Risk assessment for hypocalcemia in therapeutic apheresis for kidney transplantation
Institution where study was performed—University of Virginia Health, Charlottesville, Virginia, USA.
Abstract
Introduction
The increase in the number of kidney transplants performed in the United States has been paralleled with an increase in the utilization of therapeutic apheresis (TA) for kidney transplant indications. Hypocalcemia remains a significant contributor to the adverse event in TA. The magnitude of hypocalcemia and its risk factors are scarcely discussed in literature.
Methods
This is a retrospective cohort review of adults from 18 years and above who received TA for kidney transplant-related indications from January 1, 2017 to December 31, 2022. Data extracted included basic demographics, indication for apheresis, procedure characteristics, serum ionized calcium at the mid and end of procedure and serum creatinine at the beginning of apheresis, and so forth.
Results
Data from 131 patients and 860 sessions of TA were analyzed. Antibody-mediated rejection (69%) and recurrent FSGS (15%) were the leading indications for TA. There were 60 (7%) TA sessions complicated by hypocalcemia. Of these, 53 (88%) occurred in the first session, 5 (8%) occurred in second session while 2 (4%) occurred in the third and subsequent sessions. Female sex, elevated serum creatinine and use of fresh frozen plasma- are the risk factors for hypocalcemia with odd's ratio of 2.34, 7.42, and 5.01, respectively. Binary logistic regression showed that elevated serum creatinine at the commencement of therapy is an independent predictor of hypocalcemia (adjusted odd's ratio = 3.31, p = 0.001).
Conclusion
Hypocalcemia is prevalent in this study. Clinical vigilance and tailored procedure will avert adverse consequences.
1 INTRODUCTION
The number of patients assessing apheresis procedures for wide range of indications is increasing globally [1-3]. In the United States, forecast data suggest a possible three-fold increase of apheresis for mononuclear cell collection from approximately 43 000 annual collections in 2021 to 132 000 by 2025 [4, 5].
According to the preliminary data from the United Network for Organ Sharing (UNOS), 42 887 organ transplants were performed in the United States in 2022 indicating an increase of 3.7% over 2021; with kidney transplants achieving its first ever record of more than 25 000 procedures across the United States [6]. This rise is accompanied by increasing utility of therapeutic apheresis (TA) indication for kidney transplant as it plays critical role in improving access to donor kidneys (as a cornerstone of desensitization protocol) and in managing post-transplant complications like antibody-mediated rejection, recurrent glomerulonephritis and thrombotic microangiopathy [2, 7].
Therapeutic apheresis is a well-tolerated procedure with minimal side effects [8]. Hypocalcemia isone of the complications of apheresis with prevalence of 5%–10% in reported series [9, 10].Despite the use of supplementary calcium infusion, hypocalcemic toxicity is encountered and can manifest as tingling sensation at the digits and peri-oral region, numbness, transient nausea, light-headedness, seizures, cardiac arrhythmias, loss of consciousness and death. A previous study in Pakistan among 177 patients undergoing plasma exchange showed the prevalence of hypocalcemia was 10.1% [10]. However, there was no assessment of the possible associations between hypocalcemia and the variables in the study and no search for risk factors was made.
This paucity of studies on the risk factors for hypocalcemia in TA has created a knowledge-gap, hence the rationale behind our study. Knowledge of these risk factors will enable clinicians appropriately tailor treatment to each patient appropriately to avoid these adverse outcomes. Hence, the objectives of this study are to obtain the prevalence of hypocalcemia and identify the risk factors among a cohort of patients undergoing TA for kidney transplant indications.
2 MATERIALS AND METHODS
The study center is a teaching hospital with a transplant program that includes kidney, liver, lung, heart and stem cell transplantation. There is an in-patient apheresis unit that uses centrifuge-based apheresis hardware, the Spectra Optia (Terumo BCT). Whole blood flow rate is 70–80 mL/min, the replacement fluid is usually albumin and fresh frozen plasma for select indications like Thrombotic Thrombocytopenic Pupura. Citrate dextrose (ACD-A) is the circuit anticoagulant of choice while intravenous calcium gluconate supplementation is given prophylactically to all patients in the course of each treatment. The AC infusion rate is set at of 0.8 mL/min/L TBV while the inlet: ACD-A ratio is set at 10:1 for all patients, and modified as needed. Prophylactic continuous infusion of intravenous 10% calcium gluconate to the returning fluid is used as calcium supplementation. Prior to apheresis, at the mid-point of a treatment session and at the end of a therapy session, about 4 mL of blood is collected through the median ante-cubital vein and into the corresponding sample bottle and transported to the main laboratory through the pneumatic transport system in the apheresis unit. Serum ionized calcium is accessed using the laboratory automated machine through the ion-selective electrodes method. Results typically are generated within 10–15 min and this helps to guide clinical decision.
The standard operating protocol (SOP) for calcium supplementation in TA in the institution is prophylactic continuous infusion of intravenous calcium gluconate 2 g in 100 mL of 0.9% saline is given at an infusion rate dependent on level of ionized calcium. The infusion rate of calcium gluconate is 200 mL/h for ionized calcium less than or equal to 4 mmol/L, 175 mL/h for ionized calcium 4.1–4.5 mmol/L, 150 mL/h for ionized calcium 4.6–4.9 and 125 mL/h for ionized calcium 5–5.5 mmol/L. Therapeutic apheresis, in the center, is usually done on alternate day interval till recommended sessions in the batch of apheresis is completed. The therapy is administered at intervals stated by ASFA guidelines for each indication [2]. The prescription is done by the Medical Doctors who also supervise the procedure as delivered by trained nurses.
The study was a retrospective cohort study involving adults, age 18 years and above who underwent TA prior to or after kidney transplantation over a 6 year period, between January 1, 2017 and December 31, 2022 at the study center. Patients were excluded if they have undergone parathyroid surgery in the past or have baseline hypocalcemia from laboratory tests.
Data extracted from the medical records includes age, sex, weight, height, and blood pressure procedure data such as and diagnosis, indication, serum ionized calcium at start, mid and end of procedure, vascular access, replacement fluid and replacement volume, and the serum creatinine at the start of procedure. Based on the normal range of the respective analytes in our laboratory and standards of care, hypocalcemia was defined as serum ionized calcium less than 4.5 mg/dL in the mid or end procedure laboratory test for serum ionized calcium [11] while elevated serum creatinine was defined as serum creatinine greater than 1.3 mg/dL in men or greater than 1.1 in females [12, 13].
2.1 Data analysis
Data obtained was stored on the excel sheet and analyzed using the statistical package for social sciences (SPSS-version 2020 IBM Inc. US). Mean (±SD) was used to describe continuous variables while proportions were used to describe categorical variables. The student t-test was used to compare the means of continuous variables which were normally distributed while Chi-square test was used to determine the association between dichotomous variables and hypocalcemia. Variables found to be significant on univariate analysis were entered into a multivariate logistic regression model to determine the independent risk factors for hypocalcemia. A p-value of <0.05 was regarded to be statistically significant.
3 RESULTS
Table 1 shows some of the basic demographic and clinical characteristics of the participants. There were more males than females with a ratio of 1.5:1 and hypertension was the leading etiology of end-stage renal disease.
| Parameter | Number (%) |
|---|---|
| Sex | |
|
80 (60.8) |
|
51 (39.2) |
| Age (years) | |
| Mean age = 48.8 ± 14.8 | |
|
41 (31.3) |
|
62 (47.3) |
|
28 (21.4) |
| Body mass index (kg/m2); Mean = 30.3 ± 7.5 | |
|
2 (1.5) |
|
30 (23.0) |
|
29 (22.0) |
|
38 (29.0) |
|
17 (13.0) |
|
15 (11.5) |
| Etiology of end-stage renal disease | |
|
36 (27.4) |
|
32 (24.4) |
|
20 (15.2) |
|
7 (5.3) |
|
7 (5.3) |
|
6 (4.6) |
|
4 (3.1) |
|
3 (2.3) |
|
3 (2.3) |
|
2 (1.5) |
|
3 (2.3) |
|
1 (0.8) |
|
7 (5.6) |
- Abbreviation: IgA, Immunoglobulin A.
- a Others—Dent's disease, anti-Glomerular Basement Membrane Disease, HIV-Associated Nephropathy, Thin Basement Membrane Disease, bilateral nephrectomy, AKI from septic abortion, recurrent pyelonephritis- 0.8% each.
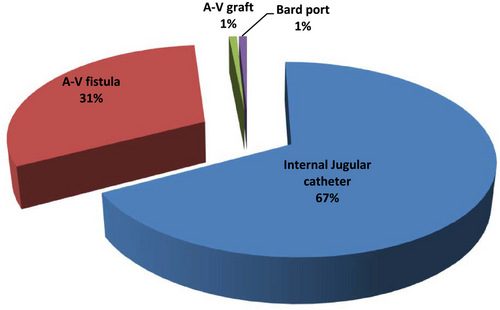
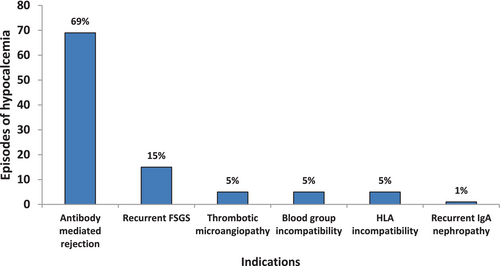
Table 2 shows some of the procedure characteristics of the apheresis sessions. Internal jugular catheters were utilized as vascular access in majority of the patients as shown in Figure 1. Indication choices for Therapeutic apheresis are as shown in Figure 2 and illustrates that antibody-mediated rejection was the leading indication for TA.
| Parameters | Number (%) |
|---|---|
| Replacement fluid | |
|
58 (44.3) |
|
51 (38.9) |
|
22 (16.8) |
| Replacement volume | |
|
68 (52) |
|
63 (48) |
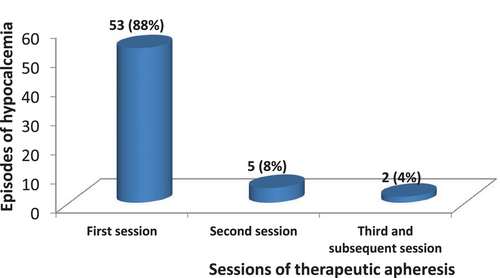
A total of 860 sessions of therapeutic apheresis for transplant indication were recorded within the study period among 131 patients, giving an average of seven sessions per patient. The number of sessions by each patient ranged from 2 to 48 sessions. Overall, there were 60 (7%) TA sessions complicated by hypocalcemia. Figure 3 shows the occurrence of hypocalcemic episodes in the different sessions of TA. It shows that out of the 60 episodes, 53 (88%) occurred in the first session, 5 (8%) occurred in second session while 2 (4%) occurred in the third and subsequent sessions.
The starting mean serum ionized calcium of patients with hypocalcemia was lower than those of patients without hypocalcemia (4.6 ± 0.3 vs. 4.80 ± 0.4); the difference was not statistically significant, t = 1.72, p = 0.092.
There was a weak negative correlation between serum creatinine at initiation of TA and serum ionized calcium and the middle of TA (r = − 0.025) and end of TA (r = − 0.12). These were not statistically significant; p = 0.78 and 0.19, respectively.
Patients with hypocalcemia were compared across different variables of interest (Table 3) to obtain the risk factors for hypocalcemia. The table showed that female sex, elevated serum creatinine and use of thawed fresh frozen plasma-containing fluids are the risk factors for hypocalcemia with odd's ratio of 2.34, 7.42, and 5.01, respectively. These variables entered in a binary logistic regression model to obtain the independent risk factors for hypocalcemia (Table 4). Only elevated serum creatinine at the commencement of therapy was found as an independent predictor of hypocalcemia adjusted odd's ratio (AOR) = 3.31 (95% CI; 1.044–5.321; p = 0.001).
| Hypocalcemia | ||||||
|---|---|---|---|---|---|---|
| N (%) | ||||||
| Parameter | Yes | No | Chi-square | OR | 95% CI | p-Value |
| 53 (40.5) | 78 (59.5) | |||||
| Sex | ||||||
|
27 (20.6) | 24 (18.3) | 5.4 | 2.34 | 1.14–4.81 | 0.02* |
|
26 (19.8) | 54 (41.2) | ||||
| Age (years) | ||||||
|
14 (10.7) | 19 (14.5) | 0.07 | 1.12 | 0.50–2.48 | 0.79 |
|
39 (28.8) | 59 (45.0) | ||||
| Body mass index (kg/m2) | ||||||
|
24 (18.3) | 377 (28.2) | 0.06 | 0.92 | 0.46–1.85 | 0.81 |
|
29 (22.1) | 40 (31.3) | ||||
| Elevated serum creatinine | ||||||
|
48 (36.6) | 44 (41.2) | 17.6 | 7.42 | 2.66–20.65 | 0.001* |
|
5 (3.8) | 34 (18.3) | ||||
| FFP-containing replacement fluid | ||||||
|
43 (32.8) | 36 (27.5) | 16.13 | 5.01 | 2.21–11.38 | 0.001* |
|
10 (7.6) | 42 (32.1) | ||||
| Replacement volume (ml) | ||||||
|
37 (28.2) | 43 (32.8) | 2.86 | 1.88 | 0.90–3.93 | 0.09 |
|
16 (12.2) | 35 (26.7) | ||||
- Abbreviation: OR, odds ratio.
- * p < 0.05.
| 95% CI for EXP (B) | |||||
|---|---|---|---|---|---|
| Variables | Exp (B) | Significance | Lower | Upper | |
| Step 1 | Plasma containing fluids | 0.501 | 0.054 | 0.310 | 1.002 |
| Elevated serum creatinine | 3.310 | 0.001 | 1.044 | 5.231 | |
| Female sex | 0.606 | 0.520 | 0.312 | 1.660 | |
- Abbreviations: CI, confidence interval; ExpB, Odd's ratio.
4 DISCUSSION
Our study shows that the prevalence of hypocalcemia is high among the cohort of patients commencing apheresis; female sex, elevated serum creatinine and use of fresh frozen plasma as are the risk factors for hypocalcemia while elevated serum creatinine is an independent predictor of hypocalcemia.
The prevalence of hypocalcemia in this study is high compared to an earlier study in Pakistan [10]. This could be due to differences in the patient population studied. The study in Pakistan had a heterogeneous mix of patients. Our study is based on patients with end-stage renal diseases in the pre-transplant phase and those at the post-transplant phase. Hypocalcemia is one of the complications of end-stage renal disease due to hypovitaminosis D and this biochemical perturbation can lower the ability of renal patient to respond to rapid changes in blood calcium level in other to restore normalcy; which otherwise other patients can easily adjust to [14]. Vitamin D enables absorption of calcium from the gastro-intestinal tract, bones and the renal tubules into the blood. Its absence can result to low levels of serum calcium. Although we excluded patients with baseline hypocalcemia from the study, those with normal calcium may have a neuro-hormonal disturbance caused by the end-stage renal disease. Second, most of our study participants commenced therapeutic apheresis as hospitalized patients and hypocalcemia has been demonstrated to be one of the leading electrolyte abnormalities in hospitalized patients and associated with in-hospital mortality risk of up to 48% [15-17].Papari et al. has reported persistent hypocalcemia associated with therapeutic apheresis for HLA antibody desensitization among three cardiac transplant recipients, but no significant manifestation among six lung transplant recipients who underwent therapeutic apheresis within the same period [18]. There is no obvious explanation for this difference in outcome and more studies are needed in transplant clime on the occurrence and magnitude of hypocalcemia.
Our data showed that the likelihood of having hypocalcemia is three times greater among patients with elevated serum creatinine at the start of apheresis than those with lower or normal serum creatinine. Some studies have demonstrated a significant inverse relationship between serum calcium and creatinine in hematopoietic transplant patients treated with foscarnet for cytomegalovirus infection [19, 20]. This may explain why serum creatinine is one of the risk factors of hypocalcemia in this study. Serum creatinine is inversely related to kidney function and the biochemical and physiological process controlling calcium balance is complex particularly in patients with kidney dysfunction [21].Ionized calcium (iCa+) is the physiologically active form of calcium and its normal range is 4.5–5.6 mg/dL [22]. For each session of apheresis, there is an 18% reduction in ionized calcium, net loss of calcium of up to 150 mg and a secondary increase in parathyroid hormonal level to 280% of its initial level [23]. The released PTH physiologically acts on the renal distal convoluted tubules and collecting ducts directly increasing calcium re-absorption and reducing phosphate excretion at the proximal convoluted tubules. The increased phosphate ions in the serum form insoluble salts with serum calcium, contributing hypocalcemia [24]. This chain of events require an intact kidneys, not a fibrotic kidneys (as in end-stage renal disease) or kidneys undergoing transplant rejection or recurrent disease as in the post-transplant kidneys in this study. Hence hypocalcemia may not get corrected quickly and may predispose a patient to hypocalcemia. Our study however, has made an important finding which has clinical implications in vigilance and individualizing apheresis to patients with elevated serum creatinine at start of apheresis.
Theoretically, hypocalcemic toxicity in apheresis has been attributed to use of citrate as anticoagulant and use of fresh frozen plasma as a replacement fluid. Citrate is used as anticoagulant in some apheresis procedures as it reversibly binds calcium (and other divalent cations such as magnesium) and removes it from circulation, thereby inhibiting clotting in the apheresis circuit [25]. There is individual variability in the metabolism of citrate [25]. Patients with renal insufficiency has impaired metabolism of citrate and may be at increased risk of citrate toxicity including hypocalcemia [26, 27]. Use of fresh frozen plasma as replacement fluid during apheresis increases the risk of hypocalcemic toxicity [27]. It contains 15% of citrate and is associated with a 4.8% incidence of hypocalcemic symptoms [9, 28]. Hence, the finding of our study is not surprising and calls for more vigilance when plasma is used as replacement fluid.
Some studies have identified female sex as a risk factor for hypocalcemia among patients who underwent sub-total thyroidectomy [29-31]. We found this observation among our renal patient cohort with female sex having twice the odds of developing hypocalcemia than their male counterparts. This study is comparable to earlier studies in reporting higher prevalence of adverse events in the first session of apheresis compared to the second and subsequent sessions. [32] Technical measures are usually taken to avoid repeat episodes of hypocalcemia in patients so affected at the earlier episodes and this can account for reduced prevalence of hypocalcemia in subsequent episodes.
4.1 Clinical relevance of the study
The most common adverse event in apheresis procedure is related to citrate toxicity especially hypocalcemia [33]. Since there is considerable individual variability in development of hypocalcemia, the risk factors for hypocalcemia need to be identified. This is especially important as this study shows that most episodes of hypocalcemia occurred in the first session of TA. Identification of these risk factors (clinical and technical) is valuable in personalizing therapy session and to avert advert consequences related to hypocalcemia such as seizures and cardiac arrhythmias and possibly cardiac arrest [25].
4.2 Study limitations
Our study has two limitations that call for caution in interpretation of its findings. All categories of patients undergoing apheresis for transplant indications were studied. There are some considerable differences related to parathyroid hormone metabolism in the pre- and post-transplant period that may have implication in the serum levels of ionized calcium. Second, we did not assess for metabolic alkalosis. Due to reduced bicarbonate excretion related to renal impairment and sodium citrate used as anticoagulant, some patients may develop metabolic alkalosis [34, 35]. Metabolic alkalosis increases calcium binding to albumin and decreases ionized calcium causing hypocalcemia.
5 CONCLUSION
Hypocalcemia is prevalent among this cohort of patients undergoing apheresis for kidney transplant indications. Female sex, elevated serum creatinine and use of fresh frozen plasma are the risk factors while elevated serum creatinine is independent risk factor in our cohort. With a baseline physiological derangement in the end-stage renal disease milieu which predisposes to hypocalcemia, the findings may help physicians increase their clinical vigilance and closely monitor these categories of patients for possible adverse events related to hypocalcemia.
FUNDING INFORMATION
This work was funded in major part by the International Society of Nephrology Fellowship grant to Chimezie Godswill Okwuonu.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




