Treatment of acute kidney injury with continuous renal replacement therapy and cytokine adsorber (CytoSorb®) in critically ill patients with COVID-19
Abstract
Introduction
This retrospective study aimed to evaluate the 30 and 60-day survival of critically ill patients with COVID-19 and AKI.
Methods
Inflammatory and biochemical biomarkers, length of intensive care unit (ICU) stay and mortality at Day 30 and Day 60 after ICU admission were analyzed. A total of 44 patients treated with continuous renal replacement therapy (CRRT) with cytokine adsorber (CA group) were compared to 58 patients treated with CRRT alone (non-CA group).
Results
Patients in CA group were younger, had better preserved kidney function prior to the beginning of CRRT and had higher levels of interleukin-6. There were no statistically significant differences in their comorbidities and in other measured biomarkers between the two groups. The number of patients who died 60 days after ICU admission was statistically significantly higher in non-CA group (p = 0.029).
Conclusion
Treatment with CRRT and cytokine adsorber may have positively influenced 60-day survival in our COVID-19 ICU patients with AKI.
1 INTRODUCTION
Acute kidney injury (AKI) affects more than 20% of hospitalized patients with coronavirus disease 2019 (COVID-19) and more than 50% of COVID-19 patients in intensive care unit (ICU) [1]. Renal injury in COVID-19 results from direct cellular infection of SARS-CoV-2 by angiotensin convertase 2 (ACE2), cytokine storm, and AKI associated with acute respiratory distress syndrome (ARDS), hypovolemia with hypoperfusion, collapsing glomerulopathy, thrombotic phenomena, and treatment complications [2-4]. The main risk factors for the development of AKI and more severe disease in COVID-19 were arterial hypertension (15%–42.3%), diabetes mellitus (7.4%–41.4%), preexisting chronic kidney disease (CKD) (0.7%–7.6%), obesity, cardiovascular disorders, higher age, and male sex [2, 3, 5].
AKI is associated with increased morbidity and mortality [6, 7]. Several authors have shown that critically ill patients with COVID-19 and renal dysfunction have a significantly higher in-hospital mortality rate, exceeding 70% [1].
SARS-CoV-2 triggers an immune response with the production of inflammatory cytokines and a weak interferon response. Cytokine storm in COVID-19 is the result of an impaired acquired immune response and an uncontrolled inflammatory innate response and is characterized by high expression of (interleukine-6) IL-6 and tumor necrosis factor ά (TNF-ά) [8]. In some observational studies, patients with elevated inflammatory markers were more likely to develop AKI and had a worse outcome [6]. A cytokine storm affects the kidneys through intrarenal inflammation, increased vascular permeability, volume depletion, and cardiomyopathy [9]. Similarly, in sepsis, the main causes of AKI are associated with hypoperfusion, shock, and ischemic injury [10]. The inflammatory response is thought to be the major host defense mechanism. However, at the Sepsis 3 Conference in 2016, sepsis was defined as a life-threatening organ dysfunction caused by a deregulated host response to infection [11] and consequently may be responsible for multiple organ failure (MOF) and poor outcomes [10].
The knowledge of these mechanisms led to the development of different treatment strategies, mainly using extracorporeal blood purification techniques (high-volume haemofiltration, high-cut-off membranes, adsorption techniques). Their aim is immunomodulation in acute inflammatory conditions [12]. Their efficacy and safety have been investigated in numerous studies, but there was still insufficient evidence to recommend the use of hemoperfusion or other blood purification techniques in sepsis in the latest 2021 Surviving Sepsis Campaign guidelines [13]. One of these adsorbents is CytoSorb® membrane (CytoSorbents Corp., USA). It is a whole blood adsorbent with a large total surface area. It can remove molecules up to 60 kDa in size, including cytokines and various bacterial toxins, without affecting important substances such as albumin [14].
All of the above mechanisms support the idea that extracorporeal blood purification techniques, such as immunoadsorption, may prove beneficial in COVID-19 patients, just as they are used in other hyperinflammatory conditions, such as sepsis [15].
The aim of our study was to evaluate the 30 and 60-day survival of patients with COVID-19 and AKI in the ICU compared to whether they were treated with continuous renal replacement therapy (CRRT) with or without cytokine adsorbers.
2 MATERIALS AND METHODS
This retrospective study included data from patients diagnosed with COVID-19 and AKI who were admitted to the ICU of a tertiary medical centre and treated with CRRT between March 1, 2020, and April 15, 2022. The study was approved by the Local Ethics Commitee.
Inclusion criteria were the following: all covid-19 patients admitted to ICU with AKI, treated with continuous veno-venous hemodialysis (CVVHD), regardless of their concomitant diseases.
There were no exclusion criteria.
The diagnosis of SARS-CoV-2 infection was made by real-time reverse transcriptase polymerase chain reaction (PCR) on a nasal swab before admission to the ICU. Patients were transferred either from an acute care COVID-19 unit of our hospital or from another regional hospital or were admitted directly from the emergency department. All patients were treated with antimicrobials (if needed), antithrombotics, vasopressants, immunomodulators, and mechanical ventilation as determined by the treating physician. AKI was defined and graded using the KDIGO guidelines [16].
CRRT method of choice in all patients was CVVHD using MultiFiltrate® (Fresenius Medical Care, Bad Homburg, Germany) with membrane surface area of 1.8 m2. CytoSorb was attached prefilter. No CytoSorb membrane had been used for more than 24 h. If longer period of cytokine adsorption was needed, the membrane was replaced by a new one. CytoSorb was always started together with CRRT, never added-on. Decision to stop CytoSorb was made in accordance with the intensivists regarding patients’ clinical improvement. Some patients, but not all of them, had only pure CRRT following CytoSorb® removal.
The procedure was prescribed by a consulting nephrologists due to AKI with or without anuria. In one group of patients, a cytokine-adsorbing membrane CytoSorb® was added to the CVVHD circuit. This was the cytokine adsorber group (CA group) compared to the group without cytokine adsorber (non-CA group), which consisted of patients treated with CVVHD only. The decision to use the cytokine adsorber was made individually for each patient through an agreement between a nephrologist and an intensivist.
Data on patients' comorbidities and pre-existing CKD were collected by reviewing our electronic medical records. Data on mean arterial pressure (MAP), body temperature, mechanical ventilation and various treatment modalities (antibiotics, steroids, remdesivir, monoclonal antibodies, noradrenaline dose, vasopressin dose, dobutamine dose) were collected from patients' paper temperature charts. Sequential organ failure assessment score (SOFA) was calculated with the use of an established formula. Inflammatory and biochemical biomarkers on admission, length of ICU stay, and mortality 30 and 60 days after ICU admission were analyzed. The biochemical biomarkers creatinine, urea, estimated glomerular filtration rate (eGFR, estimated by using the Chronic Kidney Disease Epidemiology Collaboration 2009 equation—CKD-EPI 2009)), C-reactive protein (CRP), procalcitonin (PCT), IL-6, lactate, lactate dehydrogenase (LDH), D-dimer, ferritin, and hemoglobin (Hb) were measured by standard tests at the Department of Laboratory Diagnostics of our tertiary medical centre.
2.1 Statistical analyses
Statistical analysis was performed using the Statistical Package for Social Sciences version 28.0.1.1 (SPSS Inc., Chicago, IL, USA). Probability values of p < 0.05 (double-tailed) were considered statistically significant for all comparisons. Continuous variables are presented as the mean ± standard deviation (SD) and categorical variables as absolute and relevant frequencies. Within-group comparisons for continuous variables were performed with Student's t-test for paired samples or Wilcoxon's signed-rank test, and between-group comparisons were performed with Student's t-test for independent samples or Mann–Whitney test, depending on normality. Categorical variables were compared with the chi-square test or Fisher's exact test. The events studied were fatal events resulting in death. A multivariate Cox regression model was used to assess the influence of different variables on death. We used chi-square test to analyze mortality between the two groups of patients at 30 and 60 days since the start of treatment. Kaplan–Meier survival curve was used to asses the impact of cytokine adsorption on survival.
3 RESULTS
The study included 102 critically ill COVID-19 patients, 74.5% (N = 76) were male. Their mean age was 68.1 ± 8.1 years. Their most common comorbidities are shown in Table 1. Twenty-two (21.6%) patients were transferred from another regional hospital.
| Disease | Number (%), all patients | CA group (N = 44) | Non-CA group (N = 58) | p-Value |
|---|---|---|---|---|
| Diabetes | 39 (38.2) | 21 (47.7) | 18 (31) | 0.086 |
| Hypertension | 75 (73.5) | 32 (72.7) | 43 (74.1) | 0.873 |
| Chronic kidney diesase | 26 (25.5) | 7 (15.9) | 19 (32.8) | 0.053 |
| Heart failure | 22 (21.6) | 7 (15.9) | 15 (25.9) | 0.226 |
| Coronary artery disease | 15 (14.7) | 9 (20.5) | 6 (10.3) | 0.153 |
| Dyslipidemia | 31 (30.4) | 15 (34.1) | 16 (27.6) | 0.479 |
| Previous stroke | 10 (9.8) | 4 (9.1) | 6 (10.3) | 0.833 |
| Previous malignancy | 14 (13.7) | 4 (9.1) | 10 (17.2) | 0.236 |
| Liver cirrhosis | 3 (2.9) | 2 (4.5) | 1 (1.7) | 0.404 |
| Functioning kidney transplant | 3 (2.9) | 2 (4.5) | 1 (1.7) |
All patients were treated with CVVHD, 44 patients (43.1%) in the CA group and 58 patients (56.9%) in the non-CA group. The overall method of anticoagulation during CVVHD was 4% sodium citrate in 66.7% (N = 68), low molecular weight heparin (LMWH) in 12.7% (N = 13), and unfractionated heparin (UFH) in 19.6% (N = 20).
Patients received varying numbers of CRRT procedures (1–22; mean 3.69 ± 3.5). Mean cumulative ultrafiltration (UF) during all CVVHD procedures was 9435.6 ± 11417.9 mL (0–50 150 mL). The mean cumulative duration of CVVHD treatment was 61.9 ± 57.9 h (1–240 h).
The mean time from admission to our hospital to initiation of CRRT was 15.2 ± 12.8 days (1–89 days). The mean time from admission to our hospital to initiation of cytokine adsorber treatment was 12.1 ± 9.4 days (1–33 days). Treatment with CVVHD was initiated earlier in the CA group, compared to the non-CA group (11.4 vs. 18.2 days; p = 0.007). The mean creatinine before RRT/creatinine at admission ratio was 2.36 ± 1.73 in CA group and 3.07 ± 1.90 in the non-CA group.
Patients in the CA group were younger (64.7 vs. 70.6 years; p < 0.0001), had lower serum creatinine (293.7 vs. 404.8 μmol/L; p < 0.001), urea (29.4 vs. 47.6 mmol/L; p < 0.001), higher eGFR (26.3 vs. 15.1 mL/min/1.73 m2; p = 0.003), IL -6 (1754 vs. 385 pg/mL; p < 0.001) and LDH (8.8 vs. 7.1 (μkat/L); p = 0.038). We found no significant difference between the two groups for serum lactate, ferritin, D-dimer, CRP and PCT. The results for both groups are shown in Table 2.
| CA group (N = 44) | Non-CA group (N = 58) | p-Value | |
|---|---|---|---|
| Age (years) | 64.7 ± 9.6 | 70.6 ± 5.6 | <0.0001 |
| Creatinine at admission (μmol/L) | 190.2 ± 260.1 | 199.7 ± 182.3 | 0.837 |
| Creatinine at the start of RRT (μmol/L) | 293.7 ± 145.1 | 404.8 ± 151.8 | <0.0001 |
| Urea at admission (mmol/L) | 14.9 ± 15.1 | 15.7 ± 12.9 | 0.776 |
| Urea at the start of RRT (mmol/L) | 29.4 ± 15.7 | 47.6 ± 16.8 | <0.0001 |
| eGFR at admission (mL/min/1.73 m2) | 50.6 ± 28.0 | 45.8 ± 27.9 | 0.389 |
| eGFR at the start of RRT (mL/min/1.73 m2) | 26.3 ± 21.2 | 15.1 ± 12.3 | 0.001 |
| Creatinine at discharge / death (μmol/L) | 219.1 ± 140.6 | 271.4 ± 132.5 | 0.06 |
| Urea at discharge / death (mmol/L) | 25.3 ± 17.3 | 32.9 ± 16.4 | 0.026 |
| eGFR at discharge / death (mL/min/1.73 m2) | 39.2 ± 26.8 | 27.2 ± 22.5 | 0.017 |
| CRP at admission (mg/L) | 153.5 ± 102.3 | 121.1 ± 95.0 | 0.106 |
| PCT at admission (μg/L) | 8.36 ± 23.28 | 1.97 ± 7.68 | 0.054 |
| Max. IL-6 (pg/mL) | 1753.8 ± 2125.3 | 385.2 ± 579.5 | <0.0001 |
| LDH at hospital/ICU admission (μkat/L) | 8.8 ± 5.1 | 7.1 ± 3.2 | 0.038 |
| Lactate at admission (mmol/L) | 3.0 ± 2.8 | 2.3 ± 1.4 | 0.09 |
| Ferritin at admission (μg/L) | 2585.1 ± 3941.3 | 1565.6 ± 1760.2 | 0.093 |
| D-dimer at admission (μg/L) | 8618.7 ± 16194.3 | 4438.1 ± 9999.2 | 0.114 |
| Hb at admission (g/L) | 124.8 ± 24.5 | 129.1 ± 21.2 | 0.358 |
| Time from hospital admission to start of CRRT (days) | 11.4 ± 9.5 | 18.2 ± 14.2 | 0.007 |
| Time from hospital admission to start of Cytosorb (days) | 12.1 ± 9.4 | / | |
| Total UF (ml) | 6374.7 ± 8755.5 | 11757.6 ± 12667.1 | 0.018 |
| Duration of CVVHD (hours) | 55.9 ± 58.5 | 66.4 ± 57.6 | 0.370 |
| Length of ICU stay (days) | 26.1 ± 21.9 | 28.3 ± 19.0 | 0.593 |
| Length of hospital stay (days) | 33.7 ± 28.0 | 35.6 ± 24.9 | 0.720 |
- Abbreviations: CRP, C-reactive protein; CRRT, continuous renal replacement therapy; CVVHD, continuous veno-venous hemodialysis; eGFR, estimated glomerular filtration rate; Hb, haemoglobine; ICU, intensive care unit; IL-6, interleukin-6; LDH, lactate dehydrogenase; PCT, procalcitonin; UF, ultrafiltration.
The mean length of hospital stay during treatment of COVID-19 was 34.8 ± 26.2 days (1–116 days). The average ICU stay was 27.4 ± 20.3 days (1–96 days), with no difference in length of stay between the two groups. Using Cox regression we showed no difference in ICU stay between the two groups depending on the gender (p = 0.754), age (p = 0.299), the body mass index (BMI) (p = 0.234), presence of diabetes (p = 0.951) or heart failure (p = 0.263), and eGFR (p = 0.660), CRP (p = 0.269), lactate (p = 0.462), or Hb (p = 0.312) at admission.
Not all data were available for every patient, so we included in further analyses only those for whom all data were available (data on medications, including antimicrobials, anti-inflammatory treatment and vasopressors, MAP, mechanical ventilation). In these further analyses, we included 89 patients, 76.4% of them male (N = 68). Totally 37 were in the CA group and 52 in the non-CA group. At baseline, 87 of them (97.8%) were mechanically ventilated, 86 (96.6%) received steroids, 11 (12.45%) received remdesivir, 3 (3.4%) received monoclonal antibodies and 86 (96.6%) were treated with antibiotics, with no significant differences between the two groups. There were statistically significant differences between the groups in CRP before (221.0 ± 130.9 vs. 86.7 ± 66.5 mg/L; p < 0.0001) and after the procedure (160.7 ± 111.9 vs. 79.7 ± 52.3 mg/L; p < 0.0001), PCT before the procedure (46.20 ± 92.95 vs. 7.87 ± 18.48 μg/L; p = 0.018), noradrenaline dose before (0.29 ± 0.31 vs. 0.07 ± 0.13 μg/kg/min; p < 0.0001) and after the procedure (0.28 ± 0.41 vs. 0.05 ± 0.08 μg/kg/min; p = 0.002), vasopressin dose before (1.5 ± 1.8 vs. 0.2 ± 0.7 μg/kg/min; p < 0.0001) and after the procedure (1.2 ± 1.7 vs. 0.3 ± 0.8; p = 0.005) and in the SOFA score at baseline (13.5 ± 2.4 vs. 12.3 ± 2.1; p = 0.016), but not in the SOFA score after the procedure (12.6 ± 2.9 vs. 11.7 ± 2.0; p = 0.148), as shown in Figures 1 and 2. In the CA group, we observed a statistically significant decrease in CRP (p = 0.008) and SOFA score (p = 0.044) after the procedure, but no significant differences in PCT, MAP, body temperature and inotrope and vasopressor doses. In the non-CA group, we only observed a statistically significant decrease in SOFA score after the procedure (p = 0.008).
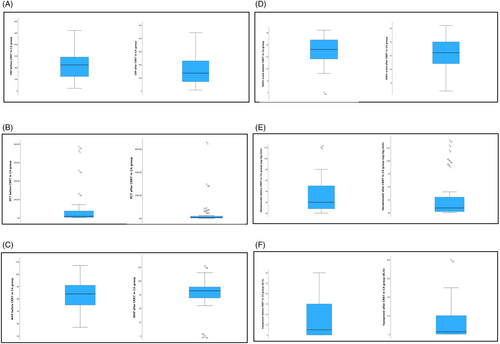
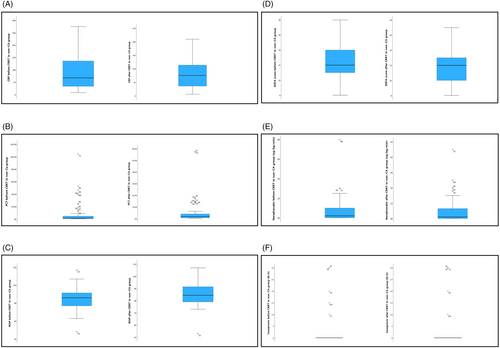
The CA group had a better renal outcome; in the CA group, mean eGFR at discharge was 39.2 mL/min/1.73 m2 vs. 27.2 mL/min/1.73 m2 in the non-CA group (p = 0.008). Forty-two (41.2%) patients were alive 30 days after ICU admission, and 24 (23.5%) patients were alive 60 days after ICU admission. Eighteen (17.6%) patients were discharged alive from the ICU. The average mortality rates 30 and 60 days after ICU admission were 58.8% and 76.5%, respectively. In the non-CA group mortality at 30 and 60 days was 65.5% and 84.5%, respectively. In the CA group, mortality after 30 and 60 days was 50% and 65.9%, respectively. A statistically significant difference between the two groups was found for the 60-day mortality (p = 0.029), but not for the 30-day mortality (p = 0.115). The most common causes of death were sepsis with MOF in 55.1% (N = 43), acute respiratory failure in 24.4% (N = 19) and cardiac arrest in 19.2% (N = 15), one patient died of intracerebral hemorrhage (Table 3). Using the Mann–Whitney test, no statistically significant difference was found between the CA group and the non-CA group in terms of cause of death (p = 0.604). Kaplan–Meier survival curve showed no positive effects of CytoSorb® on 60-day survival (Figure 3).
| CA group (N = 44) | Non-CA group (N = 58) | p-Value | |
|---|---|---|---|
| 30-day mortality | 22 (50%) | 38 (65.5%) | 1.117 |
| 60-day mortality | 29 (65.9%) | 49 (84.5%) | 0.029 |
| Causes of death | |||
| Sepsis and MOF | 25 (43.1%) | 20 (45.5%) | |
| Acute respiratory failure | 19 (32.8%) | 3 (6.8%) | |
| Cardiac arrest | 7 (12.1%) | 9 (20.6%) | |
| Intracerebral hemorrhage | 1 (1.7%) | 0 | |
- Abbreviation: MOF, multiorgan failure.

4 DISCUSSION
The aim of our study was to evaluate the 30 and 60-day survival of critically ill COVID-19 patients with AKI in the ICU depending on whether they were treated with cytokine adsorbers or CRRT alone. The majority of patients were male, which is consistent with data showing that male sex is a risk factor for the development of AKI in COVID-19 [1]. Patients in CA group were younger compared to non-CA group (64.7 vs. 70.6 years). In the study by Gupta et al, older age was shown to be a lower risk for needing CRRT for AKI, but it is possible that a more conservative treatment approach was more often used in older patients with a poor prognosis. In this way, a physician was less likely to decide for invasive procedures such as dialysis [17]. There is also a possible explanation that at the peak of the COVID-19 waves, ICUs were full of patients and required more limited triage of who should be admitted to the ICU. In this way, elderly and otherwise disabled patients were less likely to be admitted to the ICU and subsequently treated with CRRT.Our patients‘comorbidities had no effect on survival and were similarly dispersed as in the study from Bezzeras et al, [18]. However, other studies showed that some comorbidities are a risk factor for the development of AKI in COVID-19 patients. [1, 17]. These include arterial hypertension, diabetes mellitus, and preexisting CKD, which were also present in high percentages in our patients [1].
When comparing the measured parameters between the two groups, we found that patients in the CA group had lower serum creatinine and urea levels and higher serum IL-6 and LDH at the start of CRRT. All patients had been diagnosed with AKI, but this was not necessarily the main reason for treatment. Main indications for starting “regular” RRT in the ICU are uremia, hyperkalemia, hypervolemia, metabolic acidosis, or various intoxications [19]. In contrast, indications for CytoSorb® therapy, according to the manufacturer, are shocks of various etiologies, sepsis, necrotizing fasciitis, pancreatitis, burns, trauma, liver failure, rhabdomyolysis, and others [14]. We speculate that the reason for the difference in serum urea, creatinine, IL-6, and LDH between the CA and non-CA groups is the different indications for the intervention. In the non-CA group, the main indications for starting CRRT were “classical” indications (the most common being hypervolemia) and this is the reason for the higher urea and creatinine levels at the start of RRT compared with the CA group. In contrast, the patients in the CA group were patients with a cytokine storm due to COVID-19, whose main indication for the procedure, besides AKI, was the need to remove excess cytokines. If it were only for AKI, they would not yet need RRT and therefore had lower serum urea and creatinine levels at the start of RRT compared to the non-CA group. In the CA group, elevated levels of CRP, PCT, IL-6 and LDH were indicators of inflammation, which was more severe than in the non-CA group. The CA group also had higher preprocedure SOFA score and required higher doses of noradrenaline and vasopressin before and after the procedure. The state of inflamation was the main reason for treatment with cytokine adsorbers. In both groups we observed a statistically significant decline of SOFA score after the procedure. Unfortunately, there were no control measurements of IL −6 after the use of cytokine adsorbers. There have been other studies in which serum levels of IL-6 were measured before and after the procedure, such as a study from Asghapours et al, who found a statistically significant decrease in IL-6 after 3 procedures of hemoperfusion in 10 patients [20].
There were some studies using cytokine adsorbers in septic COVID-19 or non-COVID-19 patients yielding contradictory results. In some, the use of cytokine adsorber was linked to improvement in MAP, lower need for vasopressors and better haemodynamic stability [21], whereas in others there was no difference in the elimination of cytokines, development of multiple organ dysfunction syndrome (MODS), duration, and type of ventilation or survival [22-24].
In our previous study, however, we evaluated non-COVID-19 patients with septick shock and AKI treated with CRRT with cytokine adsorber membrane and found statistically significant differences at PCT, SOFA score, and MAP. Patients who underwent more than one procedure had statistically significant reductions in SOFA score, serum lactate, and vasopressin dose [25]. Results were conclusive with the results of the study of Mehta et al. [21].
Patient mortality in this study was higher compared with other studies, with a few exceptions, such as the study from Bezzeras et al. where mortality reached 90% [1, 18]. In our study, a total of 82.4% of all patients died, 58.8% of whom were dead 30 days after ICU admission and 76.5% 60 days after ICU admission. Of 24 patients who survived 60 days after ICU admission, 15 were in the CA group and 9 were in the non-CA group. Of 78 patients who died, 29 were in the CA group and 49 were in the non-CA group. The difference between the groups was statistically significant (p = 0.029). However, the Kaplan–Meier survival curve did not yield any statistically significant difference between the groups (Figure 1). There were also no differences in causes of death between both groups of patiens, regarding CytoSorb® use.
In the study from Tan et al. the survival rate of COVID patients with AKI was 67.5% [26]. The risk of death was higher in older patients, patients with more severe disease, and patients with ischemic heart disease [26]. Burke et al examined the outcome of critically ill COVID patients in relation to RRT and mortality [27]. Patients who were mechanically ventilated were more likely to require RRT. Mortality in patients who needed CVVH was 56%, and when patients with prior CKD were excluded, mortality increased to 68%. Risk factors for mortality were older age, need for mechanical ventilation, need for CVVH, and elevated CRP [27]. Ng et al. examined renal and patient survival after COVID-19. The risk of mortality was lowest in patients without AKI and rised in concordance with the severity of AKI, being the highest in patients, requiring RRT [28]. We asked ourselves why the mortality of our patients was so high. There is no simple answer to this question. The average age of the patients and concomitant diseases were similar to those in some other studies [27, 28]. One possible answer lies in the time that elapsed from admission to the ICU to the start of RRT. There was a difference between groups, but even for the CA group, this period was quite long. It is true that they were septic with MOF, but this was probably due not only to COVID-19 but also to subsequent hospital infections. The other reason was that they were, on average, highly volume overloaded. The high UF requirement proves this. It is well known that positive fluid balance leads to microcirculatory failure and consequently to higher mortality [29, 30]. We can attribute this, at least in part, to understaffing, because during the peak periods of the COVID waves, most of the physicians at COVID ICU were primarily not intensivists but physicians working in other areas of medicine.
In a study by Ng et al., 30.6% of patients who required RRT remained dialysis dependent. In patients with AKI without need for RRT, the kidneys recovered in 74.1% [28]. In our study, we observed a better renal outcome in the CA group (p = 0.020). We hypothesize that this was because renal function was already better in this group at the start of RRT, because the main indication for starting RRT was not only renal failure but cytokine storm due to COVID-19. However, it is not negligible, that their younger age and earlier start of CRRT might had contributed to that as well. In the study from Gupta et al. more than 30% of patients who required RRT due to AKI with sepsis remained on dialysis [17]. Xiang et al studied patients with a cytokine storm. They showed that CRRT reduced inflammation but had no effect on survival [31].
This study has several limitations. It is retrospective and therefore only an observational study with a relatively small sample size. The duration and frequency of treatment with cytokine adsorbers were not standardized and were at the discretion of the participating physicians. There were no serial measurements of IL-6. The temperature charts are paper-based, which means lower data fidelity. A large proportion of ICU staff during the epidemic were not trained intensivists or critical care nurses, which may have influenced their work. In general, however, there are relatively few data on the use of cytokine adsorbers in the ICU, so our study makes a small contribution to the overall picture.
5 CONCLUSIONS
In conclusion, in this retrospective study we speculate that some critically ill covid-19 patients with AKI benefited of the addition of a cytokine-adsorbing membrane to the dialysis circuit, since it may had helped in reduction of their inflammatory markers, the vasopressor requirement and improving their 60-day survival. They were also discharged with better residual renal function, but other parameters may had attributed to that as well. For more information further studies are needed.
ACKNOWLEDGMENTS
Not applicable.
FUNDING INFORMATION
There was no special funding.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
The study was approved by the Local Ethics Commitee (UKC-MB-KME-40/22).
CONSENT
Patient consent statements were not obtained, since it was a retrospective study.
Open Research
DATA AVAILABILITY STATEMENT
All data are available in hospitals' electronic medical records (Medis®) and in paper charts.




