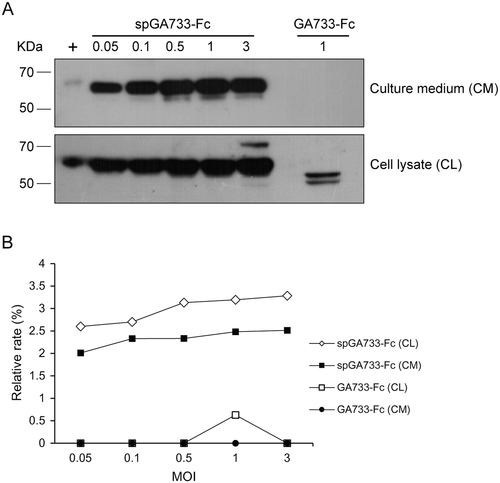
Sf9 cells (20 mL, 1 × 106/mL) were infected at different MOI values (Fig. 2H). The P3 baculovirus stock volumes were 4, 8, 37, 73, and 218 μL for spGA733-Fc and 1.75, 3.5, 17.5, 35, and 105 mL for GA733-Fc, which correspond to the MOI values 0.05, 0.1, 0.5, 1, and 3, respectively. spGA733-Fc and GA733-Fc expression levels were assessed via western blot analysis of total soluble proteins obtained from the CM and CL of Sf9 insect cells (Fig. 2H). All proteins were detected by incubation with goat anti-human IgG Fc fragment-HRP antibody.
Expression of spGA733-Fc and GA733-Fc in Sf9 CM and CL at different MOI values
After viral infection, spGA733-Fc and GA733-Fc were expressed in the CM and CL of Sf9 insect cells (Fig. 1). At the MOI of 1, GA733-Fc expression was detected in both the CM and CL. The CL bands (~58 kDa) were weaker than the CM bands. GA733-Fc expression was not detected in both CM and CL at other MOI values (data not shown). In the CM, one ~58-kDa band was detected at 48 h post-infection. On the other hand, for spGA733-Fc expression, ~58-kDa bands were observed at all tested MOI values (Fig. 4). The band intensity was found to increase with increasing MOI value until an MOI of 1. Bands in the CL were slightly stronger than those observed in the supernatant.
Discussion
The present study demonstrated the use of baculovirus titration methodology for determining the MOI value, which is essential for stable expression of recombinant proteins in a baculovirus-insect cell system. In addition, we investigated the effect of the SP on the expression of recombinant GA733-Fc protein in baculovirus-infected insect cells by placing the SP sequence at the 5′ end of the GA733-Fc gene sequence to generate spGA733-Fc.
For P3 baculovirus generation, plasmids carrying the spGA733-Fc and GA733-Fc showed no significant differences in virus transfection capability. The gene expression cassette content can affect baculovirus manipulation and infection of insect cells.
However, in this study, spGA733-Fc baculovirus showed slightly higher virus titers compared to those of GA733-Fc. Thus, during inoculation with the P3 baculovirus, the inoculum volumes for expression of both spGA733-Fc and GA733-Fc were adjusted based on MOI values such that equal amounts of the baculoviruses were used to infect the insect cells.
The signal peptide was demonstrated to be important for the expression of recombinant glycoproteins, such as the GA733-Fc protein, in a baculovirus-insect cell system (Qiao et al., 2015; Lee et al. 2016). Our results also showed that the spGA733-Fc yield was higher than the GA733-Fc yield.
In addition, the SP was demonstrated to be essential for glycosylation of the recombinant protein (Solá & Griebenow 2010). Interestingly, our results showed that the GA733-Fc protein, which does not contain a SP, was detected in the cell lysate (CL) but not in the culture medium (CM).
By contrast, the spGA733-Fc was detected both in the CL and CM, which indicated that the SP could direct the GA733-Fc to the endoplasmic reticulum and induce its secretion via the default pathway (Solá & Griebenow 2010).
Taken together, our findings demonstrated that optimization of MOI conditions is crucial for expression of the recombinant GA733-Fc in a baculovirus-insect cell system. In addition, the SP should be fused to recombinant proteins, especially when the recombinant glycoprotein is expressed in insect cells.
Acknowledgment
This work was supported by Post-Doctoral Research Program (2015) through the Incheon National University.




