In silico identification, characterization and expression analysis of attacin gene family in response to bacterial and fungal pathogens in Tenebrio molitor
Abstract
Antimicrobial peptides are effector molecules induced after microbial challenges. These form important components of innate host defense against the pathogens by exhibiting wide-spectrum antimicrobial activities. In this study, we identified three attacin-like genes from Tenebrio molitor RNASeq database using Tribolium castaneum attacin gene family as query. The T. molitor attacin gene family was annotated as TmAttacin-1a [comprising of 154 amino acids (aa)], TmAttacin-1b (150 aa) and TmAttacin-2 (164 aa), respectively. Temporal expression analysis shows that the TmAttacin-1a and -1b mRNAs are highly expressed in late larval stages, followed by a general decline in the pre-pupal stages. The mRNA level shows a decline during metamorphosis, and gets slightly overexpressed in pupal-adult transition stages. On the other hand, TmAttacin-2 is mainly overexpressed at 1-day old pupal stage. Spatial expression analysis indicates that TmAttacin-1a, −1b, and −2 mRNAs are primarily expressed in gut and fat body, but not in hemocytes and Malpighian tubules in T. molitor larvae. Interestingly, TmAttacin-1b shows more than 20-fold expression in the ovary, whereas TmAttacin-1a and −2 show similar expression patterns in gut, fat body, hemocyte, ovary, and testis in T. molitor adults. Induction pattern analysis demonstrates that the intracellular Gram-positive bacteria, L. monocytogenes elicited the strongest response by inducing ~1,000-fold expression of TmAttacin-1a mRNA. The highest level of TmAttacin-1b mRNA (~350-fold) was induced by Gram-negative bacteria, E. coli. However, the TmAttacin-2 transcripts were not induced by microbial challenges. These results indicate that TmAttacin-1a and -1b may be required for antimicrobial defenses in T. molitor.
Introduction
Antimicrobial peptides (AMPs) are effector molecules synthesized in the fat body of insects in response to microbial intrusion into the hemolymph. These directly act upon the target pathogens, showing microbiocidal action or block the microbial transcription. The induction of AMPs are characterized by microbial infection, suggesting primary role in insect immunity (Lee & Lee 2015; Rahnamaeian et al. 2015). AMPs are classified in insects into four families based on their sequence and structural heterogeneity. These includes α-helical peptides (cecropin and moricin), cysteine-rich peptides (defensin, drosomycin and thanatin), proline-rich peptides (drosocin, lebocin and formaecin), and glycine-rich peptides (attacin and gloverin) (Bulet et al. 1999; Yi et al. 2014). Over 150 AMPs have been identified and purified in insects till date. These are secreted into the hemolymph in response to broad-spectrum microorganisms. However, the precise mode of action of AMPs is still intriguing in the context of the activity close to the microbial membrane.
Earlier reports most exclusively on Drosophila melanogaster AMPs have suggested the effective role of AMPs such as drosocin and attacin against Gram-negative bacteria, defensin against Gram-positive bacteria, and drosomycin against fungus. AMPs like cecropin are induced in response to both bacterial and fungal infection. Further, the regulation of AMP genes in insects is linked to the Toll and IMD signaling pathways (Lemaitre and Hoffmann 2007; Moy and Cherry 2013). While Toll pathway (activated by both Gram-positive bacteria and fungi) controls the expression of AMPs such as drosomycin via the activation of Rel transcription factors (Dif and Dorsal), the IMD pathway controls the expression of diptericin via the activation of Relish transcription factors. Further, a synergistic interaction between Toll and IMD pathways regulates many known AMPs mainly through the convergence of the pathways at the gene-promoter level via presence of Toll and IMD κB-responsive genes (Yokoi et al. 2012; Yu et al. 2010).
The first attacin family (including attacin isoforms A – F) showing antimicrobial activity against Gram-negative bacteria, E. coli was purified from the Giant Silkmoth, Hyalophora cecropia (Engstrom et al. 1984; Hultmark et al. 1983; Kockum et al. 1984). The attacin isoforms were categorized under the basic group (Attacins A-D) and the acidic group (Attacins E and F) ranging in sizes from 20 to 23 kDa (Gandhe et al. 2006). These are widespread AMPs rich in the amino acid glycine and are considered as heat-stable antibacterial molecules. The gene is an attractive target for strain identification in Drosophila (Lazzaro and Clark 2001) and the tasar silkworm, Antheraea mylitta (Sudha et al. 2015). The transcriptional regulation of attacin in response to bacterial challenge has been demonstrated in adult fat bodies of the oriental fruit fly, Bactrocera dorsalis (Liao et al. 2015) and the beet armyworm, Spodoptera exigua (Bang et al. 2012). Attacin along with cecropin, gloverin and defensin was identified as prominent AMPs from high-throughput RNA-Seq analysis of the ghost moth, Thitarodes armoricanus (Wang and Hu 2017). Cold-inducible attacin gene was also characterized from the desert beetle, Microdera punctipennis (Li et al. 2017). Further, attacin gene with antimicrobial activity has been identified from other insects including the tse tse fly, Glossinia morsitans (Wang et al. 2008), Manduca sexta (Rao and Yu 2010), Hyphantria cunea (Kwon et al. 2008), and Trichoplusia ni (Tamez-Guerra et al. 2008). The antimicrobial activity of attacin is attributed to the helical conformation as the random coil structure in aqueous solution is unstable and susceptible to proteases (Gunne et al. 1990; Yi et al. 2014).
The mealworm beetle, Tenebrio molitor has been used as a model for the study of innate immunity in insects. The signal recognition molecules, the intracellular signaling components and the transcription factors relaying the signal into the nucleus for the expression of AMP genes have been studied in the beetle, especially in relation to Toll and IMD pathways (Patnaik et al. 2013; Patnaik et al. 2014; Seo et al. 2016). The regulatory expression of seven AMP genes by the NF-kB factor Cactin and innate immunity against both Gram-positive and Gram-negative bacteria has improved the understanding of the Toll pathway in the beetle (Jo et al. 2017). Tenecin 1 was the first AMP identified from T. molitor showing activity against fungi as well as Gram-positive and Gram-negative bacteria (Lee et al. 1998). An attacin family protein, Tenecin 4 (a 14 kDa AMP), was found to be regulated by the Toll and IMD pathways, inhibiting the growth of Gram-negative bacteria, E. coli (Chae et al. 2012). Considering the role of AMPs in controlling the microbial proliferation in the insect, an attempt was made to screen the prominent AMPs in the beetle. For in silico screening of attacin gene, Tribolium castaneum attacin gene was used as the query against the T. molitor RNA-Seq database. The attacin C-terminal region was characterized in detail using bioinformatics software and primers designed for developmental, tissue-specific, and temporal expression of the transcript after microbial challenge. This study is the first attempt to unravel the sequence characteristics and putative functions of attacin gene in T. molitor.
Materials and Methods
Identification and in silico analysis of TmAttacin gene family
To identify the T. molitor Attacin (TmAttacin) gene family, the amino acid sequences of T. castaneum Attacin gene family such as TcAttacin-1 (XP_001809216.1) and TcAttacin-2 (XP_001809637.1) were used as query searches. TcAttacin-1 and TcAttacin-2 sequences were retrieved from beetlebase (http://beetlebase.org) and NCBI (http://www.ncbi.nlm.nih.gov/) protein database. Local-tblastn analysis (Altschul et al. 1990) was performed to identify the target genes from the T. molitor RNASeq and EST database. The open reading frame (ORF) sequences of TmAttacin gene family were predicted by the Genscan web server (Burge and Karlin 1998) (http://genes.mit.edu/GENSCAN.html) and blast program. The conserved domain analysis for TmAttacin gene sequences were identified using the genome-scale protein function classification software package InterProScan 5 (Jones et al. 2014; Quevillon et al. 2005; Zdobnov and Apweiler 2001) and the signal peptide residues using the Signal 4.1 server (Petersen et al. 2011). Domain-specific (C-terminal regions) multiple sequence alignment (MSA) of TmAttacin amino acid sequences and attacins from representative insects (derived from GenBank) were aligned using CLUSTALX 2.1 (downloaded from http://www.clustal.org/clustal2/). Subsequently, the GeneDoc software was used to visualize the MSA results. The molecular phylogenetic tree was constructed by using the MEGA 6 program based on the p-distance model (Tamura et al. 2013).
qPCR-based expression analysis of target TmAttacin genes
The relative expression levels of TmAttacin genes in the developmental stages and tissues of the insect were examined by qPCR on an Exicycler 96 Real-Time Quantitative Thermal Block (Bioneer Corporation, Daejeon, South Korea). For the same, the total RNAs were isolated from the developmental stages of the insect such as late-instar larva, prepupa, pupa (day 1 to day 7), and adults (day 1 and 2). Larval and adult tissues such as gut, fat body, Malpighian tubules and hemocytes inclusive of adult ovary and testis were used to extract RNA using FavorPrep™ Tri-RNA Reagent (Favorgen Biotech Corporation Ping-Tung, Taiwan) according to manufacturer's instructions. cDNAs were synthesized using 2 μg of total RNA as the template, and Oligo (dT)12–18 primer on a MyGenie 96 thermal block (Bioneer Corporation, Daejeon, South Korea) using AccuPower® RT Pre-Mix (Bioneer). The synthesized cDNAs and TmAttacin specific primers (Table 1) were used for the relative quantitative PCR using AccuPower® 2X GreenStar qPCR Master Mix (Bioneer). The reaction program was composed of 95°C for 20 s, followed by 40 cycles of 95°C for 5 s, and 60°C for 20 s. All the primers used in qPCR were designed with the Primer 3.0 software (http://bioinfo.ut.ee/primer3-0.4.0/) and listed under Table 1. The T. molitor ribosomal protein L27a (TmL27a) was used as an internal control. The expression level of TmAttacin gene relative to TmL27a was calculated by using the comparative 2-∆∆Ct method [44]. Each reaction was conducted in three biological replicates, and each biological replicate was assessed three times.
| Name | Primer sequences |
|---|---|
|
TmAttacin-1a_qPCR_Fw TmAttacin-1a_qPCR_Rv |
5’-GAAACGAAATGGAAGGTGGA-3′ 5’-TGCTTCGGCAGACAATACAG-3′ |
|
TmAttacin-1b_qPCR_Fw TmAttacin-1b_qPCR_Rv |
5’-GAGCTGTGAATGCAGGACAA-3′ 5’-CCCTCTGATGAAACCTCCAA-3’ |
|
TmAttacin-2_qPCR_Fw TmAttacin-2_qPCR_Rv |
5’-AACTGGGATATTCGCACGTC-3′ 5’-CCCTCCGAAATGTCTGTTGT-3’ |
|
TmL27a_qPCR_Fw TmL27a_qPCR_Rv |
5’-TCATCCTGAAGGCAAAGCTCCAGT-3′ 5’-AGGTTGGTTAGGCAGGCACCTTTA-3’ |
Furthermore, to investigate the induction patterns of TmAttacin transcripts under immune challenge, pathogenic microorganisms including Escherichia coli (106 cells/larva), Staphylococcus aureus (106 cells/larva), Candida albicans (5 × 104 cells/larva) and Listeria monocytogenes (106 cells/larva) were injected into T. molitor larvae and total RNA samples were collected at different time points (3, 6, 9 and 12 h post-infection). The phosphate-buffer saline (PBS) injected group was used as the negative control. qPCR was performed to identify the relative expression levels of target TmAttacin transcripts using similar conditions as stated before.
Results
Identification and in silico analysis of TmAttacin gene family
Full-length open reading frame (ORF) sequences of TmAttacin gene family were obtained by using local-tblastn program taking T. castaneum attacin gene family (derived from beetlebase) as the query and T. molitor nucleotide database (derived from T. molitor RNA-Seq database) as the subject. TmAttacin-1a (GenBank Accession No. - MF754109), TmAttacin-1b (MF754110), and TmAttacin-2 (MF754108) were identified as full-length ORFs. TmAttacin-1a contained a 465 bp ORF encoding a putative protein of 154 amino acids (Fig. 1A). TmAttacin-1b contained 453 bp ORF encoding a putative protein of 150 amino acid residues (Fig. 1B). Further, TmAttacin-2 included an ORF of 495 bp encoding a protein of 164 amino acid residues (Fig. 1C). TmAttacin-1a, −1b, and −2 ORF sequences showed a signal peptide of 20, 19, and 17 amino acid residues, respectively suggesting their secretive nature. The attacin C-terminal region was found after 53, 43, and 52 amino acids for TmAttacin-1a, TmAttacin-1b, and TmAttacin-2, respectively.
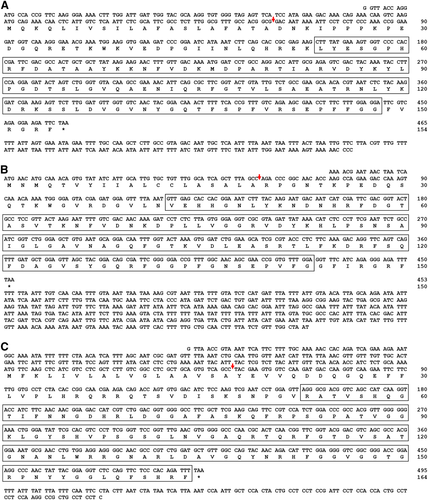
To understand the specific features and relationships of in silico derived TmAttacin amino acid sequences with other insect-derived attacins, we constructed a multiple sequence alignment, percentage identity and phylogenetic analysis using Clustal X2 program and MEGA6 program. Due to functional diversification across species, only the C-terminal regions of attacin amino acid sequences were used. The C-terminal region of attacin sequences show variations at the level of insect orders. Percentage identity and distance analysis results indicate that the T. molitor attacin amino acid sequences has low homology with each other (53 % in between TmAttacin-1a and TmAttacin-1b, 35 % between TmAttacin-2 and TmAttacin-1b, and 31 % between TmAttacin-2 and TmAttacin-1a). TmAttacin-1b and TmAttacin-2 has highest similarity with TcAttacin-2 (61 % identity) and TcAttacin-1 (73 % identity), respectively. Furthermore, the phylogenetic tree was constructed with the amino acid sequences of TmAttacin family members and attacins from representative insect orders. The phylogeny represented the sequences into two large clusters. The Tenebrio Attacin sequences were clustered with attacins from coleopteran insects. The other cluster included the Drosophila and Lepidopteron attacins with the Drosophila attacins representing a sub-cluster within the cluster (Fig. S1).
Temporal and spatial expression profiling of TmAttacin family transcripts
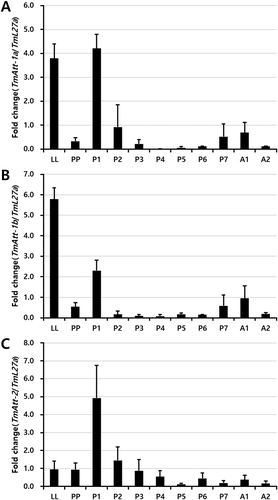
The relative expression of TmAttacin-1a, TmAttacin-1b, and TmAttacin-2 in the developmental stages of T. molitor was examined using qPCR. cDNAs were derived from late instar larvae (LL), prepupa (PP), 1–7 day-old pupa (P1 - P7), 1–2 day-old adult (A1 - A2) and amplified using qPCR with TmL27A as the internal control. As shown in Figure 2, there are differences at the level of mRNA expression between TmAttacin-1a, TmAttacin-1b, and TmAttacin-2. Developmental expression patterns are similar between TmAttacin-1a and TmAttacin-1b. TmAttacin-1a and -1b mRNAs are highly expressed in late larval stages, 1-day old pupal stage, 7-day old pupal stage, and 1-day old adult stage after ecdysis. However, TmAttacin-2 mRNA is primarily up-regulated at 1-day old pupal stage.
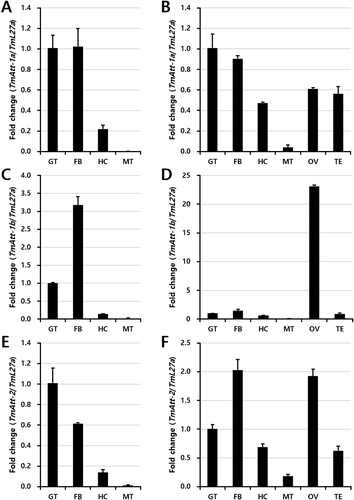
Under tissue-specific expression of the attacin transcripts, the gut, fat body, hemocytes, and Malphigian tubules from larvae and adults of the insect were considered for analysis. The adult ovary and testis was also considered for the expression analysis of TmAttacin transcripts (Fig. 3). The TmAttacin-1a transcripts were found higher in the gut and fat body of both larvae and adults of the insect. The expression of TmAttacin-1a transcripts was found moderate in the ovary and testis of the larvae (Fig. 3A). Interestingly, TmAttacin-1b showed more than 20-fold expression in the ovary but not the testis of the adult insect. In other tissues of the adult, the expression of TmAttacin-1b was found similar to the expression of TmAttacin-1a (Fig. 3B). The expression of TmAttacin-2 transcripts were found in all the tissues studied, but greater expression were noticed in the gut and fat body of the larvae and the fat body and ovary of the adult insect (Fig. 3C).
Differential induction patterns of TmAttacin family in response to bacterial and fungal pathogens
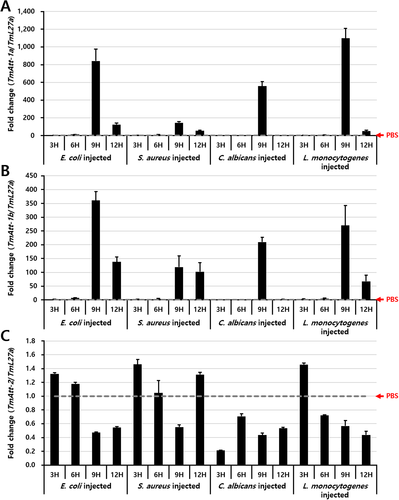
Furthermore, the relative expression levels of TmAttacin-1a, TmAttacin-1b, and TmAttacin-2 were detected by qPCR after inoculation of the Gram-negative bacterium E. coli, Gram-positive bacterium S. aureus, intracellular Gram-positive bacterium L. monocytogenes, and the fungus C. albicans into the last-instar larvae of T. molitor. The relative expression of TmAttacin-1 was upregulated after 9 h of inoculation of all the pathogens studied. L. monocytogenes followed by E. coli, and C. albicans upregulated the TmAttacin-1a transcripts to nearly 1098, 841, and 558 folds, respectively (Fig. 4A). Similar to TmAttacin-1a transcript expression, TmAttacin-1b transcripts showed an upregulation at 9 h post-inoculation of E. coli, L. monocytogenes, and C. albicans to 360, 269, and 209 fold, respectively (Fig. 4B). After 9 h post-inoculation the expression of TmAttacin-1a and TmAttacin-1b sharply declined. Interestingly, TmAttacin-2 transcripts were not induced by microbial injection (Fig. 4C). It is therefore critical to note the dynamics of TmAttacin-1a and TmAttacin-1b and not of TmAttacin-2 after microbial challenge. Hence, the microbiocidal activity regulated by AMPs in T. molitor could be attributed to the synthesis of TmAttacin-1a and TmAttacin-1b.
Discussion
AMPs extracted from insect sources are considered valuable for antimicrobials production considering the current threat of antibiotics resistance developed among the human populations. These AMPs have been considered as potential alternatives to the ‘classical antibiotics’ that leads to natural bacterial resistance. Especially, the insect AMPs have been supplemented in many types of human foodstuffs as preservative and livestock diets as an antibacterial additive (Jozefiak and Engberg 2017; Kieronczyk et al. 2016). This has opened up new possibilities for budding entrepreneurs to take up initiatives in starting insect meal production units specifically catering to animal nutrition and health.
The mealworm beetle, T. molitor is a model insect that has been elegantly explored to understand the dynamics of insect immunity with special reference to the Toll/IMD signaling pathways (Johnston et al. 2014) and pro-phenoloxidase (pro-PO) system (Lee et al. 2002). Furthermore, T. molitor AMP genes have been identified as effector molecules synthesized in the fat body showing bactericidal action and antifungal activity (Zhu et al. 2013). In this perspective, the identification of new AMP transcripts with microbiocidal action from T. molitor would be crucial for future applications in feed development and food preservatives industry. The AMPs would further substantiate the insect innate immune signaling pathways in relation to various pathogenic microbes. With the availability of T. molitor expressed sequence tag (EST) and transcriptome databases, the screening of AMPs from insects is warranted for new antibiotics development and unraveling insect immune signaling pathways in response to plethora of pathogenic microorganisms. This study was successful in identification of full-length ORFs of Attacin family genes (TmAttacin-1a, TmAttacin-1b, and TmAttacin-2) from T. molitor larvae using in silico analysis. Further, the expression of the transcripts was assessed in response to the inoculated bacterial and fungal specimens.
The TmAttacin-1a and TmAttacin-1b from T. molitor larvae were found to have a greater amino acid sequence identity and were grouped together in the phylogenetic tree. TmAttacin-2 showed a lesser degree of sequence identity with TmAttacin-1a and TmAttacin-1b. This is reflected in the deduced amino acid sequences, wherein TmAttacin-1a and TmAttacin-1b had 154 and 150 amino acids but TmAttacin-2 had 164 amino acid residues. Consistent with this report, the attacins from the desert betle, Microdera punctipennis showed 151 and 166 amino acid residues for attacin-1 and attacin-2, respectively (Li et al. 2017). The M. punctipennis attacins were cold-inducible and justified the first case of AMP expression under abiotic stress. Unlike the present report, the attacins from M. punctipennis were highly expressed in the Malpighian tubules. The attacinC from the oriental fruit fly, Bactrocera dorsalis encodes a longer amino acid sequence and was upregulated after bacterial challenge in fat bodies (Liao et al. 2015). Considering the amino acid sequences of TmAttacin-1a, TmAttacin-1b, and TmAttacin-2, a signal peptide region is noticed followed by a proline-rich region considered to be active against Gram-negative bacteria. Generally, attacins are synthesized as pre-pro-proteins and pro-attacins have no biological activity (Gunne and Steiner 1993; Rabel et al. 2004). In addition, the attacins are found upregulated after challenge with Gram-negative bacteria such as E. coli, Citrobacter freundii, and Pseudomonas cichorii (Hultmark et al. 1983; Kwon et al. 2008). This is consistent to our reports of TmAttacin-1a and TmAttacin-1b getting upregulated after E. coli challenge. TmAttacin-1a and TmAttacin-1b were also found upregulated after L. monocytogenes challenge consistent to earlier reports with Gram-positive bacteria (Bang et al. 2012). The TmAttacin-1a and TmAttacin-1b were found upregulated at high levels 9 h post-infection, compared to control larvae inoculated in PBS. The Spodoptera exigua attacin was upregulated at high levels 12 h post-infection and showed activity against both Gram-negative and Gram-positive bacteria (Bang et al. 2012). Furthermore, it would be interesting to note the antibacterial activity of T. molitor attacins and relate it to their structure. Generally, the helical conformation in the C-terminal region of the protein ascribes the antimicrobial function. Attacins with leucine-rich sequences and no helices tend to have significantly reduced or no antibacterial activity (Kwon et al. 2008; Yi et al. 2014). Having said that, the role of T. molitor attacins as an antimicrobial either independently or in combination with other synthesized AMPs is a contentious case for further research to support the food and nutrition industry.
Conclusions
The present study is an attempt to screen the antimicrobial peptide attacin from T. molitor RNA-Seq database by using T. castaneum attacin gene sequence as query. The full-length ORFs of TmAttacin-1a, TmAttacin-1b, and TmAttacin-2 were identified including their deduced amino acid sequence. The in silico analysis predicted greater sequence identity between TmAttacin-1a and TmAttacin-1b and these were grouped in one cluster. The expression of TmAttacin-1a and TmAttacin -1b was greater in the late-larval, early and late pupal, and adult stages of the insect. TmAttacin-2 transcripts were greater in the early pupal stage (1-day old pupae) of the insect. The immune organs, gut and fat body showed higher constitutive expression of TmAttacin transcripts in both the larva and adult of T. molitor. Most importantly, TmTenecin-1a and TmTenecin-1b transcripts, and not TmTenecin-2 transcripts were strikingly upregulated upon Gram-positive, Gram-negative, and fungus challenge to the host larvae. Currently, we are in the middle of developing animal feeds using various insect models. In this context, these results will be used as a basis for developing mealworm-based potential alternative natural antibiotics.
Acknowledgment
This research work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT and future planning (Grant No. 2015R1A2A201005301).




