Effect of plant secondary metabolites on common cutworm, Spodoptera litura (Lepidoptera: Noctuidae)
Abstract
The effect of various flavonoids, lectins and phenyl β-D-glucoside on larval survival, weights and the activities of digestive (total serine protease and trypsin) and detoxifying (esterase and glutathione-S-transferase) enzymes of Spodoptera litura larvae at 7 days after treatment was studied through diet incorporation assay. Flavonoids (rutin, chlorogenic acid, quinic acid, caffeic acid, naringenin, quercitin, kaempferol, myricetin, catechin, and ferulic acid) were incorporated in artificial diet at 100, 500 and 1000 ppm, lectins: groundnut leaf lectin (GLL), concavalin A (ConA) and phenyl β-D-glucoside at 1, 2 and 5 μg/mL. Flavonoids such as rutin, quercitin and kaempferol at 1000 ppm were more toxic to S. litura larvae than quinic acid, caffeic acid, naringenin, myricetin, catechin, and ferulic acid. Larval growth and development were significantly reduced in S. litura larvae fed on a diet with GLL and ConA at 5 μg/mL compared to the larvae fed at 2 and 1 μg/mL concentrations. The larvae fed on flavonoid-treated diets showed significant reduction in serine protease, trypsin and esterase activities. The flavonoids such as rutin, chlorogenic acid, quinic acid, naringenin, quercitin, kaempferol and myricetin, and lectins, GLL and ConA can be utilized in insect control programs.
Introduction
Plants produce a number of defensive secondary metabolites in response to insect herbivory, pathogen infection and other stresses. The functions of secondary metabolites are very diverse including defense, reproduction, color, scent, signaling (Boerjan et al. 2003). Among the secondary metabolites, plant phenols constitute one of the most common and widespread groups of defensive compounds, which play a major role in host plant resistance against herbivores, including insects (Sharma et al. 2009; Ballhorn et al. 2011). Qualitative and quantitative alterations in secondary metabolites and increase in activities of oxidative enzymes in plants in response to insect attack is a general phenomenon (Barakat et al. 2010; He et al. 2011; War et al. 2012, 2013). Phenols mediate both direct and indirect defenses. The direct defenses are mediated by their toxic or deterrent activity against insect pests and the indirect defenses by their effect on natural enemies of insect pests (Heil 2008; Sharma et al. 2009; Barakat et al. 2010; War et al. 2012). Condensed tannins are the potent plant defensive compounds implicated against insect pests and have been reported to reduce the growth and survivorship in many insect pests (Grayer et al. 1992; Sharma et al. 2009). Flavonoids play a central role in various facets of plant life, especially in plant–environment interactions (Treutter 2006). These defend plants against biotic and abiotic stresses, including ultraviolet radiation, pathogen infection and herbivore damage (Simmonds 2003).
Evidence suggests the role of flavonoids in plant defense against insect pests (Treutter 2006; Atteyat et al. 2012; War et al. 2013). Isoflavonoids (judaicin, judaicin-7-O-glucoside, 2-methoxyjudaicin, and maackiain) isolated from the wild relatives of chickpea act as antifeedants against Helicoverpa armigera at 100 ppm (Simmonds & Stevenson 2001). The flavonoids, quercetin dehydrate, rutin hydrate and naringine at 1000 ppm showed the mortality of 85, 93 and 86 %, respectively, in Eriosoma lanigerum (Haus.) in a twig dip assay (Atteyat et al. 2012). In addition, flavonoids scavenge the free radicals in plants, including reactive oxygen species (ROS), and reduce their formation by chelating metals (Treutter 2006). However, some flavonoids have been found to act as feeding stimulants (van Loon et al. 2002). Lectins are carbohydrate-binding (glyco) proteins and are involved in plant defense against a range of insect pests (Chakraborti et al. 2009; Vandenborre et al. 2011). The strong insecticidal potential of lectins is attributed to their binding to the membrane glycosyl groups lining the digestive tract of the herbivores (Chakraborti et al. 2009; Vandenborre et al. 2011). The β-glucosides are also the important toxic compounds involved in plant defense against insect pests (Zagrobelny et al. 2004; War et al. 2013).
The common cutworm, Spodoptera litura Fab. (Lepidoptera, Noctuidae) is well known as a serious cosmopolitan pest with extensive host range of economically important crops such as cotton, groundnut, tobacco, sunflowers, tomato, brassicas, and many other crops (Sahayaraj & Paulraj 1998). S. litura has been shown to be resistant to a wide range of insecticides, which has led to sporadic out breaks of the pest and failure of crops in several countries (Ahmad et al. 2007a). The control of this pest in China has depended mostly on application of various insecticides. As a result, many field populations of this pest have developed high resistance against wide variety of insecticides (Su et al. 2012; Tong et al. 2013; Sang et al. 2016). The efficient and acceptable methods of controlling this pest is lacking because of the development of the insecticide resistance and higher application rates in turn creating environment pollution. Under such situations, plants and plant derived products offered a tremendous advantage over synthetic pesticides in use as control agents for the pests of agriculture, veterinary and public health since plant kingdom is the most efficient producer of chemical compounds, synthesizing many products that are used in defense against insects (Ibanez et al. 2012). The objective of the present investigation was to study the effect of some flavonoids, lectins and phenyl β-D-glucoside on the growth and development and midgut enzyme activities of S. litura, to be used as a component of integrated pest management (IPM) for this polyphagous pest. This will give us an insight into the mechanism of toxicity of flavonoids and related compounds on insect pests.
Materials and methods
Insects
The population of S. litura at fourth instar larvae was collected from cotton fields of Yangtze University, Hubei Province, China, and brought to the laboratory. Then the larvae were reared on semi-synthetic diet (Ahmad et al. 2008), in the laboratory at 25 ± 2°C and 65 ± 5 % RH in glass jars for at least two generations before the bioassays were carried out. The diet consisting of soybean powder 300 g, wheat germ 240 g, dried brewer's yeast 80 g, agar 80 g, casein 40 g, ascorbic acid 24 g, sorbic acid 6 g, methyl 4-hydroxybenzoate 15 g, cholesterol 0.6 g, choline chloride 3 g, inositol 0.6 g, vitamin mix 0.2 g, streptomycin 2 g, 37.5 % formaldehyde 3 mL and distilled water to 3000 mL total. Diet was replaced after 24 h and pupae were collected on alternate days. Moths were shifted to glass cages with mesh sides for ventilation and fed on a solution containing 10 % honey soaked onto cotton wool ball (Ahmad et al. 2007b). The neonate larvae were fed with a semi-synthetic diet. The population was reared in the laboratory to accommodate to laboratory conditions and to obtain sufficient insect numbers for bioassays.
Chemicals
The chemicals used in this study were of analytical grade. Ethylene diamine tetra acetic acid (EDTA), sodium dodecyl sulphate (SDS), azocasein, glycine, sodium hydroxide (NaOH), N-a-benzoyl-DL-arginyl-pnitroanilide (BApNA), naphthyl acetate, fast blue B, reduced glutathione (GSH), trichloroacetic acid (TCA), flavonoids, lectin, Concavalin A (ConA), and phenyl β-D-glucoside were obtained from Sigma Aldrich (St Louis, MO, USA). Acetic acid, disodium hydrogen phosphate, sodium dihydrogen phosphate and 1-chloro-2,4-dinitrobenzene (CDNB) were bought from the Beijing Chemical Reagent Company (Beijing, China).
Bioassays
The effect of flavonoids on S. litura growth and development was studied by feeding the third instar larvae on flavonoids incorporated artificial diet. Ten flavonoids: rutin, chlorogenic acid, quinic acid, caffeic acid, naringenin, quercitin, kaempferol, myricetin, catechin, and ferulic acid were bioassayed using diet incorporation assay as previously described (Su et al. 2012). The flavonoids were weighed and mixed with the diet (mg/mL) just after its preparation. Larvae of S. litura were released on the diet containing three concentrations of each flavonoid (100, 500 and 1000 ppm). These doses of flavonoids are within the physiological range (20–1600 ppm) of their natural production from various plant sources (Rothwell et al. 2013). One larva was released in each cell well in a 20-well plastic plate. Four replications were maintained for each treatment with ten larvae in each replication thus total numbers of tested larvae per concentration were 40. Larvae fed on untreated diet were maintained as a control. At 7 days after treatment (DAT), larvae weights and survival were recorded. The larvae after 7 days of treatment were used to study the effect of flavonoids on gut enzyme activities such as serine protease, trypsin, esterase (EST) and glutathione-S-transferase (GST).
Groundnut leaf lectin (GLL), ConA and phenyl β-D-glucoside were also incorporated into the artificial diet to study their effects on biology of S. litura. The concentrations used were 1, 2 and 5 μg/mL of diet. These concentrations are typical of those generally used in lectin bioassays in Lepidoptera pests (War et al. 2013). Four replications were maintained for each treatment and ten larvae placed individually in cell wells in each replication. The larvae fed on untreated diet were maintained as a control. At 7 DAT, larvae weights and survival were also recorded, and the larvae were used to study insect gut enzymes (EST, GST, trypsin, and serine protease).
Total serine protease assay
The larvae were dissected and midguts were extracted in 0.2 M sodium phosphate buffer (pH 7.5). The midguts were removed and homogenized in 0.1 M glycine–NaOH buffer (pH 10), containing 1 mM EDTA. The homogenate was centrifuged at 10,000 rpm for 20 min at 4°C. The supernatant was collected and used as enzyme source for serine protease and trypsin activity. Serine protease activity of insect midgut was estimated by following the method of Hegedus et al. (2003) using azocasein as a substrate. To 0.04 mL of midgut supernatant, 0.3 mL of 1 % azocasein solution (prepared in 0.05 M glycine–NaOH buffer, pH 10) was added. The reaction mixture was incubated at 28°C for 15 min, and then 0.34 mL of 10 % TCA was added to it. The reaction mixture was incubated again for 1 h at room temperature and centrifuged at 12,000 rpm for 10 min. The supernatant was collected in a separate tube and 0.68 mL of 1 M NaOH added to it. Absorbance was read at 495 nm. Total midgut serine protease activity (SP) was calculated by subtracting the azocasein blank absorbance from sample absorbance divided by incubation time in minutes multiplied by 1000. Results are expressed in tryptic activity (mU) per min of incubation per mg insect body weight (mU/min /mg protein), and treatment values are the mean of six replicates.
Trypsin assay
Trypsin activity of the insect midgut was determined as the method described by Perlmann and Lorand (1970). Larvae midgut extract (0.15 mL) was added to 1 mL of 1 mM BApNA (in 0.2 M glycine–NaOH buffer, pH 10). The reaction mixture was incubated at 37°C for 10 min. The reaction was terminated by adding 0.2 mL of 30 % acetic acid. Absorbance was read at 410 nm and the enzyme activity was expressed as μmol/min/mg protein, and treatment values are the mean of six replicates.
Esterase assay
The larvae were dissected in 0.1 M sodium phosphate buffer (pH 7.5), then the midguts were removed and homogenized in 0.1 M sodium phosphate buffer (pH 7.5) containing 1 mM EDTA. The homogenate was centrifuged at 12,000 rpm for 15 min at 4°C. The supernatant was collected and used as an enzyme source for EST and GST. The EST activity was determined by the following method. To 2 mL of 1.5 mM 1-naphthyl acetate solution, 0.1 mL of diluted enzyme sample (10 times with 0.1 M sodium phosphate buffer) was added. This mixture was incubated at 25°C for 30 min. The reaction was stopped by addition of fast blue B (in 5 % SDS) staining solution. The reaction mixture was incubated for 15 min and absorbance recorded at 490 nm. The concentration of hydrolyzed substrate was determined from the standard curve of 1-naphthol. Specific activity was expressed as μmol of 1-naphthol formed/min/mg protein, and treatment values are the mean of six replicates.
Glutathione-S-transferase assay
GST activity was determined using 1-chloro-2,4-dinitrobenzene (CDNB) and reduced GSH as substrates according to Habig et al. (1974) with slight modifications. To 1 mL of phosphate buffer (pH 7.5), 0.1 mL of CDNB (25 mM) and 1.6 mL of distilled water were added. The reaction was started by adding 0.1 mL of diluted enzyme solution (the stock solution was diluted 10-fold with 0.1 M sodium phosphate buffer, pH 7.5). The reaction mixture was incubated at 37°C for 5 min and 0.1 mL of 20 mM GSH was added. Optical density at 340 nm was recorded at 30 s intervals for 3 min. The enzyme activity was calculated with an extinction coefficient of 9.6 mM/cm for CDNB. Specific activity was expressed as nmol of CDNB conjugate formed/min/mg protein, and treatment values are the mean of six replicates.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) sing SPSS ver.17 (SPSS, Inc., Chicago, IL, USA). Tukey's test was used to separate the means when the treatment effects were statistically significant (P < 0.05).
Results
Effect of plant secondary metabolites on S. litura growth and development
Among the flavonoids tested, higher larvae mortality of S. litura at 7 DAT was observed in larvae fed on diet treated with 1000 ppm of rutin (47.5 %) followed by quercitin (42.5 %), kaempferol (35.0 %), chlorogenic acid (32.5 %), myricetin (27.5 %), caffeic acid (25.8 %) and naringenin (22.5 %) (Table 1). At 500 ppm, significantly higher mortality was observed in larvae fed on the diets treated with quercitin (32.5 %) and kaempferol (30.0 %) as compared to the rest of the treatments. There were no significant effects on adult mortality for most of the flavonoids at 100 ppm (Table 1).
| Concentration (ppm) | |||
|---|---|---|---|
| Treatment | 100 | 500 | 1000 |
| Rutin | 17.5 ± 2.3a | 27.5 ± 3.9a,b | 47.5 ± 6.3a |
| Chlorogenic acid | 15.0 ± 2.8a | 25.0 ± 3.7b,c | 32.5 ± 2.5b |
| Quinic acid | 5.0 ± 1.2b | 12.5 ± 2.3d | 20.5 ± 3.3d |
| Caffeic acid | 12.5 ± 2.1a | 20.0 ± 4.0c,d | 25.8 ± 4.3c,d |
| Naringenin | 13.5 ± 2.2a | 17.6 ± 3.2c,d | 22.5 ± 3.6c |
| Quercitin | 15.0 ± 3.2a | 32.5 ± 4.6a | 42.5 ± 5.2a |
| Kaempferol | 17.5 ± 2.6a | 30.0 ± 4.4a | 35.0 ± 4.8b |
| Myricetin | 15.8 ± 2.6a | 22.5 ± 3.5c | 27.5 ± 4.7c |
| Catechin | 7.5 ± 1.4b | 12.5 ± 1.8d | 15.2 ± 2.9d |
| Ferulic acid | 12.5 ± 2.4a | 15.5 ± 2.8d | 17.5 ± 2.5d |
| Control | 2.5 ± 0.8c | 2.5 ± 0.8e | 2.5 ± 0.8e |
- Values (mean ± SE) with similar letters within a column do not differ significantly at P < 0.05 (Tukey's HSD test).
At 7 DAT, quercitin showed significantly greater reduction in S. litura larvae weight (mg per five larvae) (390.3, 228.4 and 110.9), followed by kaempferol (461.1, 251.6 and 160.4) at 100, 500 and 1000 ppm, respectively, than the rest of the treatments (Table 2). As compared to the larvae fed on untreated control diet, larvae fed on flavonoid-treated diets showed reduced adult weights. The diet containing GLL, ConA and phenyl β-glucoside did not cause significant larvae mortality in S. litura (data not shown). However, the larvae weights were reduced when these insects were fed on the treated diets (Table 3). At 7 DAT, S. litura larvae fed on the diets treated with GLL, ConA and phenyl β-glucoside (5 μg/mL each) showed body weight reduction by 4.5-, 2.7- and 1.7-fold, respectively, as compared to those fed on the control diet (Table 3).
| Concentration (ppm) | |||
|---|---|---|---|
| Treatment | 100 | 500 | 1000 |
| Rutin | 439.2 ± 12.3d | 292.3 ± 6.2d | 168.6 ± 5.2d |
| Chlorogenic acid | 480.4 ± 9.2c | 360.6 ± 9.4c | 201.2 ± 8.1c,d |
| Quinic acid | 490.3 ± 10.7c | 349.8 ± 9.2c | 239.5 ± 8.6c |
| Caffeic acid | 448.4 ± 9.4d | 361.2 ± 9.2c | 213.4 ± 8.9c,d |
| Naringenin | 497.9 ± 11.2c | 310.3 ± 10.7c,d | 183.7 ± 7.9d |
| Quercitin | 390.3 ± 10.8e | 228.4 ± 8.4e | 110.9 ± 6.8e |
| Kaempferol | 461.1 ± 10.2c,d | 251.6 ± 9.9d,e | 160.4 ± 5.3d |
| Myricetin | 470.4 ± 8.5c | 273.8 ± 10.3d | 173.6 ± 8.5d |
| Catechin | 510.2 ± 10.2c | 360.6 ± 9.6c | 201.5 ± 8.4c,d |
| Ferulic acid | 600.4 ± 9.7b | 470.4 ± 8.8b | 305.2 ± 7.5b |
| Control | 680.3 ± 12.8a | 686.7 ± 12.3a | 683.6 ± 11.6a |
- Values (mean ± SE) with similar letters within a column do not differ significantly at P < 0.05 (Tukey's HSD test).
| Concentration (μg mL−1) | |||
|---|---|---|---|
| Treatment | 1 | 2 | 5 |
| Groundnut leaf lectin | 356.8 ± 9.3c | 243.6 ± 8.5c | 147.4 ± 5.6c |
| Concavalin A | 386.2 ± 10.2c | 310.6 ± 9.4b | 252.8 ± 8.1b |
| phenyl β-glucoside | 452.6 ± 11.7b | 420.7 ± 9.9b | 396.9 ± 9.4b |
| Control | 676.4 ± 12.2a | 678.9 ± 12.8a | 673.9 ± 12.4a |
- Values (mean ± SE) with similar letters within a column do not differ significantly at P < 0.05 (Tukey's HSD test).
Effect of plant secondary metabolites on S. litura midgut enzyme activities
Effect of flavonoids
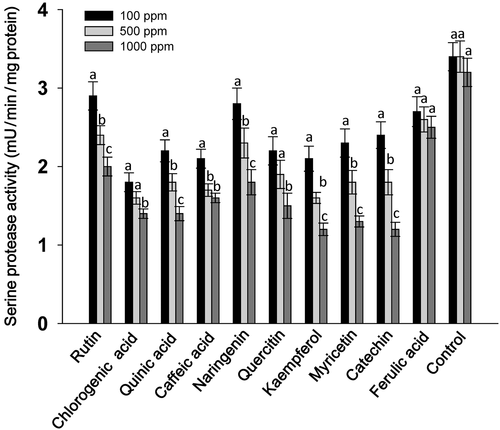
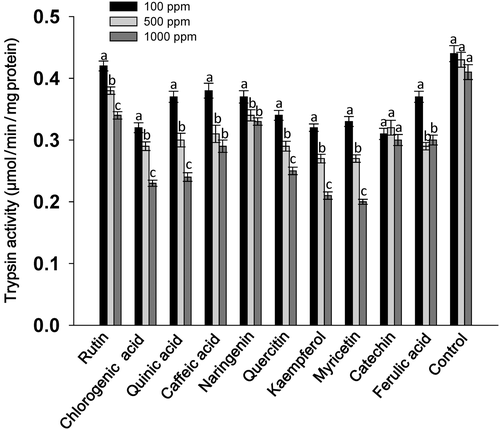
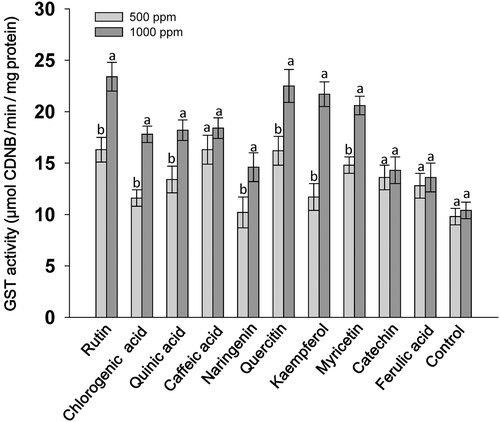
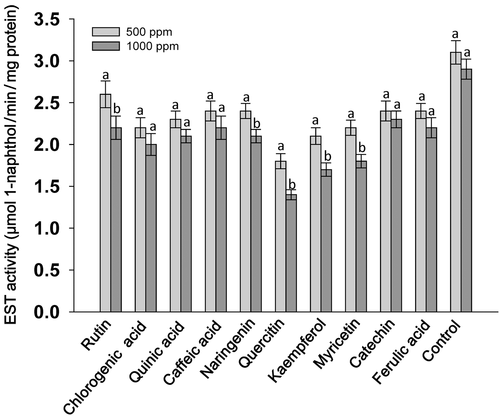
A considerable effect of flavonoids at 1000 ppm concentration was observed on the serine protease and trypsin activities of S. litura larvae, however, the levels of significance varied across the treatments. Larvae fed on flavonoid-treated diets at 1000 ppm showed significantly lower levels of serine protease activity in almost all the treatments except in diets with caffeic acid and ferulic acid as compared to those fed at 100 and 500 ppm concentration of their respective compounds (Fig. 1). Across the treatments, larvae fed on rutin, chlorogenic acid, quinic acid, naringenin, quercitin, kaempferol, myricetin and catechin at 1000 ppm showed lower serine protease activity than those fed on the rest of the treatments, and the untreated control diet (Fig. 1). The trypsin activity of larvae fed on the flavonoid-treated diet at 1000 ppm was significantly lower than the larvae fed at 100 ppm of the respective compounds (Fig. 2). Larvae fed on diets with 1000 ppm of rutin, chlorogenic acid, quinic acid, quercitin, kaempferol and myricetin had significantly lower trypsin activity than the larvae fed on the rest of the treatments, and the untreated diet. The GST activity of S. litura larvae was significantly higher when fed on the diets treated with rutin, chlorogenic acid, quinic acid, naringenin, quercitin, kaempferol and myricetin at 1000 ppm than at 500 ppm, and the larvae fed on control diet (Fig. 3). Across the treatments, rutin, quercitin, kaempferol and myricetin induced higher levels than the rest of the treatments at 1000 ppm, and larvae fed on untreated control diet had lower levels of GST activity (Fig. 3). The S. litura larvae fed on flavonoid-treated diets showed lower levels of EST activity in diets with 1000 ppm of rutin, naringenin, quercitin, kaempferol and myricetin than those fed at 500 ppm (Fig. 4). Quercitin, kaempferol and myricetin fed larvae at 1000 ppm had significantly lower levels of EST activity than the rest of the treatments (Fig. 4).
Effect of GLL, ConA and phenyl β-glucoside
Spodoptera litura larvae fed on the diets containing 5 and 2 μg/mL GLL showed significantly lower levels of total serine protease and trypsin activities (F2,8 = 16.4 and 17.9 respectively, P < 0.05) than those fed on diets with 1 μg/mL GLL (Table 4). Similarly, larvae fed on ConA treated diets at 5 and 2 μg/mL concentration had significantly reduced total serine protease and trypsin activities (P < 0.05). Larvae fed on the phenyl β-glucoside-treated diet showed reduced serine protease activity at 5 and 2 μg/mL concentrations (F2,8 = 14.2, P < 0.05), but did not exhibit any significant differences in trypsin activity across concentrations (Table 4). Across treatments, larvae fed on a GLL-treated diet at 5 and 2 μg/mL concentrations had significantly reduced serine protease and trypsin activities (P < 0.05).
| Serine protease (mU min−1 mg−1 protein) | Trypsin (μmol min−1 mg−1 protein) | |||||
|---|---|---|---|---|---|---|
| Concentration (μg mL−1) | ||||||
| Treatments | 1 | 2 | 5 | 1 | 2 | 5 |
| Groundnut leaf lectin | 1.68 ± 0.08a | 1.40 ± 0.06b | 1.32 ± 0.07b | 0.53 ± 0.02a | 0.38 ± 0.01b | 0.36 ± 0.01b |
| Concavalin A | 1.79 ± 0.09a | 1.50 ± 0.07b | 1.33 ± 0.09b | 0.54 ± 0.06a | 0.46 ± 0.05a,b | 0.44 ± 0.04a,b |
| phenyl β-glucoside | 1.91 ± 0.14a | 1.55 ± 0.12b | 1.44 ± 0.08b | 0.50 ± 0.05a | 0.48 ± 0.04a,b | 0.46 ± 0.02a,b |
| Control | 1.88 ± 0.1a | 1.85 ± 0.09a | 1.83 ± 0.09a | 0.58 ± 0.08a | 0.56 ± 0.08a | 0.55 ± 0.08a |
- Values (mean ± SE) with similar letters within a column do not differ significantly at P < 0.05 (Tukey's HSD test).
The S. litura larvae fed on GLL showed increased GST activity at 2 and 5 μg/mL concentrations as compared to those fed on a diet with 1 μg/mL concentrations (F2,8 = 13.8, P < 0.05; Table 5). Although there was an increase in GST activity of the larvae fed on ConA and phenyl β-glucoside-treated diets, the differences were not statistically significant (P > 0.05). Across the treatments, no significant differences were observed in GST activity of S. litura larvae in all the concentrations tested (P > 0.05). The S. litura larvae fed on the GLL and ConA treated diets showed reduced EST activity at 5 μg/mL concentration (F2,8 = 10.2 and 11.8 respectively, P < 0.05) compared to those fed on diets with 2 and 1 μg/mL concentrations (Table 5). The larvae fed on diets containing phenyl β-glucoside did not show any significant effect on EST activity of S. litura larvae (P > 0.05). Across treatments, the larvae fed on the GLL-treated diet at 5 μg/mL showed significantly reduced EST activity (F3,12 = 20.5, P < 0.05) as compared to those fed on ConA and phenyl β-glucoside-treated diets.
| GST protease (μmol CDNB min−1 mg−1 protein) | EST (μmol 1-naphthol min−1 mg−1 protein) | |||||
|---|---|---|---|---|---|---|
| Concentration (μg mL−1) | ||||||
| Treatments | 1 | 2 | 5 | 1 | 2 | 5 |
| Groundnut leaf lectin | 17.4 ± 1.3b | 22.9 ± 1.8a,b | 22.8 ± 1.5a | 2.7 ± 0.28b | 2.8 ± 0.27b | 1.8 ± 0.28c |
| Concavalin A | 19.0 ± 1.6a,b | 21.2 ± 1.5a,b | 21.5 ± 1.7a | 3.3 ± 0.27b | 2.9 ± 0.23b | 2.2 ± 0.26c |
| phenyl β-glucoside | 23.4 ± 2.1a | 24.6 ± 2.8b | 23.5 ± 1.9a | 4.0 ± 0.25a | 3.8 ± 0.26a | 3.3 ± 0.24b |
| Control | 18.9 ± 2.4a,b | 18.9 ± 2.2a | 19.1 ± 2.2a | 4.4 ± 0.28a | 4.2 ± 0.28a | 4.2 ± 0.28a |
- Values (mean ± SE) with similar letters within a column do not differ significantly at P < 0.05 (Tukey's HSD test).
Discussion
In many countries, plant derived products have been used by farmers since ancient times and it has triggered scientists to search for ecofriendly insecticides from the plant kingdom. Flavonoids are important plant secondary metabolites involved in defense against a number of stresses including herbivory (Treutter 2006; Sharma et al. 2009). Plants contain more than 5000 flavonoids, which play a central role in plant–environment interactions (Treutter 2006). Flavonoids and isoflavonoids directly affect the insect behavior, growth and development (Simmonds 2003). Various flavonoids including isorhamnetin-3-sophoroside-7-glucoside and kaempferol-3,7-diglucoside have been reported as feeding deterrents against Mamestra configurata (Walk.) (Onyilagha et al. 2004).
The present study revealed that rutin, quercitin and kaempferol when incorporated into an artificial diet at 1000 ppm were more toxic to S. litura larvae at 7 DAT than quinic acid, caffeic acid, naringenin, myricetin, catechin, and ferulic acid (Table 1). The weights of the larvae fed on flavonoid-treated diets were significantly lower as compared to those fed on the control diet (Table 2). In addition, total serine protease and trypsin activities were reduced in S. litura larvae fed on diets treated with rutin, chlorogenic acid, quinic acid, naringenin, quercitin, kaempferol and myricetin at 1000 ppm (Figs 1 and 2). The GST activity increased in larvae fed on diets treated with 1000 ppm of these compounds (Fig. 3), and EST activity showed a reduction in S. litura larvae fed on flavonoid-treated diets at 1000 ppm, although the levels of reduction varied across the treatments (Fig. 4). Thus, flavonoids at 1000 ppm concentration are promising for insecticide use for S. litura control. Our results are in line with earlier reports, which have shown a significant increase in GST activity in larvae fed on natural host plant diet with prooxidant allelochemicals (Vanhaelen et al. 2003; Krishnan & Kodrík 2006) and/or fed on the artificial diet containing plant allelochemicals (Morimoto et al. 2000; Atteyat et al. 2012; War et al. 2013). Leaf discs treated with various flavonoids also reduce larval growth and development of Trichoplusia ni (Hub.) (Sharma & Norris 1991). Caffeic and chlorogenic acids are highly toxic to insect pests and have been reported to cause gut toxicity due to protein oxidation and free ion release (Summers & Felton 1994). Chlorogenic acid reduces the nutritional quality of plant tissues by decreasing their digestibility due to the binding of chlorogenoquinone, an oxidative product of chlorogenic acid, to free amino acids and proteins (Felton et al. 1992), and reduces the growth and development of many insect pests including T. ni (Beninger et al. 2004), Heliothis zea (Bod.) (Isman & Duffey 1982; Felton & Duffey 1990), leaf beetles, leaf hoppers and aphids (Ikonen et al. 2002; Jassbi 2003).
Lectins are regarded as potent plant defensive proteins that bind to soluble carbohydrates or to carbohydrate of the glycoproteins, and limit their availability to insects (Peumans & Vandamme 1995), thus depriving the insects from essential nutrients and resulting in reduced growth and development. There are a number of reports that have shown the deleterious effects of lectins on insect pests (Zhu-Salzman et al. 1998; Gatehouse et al. 1999; Arora et al. 2004; Shukla et al. 2005). Lectins are also induced in plants in response to herbivory and/or elicitor application and play an important role in signaling the transduction pathways in plants (Van Damme et al. 2003; Lannoo et al. 2006). Our results showed that larval growth and development were significantly reduced in S. litura larvae fed on a diet with GLL and ConA at 5 μg/mL compared to the larvae fed at 2 and 1 μg/mL concentrations. Larvae fed on lectin-treated diets exhibited lower larval weights at 7 DAT (Table 3). Moreover, there was a considerable reduction in the total serine protease and trypsin activities of S. litura larvae fed on GLL, ConA and phenyl β-glucoside-treated diets at 5 μg/mL (Table 4). However, reduction in trypsin activity was greater in larvae fed on the GLL-treated diet than the larvae fed on the ConA and phenyl β-glucoside-treated diets at 5 μg/mL. The GST and EST activities were also altered significantly (Table 5). Significant inhibitory activity on insect growth has been observed in Callosobruchus maculatus (F.) fed on a diet treated with Maclura pomifera (Raf.) derived lectin and galactose-binding peanut lectin (Murdock et al. 1990). Jacalin and M. pomifera lectin reduced the larval growth of Diabrotica undecimpunctata Barber (Czapla & Lang 1990). ConA lectin, when incorporated in the artificial diet, resulted in 90 % larval mortality of tomato moth, Lacanobia oleracea (L.), and also reduced the size of M. persicae by about 30 %. Expression of ConA in potato plants reduced the larval weight by 45 % (Gatehouse et al. 1999).
Spodoptera litura has emerged as a serious pest of cash crops in China. Although various high dosage synthetic insecticides have been used to protect field vegetables, the development of a broad-spectrum resistance to insecticides has complicated its chemical control (Su et al. 2012; Tong et al. 2013; Sang et al. 2016). Hence, it is very important to use some safe and environment friendly methods to control this pest. From the results of this article, we propose that the flavonoids such as rutin, chlorogenic acid, quinic acid, naringenin, quercitin, kaempferol and myricetin, and lectins, GLL and ConA, are highly toxic to S. litura and can form an important component of insect pest management against S. litura. Our results also show that rutin and quercitin are more promising insecticide candidates used for S. litura control. Further studies are needed to isolate and identify the active the antifeedant principle present in plants on insect toxicity and, simultaneously, to determine the effects of these components on gene expression in the insect midgut and fat body.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31501641, 31601638) and Hubei Provincial Natural Science Foundation of China (2016CFB304).
Conflict of interests
The authors declare that they have no competing interests.




