Characterization of integrins in cellular immunity of the oriental armyworm, Mythimna separata
Abstract
Insect hemocytes eliminate foreign substances from the hemocoel through various immune reactions. Integrins, receptor proteins present on the cell membrane, are formed as a heterodimer from α and β subunits and are known to be involved in various immune reactions. To elucidate the role of integrins in the immunity of the lepidoptera Mythimna separata, genes encoding integrins were screened from the genome, resulting in the identification of eight α and four β integrin genes. The expression levels of the integrin genes did not change in response to the injection of small abiotic beads undergoing phagocytosis in M. separata larvae. However, significant inductions of some integrin gene expressions were observed in hemocytes that formed capsules around large abiotic beads during encapsulation, especially in MysIntα2. Under biotic stimulation, induction of the MysIntα2 was evident after exposures to Gram-negative bacteria (Escherichia coli) and entomopathogenic nematodes (Steinernema carpocapsae), but not to Gram-positive bacteria (Micrococcus luteus). Immunostaining analysis revealed that MysIntα2 was specifically localized to hemocytes surrounding the beads during the encapsulation reaction. Furthermore, the spreading and encapsulation abilities of hemocytes were significantly inhibited by incubation with MysIntα2 antibodies. Suppression of MysIntα2 expression in M. separata larvae by injecting double-stranded RNA also resulted in a decrease in encapsulation activity. Collectively, these results indicate that MysIntα2 plays pivotal roles in the cellular immune response of M. separata, particularly during encapsulation. This likely occurs through the regulation of hemocyte spreading activity, thereby facilitating the formation of multilayered capsules around large invaders.
Introduction
Insects utilize hemocytes as a direct defense mechanism against pathogens. In Lepidoptera larvae, there are five typical types of hemocytes: granulocytes. plasmatocytes, prohemocytes, oenocytoids, and spherulocytes. Among these, plasmatocytes and granulocytes, as adhering hemocytes, are considered crucial for cellular defense (Lavine & Strand, 2002; Ribeiro & Brehélin, 2006). The cellular defense reactions encompass phagocytosis, nodulation, and encapsulation (Dubovskiy et al., 2016). Phagocytosis and nodulation serve to scavenge small foreign substances, such as bacteria and fungi (Hillyer, 2015). Encapsulation, on the other hand, involves the formation of overlapping capsules by hemocytes around larger invaders, such as nematodes and parasitoids, which cannot be phagocytosed by a single hemocyte (Dubovskiy et al., 2016). Activation of such immune reactions is thought to be dependent on the recognition of conserved pathogen-associated molecular patterns (PAMPs) present on the surface of pathogens or released by pathogens, through cellular and humoral pattern-recognition receptors (PRRs) (Lavine & Strand, 2002). Integrins, which are among the most prevalent proteins found in all metazoans, are PRRs with crucial adhesion and signaling roles in insect cellular immunity (Hynes, 2002; Kausar et al., 2022).
Integrins are type I transmembrane glycoproteins located on the cell surface (Tamkun et al., 1986; Hynes, 2002). They exist as heterodimers with noncovalently bound α and β subunits, each comprising an extracellular domain, a transmembrane domain, and a cytoplasmic domain (Burke, 1999; Hughes, 2001). The extracellular domains of both integrin subunits are responsible for binding to various extracellular ligands, with α integrins particularly crucial for ligand specificity (Humphries et al., 2006; Campbell & Humphries, 2011; Lv et al., 2020). Meanwhile, the cytoplasmic domain of β integrins can bind with cytoskeletal proteins, facilitating their connection to actin and resulting in altered cellular morphology (Blystone, 2004). Through this ligand-binding mechanism, integrins transduce signals in a bidirectional manner, thereby mediating not only immune processes but also developmental pathways (Hynes, 2002; Giancotti, 2003).
Integrin functions related to cellular immunity have been studied in both lepidopteran and dipteran insects. In Bombyx mori, two β integrins, β1 and β3, were reported to directly bind to bacteria and enhance the phagocytic activity of hemocytes (Zhang et al., 2017; Li et al., 2021a). Knockdown of four integrin genes (α1, α2, α3, and β1) in Manduca sexta through specific dsRNA injection resulted in hemocytes losing their ability to encapsulate diethylaminoethyl (DEAE) Sephadex beads (Levin et al., 2005; Zhuang et al., 2008). Furthermore, in Drosophila melanogaster, the deletion of a β integrin gene, βPS, resulted in lamellocytes (equivalent to plasmatocytes in lepidopteran insects) failing to form encapsulation capsules around parasitoid wasp larvae (Irving et al., 2005).
The oriental armyworm, Mythimna separata, a severe pest in certain crops, is commonly used as a model organism to study insect immune responses (Ishihara et al., 2017; Yokoi et al., 2018; Li et al., 2021b), and the interaction between invading parasitoids and the host immunity (Yamashita et al., 2019; Sawa et al., 2021; Schwier et al., 2021). Despite the potential central roles of integrins in recognizing foreign substances and regulating immune cell dynamics, the precise mechanism of their involvement in these immune responses is yet to be fully elucidated. Therefore, this study aimed to identify all members of the integrin family coded in the genome of M. separata and elucidate the function of one of these integrins, presumed to be involved in immunity, in defense against the intrusion of foreign substances.
Materials and methods
Experimental insects
Mythimna separata was reared at the Laboratory of Applied Entomology and Zoology, University of Tsukuba. The larvae were fed an artificial diet, Silkmate (Nihon Nosan Kogyo, Yokohama, Japan), and maintained under controlled conditions of 23 ± 3 °C temperature, 40%–60% humidity, and L 16: D 8 photoperiod. All experiments were conducted using sixth instar larvae.
Screening of integrin family genes from reference genome sequences of M. separata
Potential genes encoding α and β integrins were obtained from functional annotations of M. separata genes (https://doi.org/10.6084/m9.figshare.21257625). Domain prediction was conducted to confirm the potential integrins, utilizing the Pfam (protein family database) (http://pfam.sanger.ac.uk/) and SMART (simple modular architecture research tool) tools (http://smart.embl-heidelberg.de). The presence of signal peptide was predicted using SignalP 3.0 (https://services.healthtech.dtu.dk/services/SignalP-3.0/). The TMHMM (TransMembrane prediction using Hidden Markov Models) program (http://www.cbs.dtu.dk/services/TMHMM/) was used to assess the presence or absence of the transmembrane region. The results of assembling the conserved domains were plotted using an online IBS (illustrator of biological sequences) tool (http://ibs.biocuckoo.org/online.php) (Liu et al., 2015).
Phylogeny analysis of integrins
The CLUSTAL W program was used to align the amino acid sequences of integrins from M. separata, B. mori, D. melanogaster, Homo sapiens, and other species. The neighbor-joining method with 1000 bootstrap replicates was used to create phylogenetic trees for α and β integrins using the MEGA (molecular evolutionary genetics analysis) 11.0 program (Tamura et al., 2021), followed by online editing via the iTOL tool (http://itol.embl.de/) (Letunic & Bork, 2021). The GenBank accession numbers of integrin genes to construct the phylogenetic tree were listed in Table S1.
cDNA cloning of integrin genes
Total RNA was extracted from the hemocytes of M. separata using the Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA). SuperScript IV Reverse Transcriptase (Thermo Fisher Scientific) was used to synthesize cDNA after the remaining genomic DNA was removed using RQ1 RNase-Free DNase (Promega, Madison, WI, USA). Partial cDNA sequences for M. separata integrin genes were amplified using EmeraldAmp MAX PCR Master Mix (Takara Bio Inc., Shiga, Japan) through polymerase chain reactions (PCR). Each integrin gene's PCR product was introduced into the pMD20 T-vector (Takara Bio Inc., Shiga, Japan), and their accuracy was confirmed through nucleotide sequencing with an ABI 3130 genetic analyzer (Applied Biosystems, Foster City, CA, USA). Primer sets for reverse transcription-quantitative real-time PCR (RT-qPCR) were listed in Table S2.
Gene expression analysis in cellular immune responses
Small polystyrene beads (φ 1 μm, Polysciences, PA, USA), serving as targets for phagocytosis, were suspended in phosphate-buffered saline (PBS) and injected into the hemocoel of ice-anesthetized larvae. After 30 min, the hemocytes were collected. Larvae that received PBS injections were prepared as controls. As targets of encapsulation, large polystyrene beads (φ 600 μm) were implanted from the first abdominal leg of ice-anesthetized larvae, after which the leg was promptly secured with thread to avoid hemolymph leakage (Yamashita et al., 2019). The larvae were dissected 24 h after transplantation, and the encapsulated beads and circulating hemocytes were collected separately. As controls, circulating hemocytes from naïve larvae or mock-injected larvae (where the first abdominal was cut and tied with thread immediately, without the implantation of polystyrene beads) were prepared. For the pathogen treatment, Gram-negative bacteria (Escherichia coli, 107 cells) and Gram-positive bacteria (Micrococcus luteus, 107 cells) were both individually suspended in 10 μL of PBS. The suspension was then injected into larvae, and circulating hemocytes, including phagocytosing hemocytes, were collected from the larvae at 2 h, 12 h, and 24 h post-injection. Additionally, in the nematode treatment, 100 individuals of entomopathogenic nematode (Steinernema carpocapsae) suspended in 10 μL PBS, were similarly injected into larvae as targets for encapsulation. Hemocytes forming encapsulation capsules around nematodes were retrieved separately 24 h postinjection. Each treatment was performed in four replicates. The PBS group served as a control.
Total RNA extraction and cDNA synthesis were performed according to the previously described procedure. RT-qPCR was used to detect the integrin transcriptions in circulating hemocytes as well as in the hemocytes that formed capsules around the polystyrene beads and nematodes. The RT-qPCR procedures used a Thermal Cycler Dice Real Time System (Takara Bio Inc., Shiga, Japan) with Luna Universal qPCR Master Mix (New England Biolabs Japan Inc., Tokyo, Japan) under the following parameters: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and 60 °C for 30 s. As a reference gene, the housekeeping gene rp49 of M. separata (RpL32, accession number: AB669190) was employed (Yamaguchi et al., 2012). The 2−∆∆Ct method was utilized to analyze the relative expression abundance of the integrin genes (Livak & Schmittgen, 2001).
Production of polyclonal antibody against MysIntα2
A polyclonal antibody was produced against a partial peptide sequence for MysIntα2 “CQFDKNPRNNEDRQGRPVD” (amino acids 83 to 100) of M. separata (hereinafter referred to as “MysIntα2 antibody”) by immunizing a rabbit (Cosmo Bio Co., Ltd., Tokyo, Japan).
The specificity for the antibody against MysIntα2 was confirmed by Western blot analysis using recombinant MysIntα2, which was produced by the E. coli expression system. A gene fragment of MysIntα2 (encoding amino acids 18 to 440) was inserted into the pET22b(+) expression vector (Merck KGaA). The expression vector was used for the transformation of E. coli BL21 competent cells (Takara Bio Inc., Shiga, Japan), which were cultured for 14 h at 37 °C in Overnight Express medium (Merck KGaA). Recombinant MysIntα2 fused with a 6× His-tag was purified with Ni-NTA agarose (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) following the manufacturer's instructions. For Western blot analysis, the protein of intact E. coli BL21 (DE3) cells and the recombinant MysIntα2 (2 μg each) were loaded onto a 7.5% sodium dodecyl sulfate-polyacrylamide gel (TGX FastCast Acrylamide Kit, 7.5%, Bio-Rad, USA). The separated proteins were electronically transferred onto a polyvinylidene difluoride membrane (Merck Millipore Ltd., Ireland). The membrane was incubated with the MysIntα2 antibody (1.: 5000) as the primary antibody, and alkaline phosphatase-conjugated affinipure goat anti-rabbit IgG(H+L) (1: 5000) (Cosmo Bio Co., Ltd) as the secondary antibody. The protein band that reacted with the antibody was detected using a CDP-Star chemiluminescent substrate (Roche Diagnostics GmbH, Mannheim, Germany).
Immunofluorescence staining analysis
Polystyrene beads (φ 600 μm) were transplanted to M. separata larvae, followed by the collection of circulating hemocytes and encapsulated beads from each larva 24 h post-transplantation. Beads encapsulated by hemocytes were fixed in MeOH in microtubes for 15 min on ice. Sections of encapsulated beads were prepared by embedding in Tissue-Tek O.C.T. Compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan), and sliced into 15 μm sections using a CM1860 UV cryostat (Leica Biosystems, Germany). Circulating hemocytes were incubated on a slide for 30 min and fixed with MeOH. Intact encapsulation capsules, sections of the encapsulation capsules, and fixed hemocytes were blocked for 1 h at 25 °C with 1% BSA-containing Phosphate Buffered Saline (PBST; 0.1% Tween-20 in PBS, pH 7.4) and then treated with 0.1% MysIntα2 antibody in PBST as the primary antibody at 4 °C overnight and 0.1% Alexa Fluor 546 goat anti-rabbit IgG (H+L) (Thermo Fisher Scientific) in PBST as the secondary antibody at 25 °C for 1 h. Following three PBS washes, fluorescent signals were observed using Leica fluorescent microscopy (Leica Microsystems, Wetzlar, Germany).
In vitro encapsulation assay
For the in vitro encapsulation assay, 30 μL of hemolymph from M. separata larvae was mixed with 60 μL of either MysIntα2 antibodies solution or the solvent-only PBS containing 15 ppm ProClin 300 (Sigma-Aldrich, St. Louis, MO, USA), and incubated on ice with inverted mixing for 30 min. Subsequently, 10 μL of saturated 2-phenylthiourea and 100 polystyrene beads (φ 90 μm) were added. After 6 h, the beads were retrieved and examined using a Leica phase-contrast microscope (Leica Microsystems, Wetzlar, Germany) to determine whether they were encapsulated. Encapsulated beads are completely encapsulated by multilayer or one layer of hemocytes, whereas non-encapsulated beads are partially encapsulated or without any hemocytes adhering.
RNA interference (RNAi) assay
A DNA fragment of MysIntα2 (462 bp) was amplified and inserted into the T-vector pMD20 using the specific primers, MysIntα2 forward: 5′-GCTGCACTTAAAGGCTTGGTC-3′ and MysIntα2 reverse: 5′-GGTGGTCAGAGAGGGAGAGAT-3′. Sequences corresponding to the T7 polymerase promoter were added to the 5′ ends of both strands of the insert region by PCR with the following primer pair: T7-MCS Forward: 5′-taatacgactcactatagggTAGTCATATG-3′ and T7-MCS Reverse: 5′-taatacgactcactatagggCCGGGGATCC-3′ (lowercase indicates T7 RNA polymerase promoter sequences, and uppercase indicates pMD20 T-vector multiple cloning site sequences). Then, double-stranded RNA (dsRNA) was synthesized using the MEGAscript RNAi kit (Thermo Fisher Scientific) with each T7 promoter-tagged cDNA fragment as a template. As a negative control, a dsRNA fragment containing a partly enhanced green fluorescent protein (EGFP) sequence from the pEGFP plasmid (Clontech, Mountain View, CA, USA) was prepared by using primers (T7-EGFP forward: 5′-taatacgactcactatagggATGGTGAGCA-3′ and T7-EGFP reverse: 5′-taatacgactcactatagggTTACTTGTAC-3′). The synthesized dsRNA targeting MysIntα2 and EGFP were diluted to 500 ng/μL with the elution buffer and stored at −20 °C.
The dsRNA was injected into larvae that were on the second day of the sixth instar. After the larvae were anesthetized on ice, each larva received an injection of 10 μL (5 μg) of either MysIntα2 dsRNA or EGFP dsRNA into their hemocoel using a microinjector CellTram (Eppendorf, Hamburg, Germany) equipped with a glass capillary needle. After injection, the larvae were reared normally as previously described. After 48 h, approximately 150 polystyrene beads (φ 90 μm) suspended in 10 μL PBS were injected into each dsRNA-injected larva. Circulating hemocytes were collected from individual larvae 2 h after the bead treatment to assess MysIntα2 expression level through RT-qPCR. The injected beads were recovered to calculate the degree of encapsulation using a Leica phase-contrast microscope.
Statistical analysis
GraphPad Prism 9 Software (GraphPad Software, San Diego, CA, USA) was used to analyze all the data, and one-way analysis of variance (ANOVA) or the t-test was used to determine any significant differences. The results are represented as the standard error of the mean (SEM) ± mean (n = 4). Significant differences of P < 0.05 and P < 0.01 were marked by a single asterisk (*) and a double asterisk (**), respectively.
Results
Identification of integrin family members in M. separata
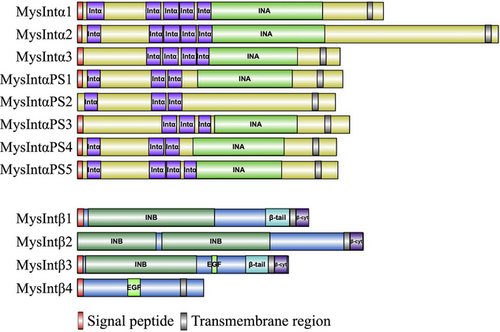
Based on reported criteria, α integrins typically possess conserved domains on their extracellular region with 2–5 integrin alpha repeats (Intα) and an integrin_alpha2 (INA, PF08441) domain, whereas β integrins contain an extracellular integrin_beta (INB, PF00362) domain, and both α and β integrins encompass a transmembrane region (Barczyk et al., 2010; Lv et al., 2020). We screened α and β integrins from the M. separata genome published by Yokoi et al. (2022). As a result, 12 integrins, comprising eight α subunits and four β subunits were found, correspondingly designated MysIntα1–MysIntαPS5 and MysIntβ1–MysIntβ4 (Table 1). Consistent with integrin structures found in other species, all M. separata integrin proteins contained three major domains: a large extracellular region, a transmembrane region, and a short cytoplasmic region (Fig. 1). The extracellular domains of α integrins contain 3–5 Intα domains and a conserved INA domain (except MysIntαPS2), whereas all the β integrins were predicted to have conserved extracellular INB domains (except MysIntβ4). Additionally, in the cytoplasmic region, all M. separata α integrins harbored a conserved GF[F/L]XR motif at the C-terminus (Fig. 2). In addition, the C-terminus of all M. separata β integrins (except MysIntβ4) harbored two conserved NPX[Y/F] motifs (Fig. 2). Collectively, these findings suggest that the mechanisms of signaling and self-activation participated in by M. separata integrins are highly conserved.
| Subfamily | Target ID | Gene name | Accession number (DDBJ) | #Contig | Position | No. of exons | Number of amino acid residues | Molecular weight (KDa) |
|---|---|---|---|---|---|---|---|---|
| α | anno2.g19709.t1 | MysIntα1 | LC806181 | 1321 | 448535–578364 | 17 | 1122 | 123.95 |
| anno2.g17869.t1 | MysIntα2 | LC806182 | 1258 | 166595–300338 | 25 | 1543 | 165.8 | |
| anno1.g21398.t1 | MysIntα3 | LC806183 | 1339 | 2947334–2969571 | 21 | 963 | 106.05 | |
| anno2.g22497.t1 | MysIntαPS1 | LC806184 | 79 | 802056–822392 | 25 | 973 | 110.02 | |
| anno2.g2142.t1 | MysIntαPS2 | LC806185 | 1337 | 68418–106292 | 24 | 946 | 106.7 | |
| anno2.g22502.t1 | MysIntαPS3 | LC806186 | 79 | 884565–913147 | 25 | 998 | 111.6 | |
| anno2.g22498.t1 | MysIntαPS4 | LC806187 | 79 | 833146–860012 | 24 | 950 | 106.31 | |
| anno2.g22496.t2 | MysIntαPS5 | LC806188 | 79 | 776125–799116 | 25 | 955 | 107.03 | |
| β | anno1.g7818.t1 | MysIntβ1 | LC806189 | 22 | 98079–114343 | 17 | 848 | 92.95 |
| anno2.g16643.t1 | MysIntβ2 | LC806190 | 1325 | 1657349–1676515 | 13 | 1048 | 116.62 | |
| anno1.g2373.t1 | MysIntβ3 | LC806191 | 1325 | 1688576–1695965 | 8 | 774 | 86.42 | |
| anno2.g16645.t1 | MysIntβ4 | LC806192 | 1325 | 1704483–1758023 | 8 | 463 | 51.45 |

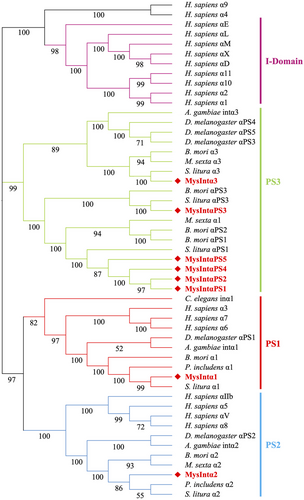
Phylogenetic analysis of integrins in M. separata
To explore the evolutionary relationship of integrins among M. separata and other species, phylogenetic trees were built for α and β integrins separately. α integrins are usually divided into four distinct groups: the PS1 and PS2 groups, which exist in both vertebrates and invertebrates; the I-DOM group, found only in vertebrates; and the PS3 group, which is exclusive to insects. Eight M. separata α integrins were distinctly classified into PS1, PS2, and PS3, as observed in other insects (Fig. 3). MysIntα1 in the PS1 group was closely related to Spodoptera litura α1 (identity: 96.17%), P. includens α1 (identity: 91.45%), and B. mori α1 (identity: 76.79%). MysIntα2 belonged to the PS2 and was closely related to P. includens α2 (identity: 83.39%), S. litura α2 (identity: 75.50%), and B. mori α2 (identity: 52.43%). The other six α integrins were all classified into the PS3 group. MysIntα3 shared 70.20% identity with S. litura α3, and MysIntαPS3 shared 46.20% identity with S. litura αPS3 and 32.48% with B. mori αPS3. MysIntαPS1, MysIntαPS2, MysIntαPS4, and MysIntαPS5 were clustered into the same branch and were closely related to S. litura αPS1, with 49.74%, 46.82%, 47.32%, and 49.95% shared identity, respectively (Fig. 3).
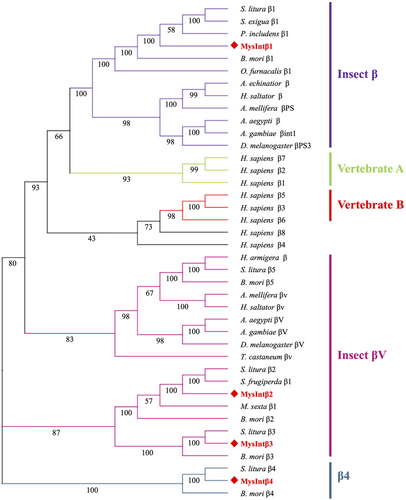
In the phylogenetic tree constructed by Hughes (2001), the β integrins were divided into invertebrate and vertebrate branches, with the vertebrates further divided into two sub-branches: vertebrate A and vertebrate B. β integrins in invertebrates exhibit more obvious phylum-lineage features, such as insect βV, early metazoan β, chordate β, and protostome β, among others (Johnson et al., 2009; Lv et al., 2020). In addition, β integrins from different insect orders have been subdivided into insect β, insect βV, and β4 (Zhang et al., 2014b; Chen et al., 2023). The phylogenetic analysis showed that four β integrins in M. separata were segregated into three groups (Fig. 4). MysIntβ1 was clustered into the insect β group and was closely related to B. mori β1 (identity: 82.90%). MysIntβ2 and MysIntβ3 were clustered with the insect βV. MysIntβ4 was related to S. litura β4 (identity: 56.87%) and B. mori β4 (identity: 29.98%), which were clustered together in the β4 group as a specific branch (Fig. 4).
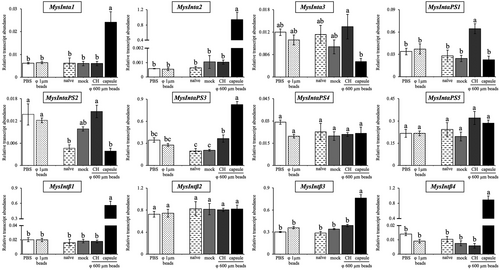
Integrin gene expressions against abiotic stimulation
To investigate the involvement of M. separata integrins in immune responses, a gene expression analysis of integrins was conducted using RT-qPCR. To collect phagocytosed hemocytes, small polystyrene beads (φ 1 μm) were injected into the hemocoel of larvae. After 30 min, the larval hemocytes were collected, with nearly all granulocytes having phagocytosed the beads. However, expressions of any integrin genes were not affected by the treatment (Fig. 5). On the other hand, expression levels of many integrin genes were altered in hemocytes forming encapsulation capsules and circulating hemocytes retrieved from the larvae 24 h after transplantation of large beads (φ 600 μm). Expressions of MysIntα1, MysIntα2, MysIntαPS3, MysIntβ1, MysIntβ3, and MysIntβ4 were significantly increased specifically in hemocytes forming encapsulation capsules of the large beads, though their expressions in the circulating hemocytes were unaffected. On the contrary, MysIntαPS1 was highly expressed in circulating hemocytes, despite the lack of change in hemocytes forming capsules. In addition, MysIntα3 showed a decreasing trend in hemocytes forming capsules, and MysIntαPS2 exhibited increased trends in circulating hemocytes from mock-injected and large beads-transplanted larvae. Expression levels of MysIntαPS4, MysIntαPS5, and MysIntβ2 were not affected by any treatments.
MysIntα2 expressions against biotic stimulation
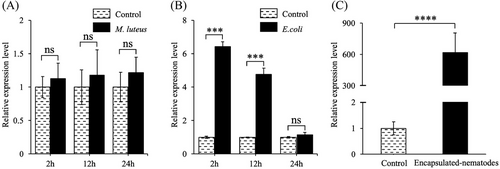
Since the highest increase in expression was seen in MysIntα2, we examined its expression pattern following infection by M. luteus (Gram-positive bacteria), E. coli (Gram-negative bacteria), and pathogenic nematode S. carpocapsae. Although both M. luteus and E. coli were phagocytosed by hemocytes, MysIntα2 expression in hemocytes was only induced by E. coli, with a 6.5 fold increase at 2 h and a 4.5 fold increase at 12 h, followed by a return to normal levels at 24 h (Fig. 6B). Notably, MysIntα2 was highly expressed in hemocytes involved in the encapsulated S. carpocapsae, with about a 600 fold increase, displaying a similar expression pattern as the φ 600 μm beads treatment, in contrast to the PBS injection (Fig. 6C).
Localization of hemocytes expressing MysIntα2
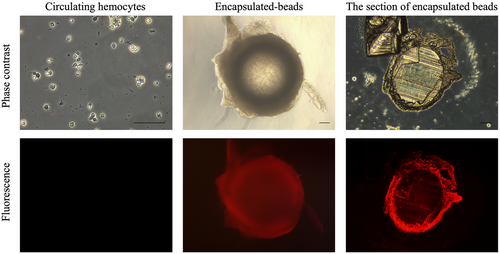
To investigate the localization of MysIntα2 in the capsules formed around large beads in M. separata larvae, an antibody against MysIntα2 was generated, and its specificity was confirmed by Western blot analysis against recombinant MysIntα2 expressed in E. coli (Fig. S1). As shown in Fig. 7, fluorescent signals indicating the localization of the MysIntα2 antibody were detected on the hemocytes around the encapsulated beads ubiquitously, whereas almost no fluorescence signals were observed in circulating hemocytes. To further understand the specific position of MysIntα2 within the capsule, frozen sections of encapsulated polystyrene beads (φ 600 μm) were prepared and subjected to immunofluorescence analysis. Strong fluorescence signals were observed in all layers of encapsulation capsules surrounding the beads.
Effect of MysIntα2 on encapsulation process in the in vitro and in vivo conditions
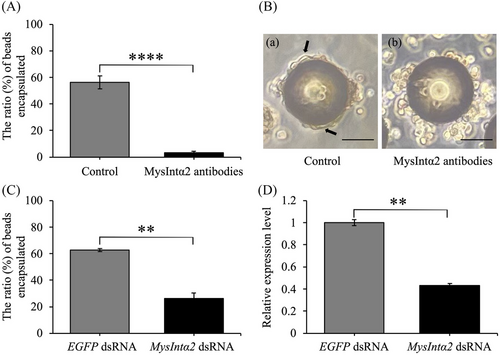
In the in vitro condition, MysIntα2 antibodies had a remarkable effect in nearly entirely inhibiting the encapsulation process (Fig. 8A). The control group's hemocytes attached to the polystyrene beads (φ 90 μm) and flattened out to encapsulate them (Fig. 8B-a). Over half of the beads were enclosed by hemocytes, with most of these encapsulations made up of multiple layers of hemocytes. For hemocytes to develop a capsule, they must undergo a distinct transition from roughly nonadherent spherical cells to adherent flat cells (Tanaka, 1987; Schmidt et al., 2001). However, when hemocytes were incubated with MysIntα2 antibodies before they were mixed with the beads, although some hemocytes adhered to the beads, they were unable to change their shape (Fig. 8B-b). Only a small number of beads were surrounded by two or three layers of hemocytes. In the in vivo encapsulation assay, 62.80% ± 1.08% of the beads (φ 90 μm) retrieved from the larvae injected with EGFP dsRNA were encapsulated (Fig. 8C). In contrast, the knockdown of MysIntα2 drastically reduced the rate of encapsulation, with only 26.04% ± 4.39% of beads encapsulated (Fig. 8C). Since RT-qPCR verified the effective reduction of MysIntα2 transcripts in hemocytes (Fig. 8D), suggesting that MysIntα2 dsRNA-induced inhibition of MysIntα2 expression reduced the hemocytes' capacity to encapsulate. These results suggested that MysIntα2 influenced the morphological changes of hemocytes, which are important aspects of their adhesion and spreading activities that lead to the formation of multilayered encapsulation capsules.
Discussion
In this study, we identified eight α and four β subunits based on the conserved domains of integrins encoded in the M. separata genome. The number of integrins in M. separata is comparable to that found in other insects, such as D. melanogaster with five α and two β subunits, B. mori with six α and five β subunits, and S. litura with five α and five β subunits (Narasimha & Brown, 2013; Zhang et al., 2014b; Chen et al., 2023). All M. separata integrins, like integrins of other species (Johnson & Chouhan, 2014; Zhang et al., 2014b), possessed extracellular, transmembrane, and cytoplasmic domains. The extracellular domain includes conserved domains such as INA/Intα in α integrins and INB in β integrins (except MysIntβ4). The INA and INB domains are essential for maintaining the ligand-binding activity of integrins and mediating important signaling pathways and cellular processes (Campbell & Humphries, 2011). All M. separata α integrins contained a GF[F/L]XR motif, and almost all M. separata β integrins (MysIntβ1–MysIntβ3) have a HDRK motif in each of their respective cytoplasmic regions. For the heterodimer of H. sapience αIIb and H. sapience β3, a salt bridge between arginine (R) in the GF[F/L]XR motif and aspartate (D) in the HDRK motif is crucial for maintaining the inactive state (Hughes et al., 1996; Hynes, 2002; Vinogradova et al., 2002). MysIntβ1–MysIntβ3 also contained conserved motifs for NPX[Y/F], which is a typical phosphotyrosine binding site (PTB) associated with intracellular-to-extracellular signaling and integrin activation (Calderwood et al., 2002, 2003; Hynes, 2002). Therefore, integrins of M. separata, like those of other species, are likely to be localized on the cell membrane and regulate intracellular signaling through the binding of ligands to specific domains on the cell surface.
Based on the phylogenetic relationships analyzed for integrins in the human and insect genomes, the eight α integrins from M. separata were divided into PS1 (MysIntα1), PS2 (MysIntα2), and PS3 (MysIntα3, and MysIntαPS1–MysIntαPS5) groups. The α integrins within the PS1 and PS2 clusters aid in phagocytosis and encapsulation (Zhuang et al., 2008; Zhang et al., 2022a). The PS3 appears to be exclusive to insects, and integrins in this group, such as those found in B. mori, are hypothesized to be involved in the development of hemocyte and cellular immunity (Johnson et al., 2009; Zhang et al., 2014b). Regarding integrins belonging to the PS3 group, four M. separata α integrins in the PS3 group (MysIntαPS1, and MysIntαPS3–MysIntαPS5) shared similar structure and size, and the genes encoding these were located within 140 kb of the genome, suggesting that these α integrins were caused by gene duplication. The β integrins were categorized into insect β (MysIntβ1), insect βV (MysIntβ2 and MysIntβ3), and β4 (MysIntβ4) groups. In insects, the integrin β1, to which MysIntβ1 belongs, appears to be involved in physiological processes and is required for cellular immune responses (Kausar et al., 2022). MysIntβ2 and MysIntβ3 belong to the insect βV branch and are closely related to B. mori β2 and β3, respectively. B. mori β2 and β3, are exclusively expressed in plasmatocytes (Zhang et al., 2022b), and their expression levels increased by 20-hydroxyecdysone injections (Zhang et al., 2014b). B. mori β3 has been suggested to enhance the phagocytic activity of hemocytes through direct binding to bacteria (Zhang et al., 2017). The sequence and structure characteristics of the β4 group diverge significantly from other β integrin groups, forming a novel phylogenetic branch with S. litura β4 and B. mori β4. S. litura β4 showed heightened expression on the sixth day of the sixth instar larvae during testicular fusion (Chen et al., 2023), whereas the injection of 20-hydroxyecdysone upregulated the expression of B. mori β4 (Zhang et al., 2014b). Based on this information, it is evident that M. separata possess a diverse array of integrins, which potentially regulate not only the immune system but also various developmental processes.
While expression levels of some integrin encoding genes were significantly upregulated in hemocytes in response to the transplantation of large beads, no induction was seen in gene expression in any of the integrins in response to the injection of small beads. This phenomenon aligns with findings by Ishihara et al. (2017), who reported similar behavior in the expression of epl, a C-type lectin gene in M. separata. The phenomenon seen in integrin gene expression wherein inducibility changes with different sizes of the same surface molecule strengthens the hypothesis that M. separata can recognize foreign substances by not only the mechanism of the PAMPs-PRRs system but also by other unexplored (possibly size-recognition) mechanisms. Among integrin genes that induced their expression in hemocytes that participated in forming encapsulation capsules, MysIntα2 showed the highest relative expression compared to the controls. Thus, we focused on MysIntα2 and examined its expression levels in response to bacterial infection. Interestingly, the expression level of MysIntα2 was elevated in response to E. coli, even though it did not respond to small E. coli-sized beads. This suggests that MysIntα2 is not unresponsive to all small foreign substances, indicating a potential regulatory role of the PAMPs-PRRs system in its expression modulation.
Since integrins function by forming α-β heterodimers through coexpression of α and β integrin genes under stress or during development (Barczyk et al., 2010; Lv et al., 2020), co-expression of partner β integrin genes is required for MysIntα2 to work. Although the expressions of all β integrin genes in M. separata were not affected after small beads injection, co-expression of at least one β integrin with MysIntα2 may be necessary for situations where hemocytes are phagocyting bacteria or forming encapsulation capsules. In other insects, some β integrin genes have been reported to be upregulated in response to the invasion of microorganisms subjected to phagocytosis. When exposed to E. coli or fungi (Beauveria bassiana), S. exigua significantly induce S. exigua β1 expression, and this gene exhibits a similar tendency to increase in expression when exposed to components from microorganisms, such as lipopolysaccharide or laminin (Surakasi et al., 2011). After being challenged with various bacteria and their components, B. mori exhibited a significant increase in the expression of B. mori β1 and β3 (Zhang et al., 2017; Li et al., 2021a). Furthermore, A. gambiae βV (BINT2) acts as a receptor for phagocytosis of E. coli in the Anopheles gambiae cell line (Moita et al., 2006), and D. melanogaster βν generates phagocytosis against bacteria (Shiratsuchi et al., 2012). In addition, a β integrin of C. capitata acts as a receptor for the phagocytosis of both Gram-positive and Gram-negative bacteria (Lamprou et al., 2007; Mamali et al., 2009). These β integrins generally cooperate with their partner α integrins to regulate phagocytosis, and therefore, MysIntα2 may similarly regulate phagocytosis against bacteria by interacting with MysIntβ subunits. During encapsulation, the upregulation of gene expressions of MysIntβ1, MysIntβ3, and MysIntβ4, within the encapsulated capsules suggests that they could potentially serve as partners for MysIntα2.
The relative expression level of MysIntα2 reached approximately a thousand fold in hemocytes around the large beads. Against large beads injection, P. includens α2 and O. furnacalis β1 showed an approximately 90 fold and 10 fold increase, respectively, in hemocytes forming encapsulation capsules (Lavine & Strand, 2003; Xu et al., 2012). Other than integrin genes, various genes encoding proteins that regulate hemocyte adhesion or spreading are activated with large beads injection, such as thermally stable proteins (Hu et al., 2021), lectins (Ishihara et al., 2017; Wang et al., 2017; Song et al., 2020), hemolin (Jung et al., 2019), Ha-DFP1 (Hu et al., 2017), and calcium and integrin-binding proteins (Zhang et al., 2022a). Several other genes, such as transglutaminase and matrix metalloproteinases, were also highly expressed only in hemocytes that form the multilayered encapsulation capsules (Yokoi et al., 2022). This suggests that MysIntα2 may also be expressed using a common pathway with the aforementioned genes.
Similar to the response to large abiotic beads, the hemocytes that encapsulated the entomopathogenic nematode revealed MysIntα2 expression levels that were upregulated several hundred folds. In H. armigera, a C-type lectin, HaCTL3, has been reported to directly bind nematodes and Haβ-integrin may act as a receptor for HaCTL3 to promote the encapsulation response (Wang et al., 2017). However, Ono et al. (2020) reported that M. separata hemocytes can encapsulate nematodes in the absence of plasma. As this is the first observation of a direct relationship between integrins and nematodes in insects, it warrants further studies to determine whether the hemocytes of M. separata can directly recognize and encapsulate nematodes independently of other factors, possibly via integrins.
Immunofluorescent analysis using MysIntα2 antibody indicates that MysIntα2 localized in the hemocytes surrounding the beads. In encapsulation capsules of lepidopteran insects, the inner- and outer-most layers of the encapsulation capsule are composed of granulocytes, while the interior is filled with plasmatocytes (Pech & Strand, 1996). Thus, MysIntα2 is extensively present in both plasmatocytes and granulocytes throughout the capsule formation process. This localization characteristic is common to integrins in other insects. In B. mori, β1 is present in the membranes of hemocytes except for spherulocytes, and β2 and β3 are specifically expressed in plasmatocytes, whereas αPS3 is found only in granulocytes (Zhang et al., 2014a; Li et al., 2021a; Zhang et al., 2022b). M. sexta α1 and β1 are mainly expressed by plasmatocytes, whereas M. sexta α3 and P. includens α3 are mainly expressed in granulocytes, and M. sexta α2 and P. includens α1, α2 and β1 can be expressed in both hemocyte types (Lavine & Strand, 2003; Zhuang et al., 2008). In addition, no signal was observed from circulating hemocytes, suggesting that MysIntα2 is not localized on nonadhesive hemocyte types, such as prohemocytes, oenocytoids and spherulocytes.
Functional assays in the in vitro and in vivo conditions revealed that MysIntα2 mediates hemocytes to form encapsulation capsules encasing the beads. Encapsulation is an immune reaction involving changes in hemocyte morphology. As changes in cell morphology are generally regulated by intracellular actin, the encapsulation response is associated with the actin movement. In M. sexta, depolymerization actin in larval hemocytes resulted in the suppression of encapsulation (Amaya et al., 2005). The cytoplasmic domains of integrins are known to assemble large focal adhesion proteins linked to actin filaments, promoting cytoskeleton rearrangement (Hynes, 2002; Vicente-Manzanares et al., 2009). In humans, integrins trigger the rearrangement of filamentous actin (F-actin), which is crucial for numerous T-cell-dependent processes (Porter et al., 2002). In the lepidoptera, O. furnacalis, β1 can regulate F-actin polymerization to affect the spreading behaviors of plasmatocytes (Xu et al., 2012). Hence, it is hypothesized that in M. separata, an intracellular signal is activated and transmitted upon recognition of “large” invaders via MysIntα2, and the signal induces F-actin to reassemble, subsequently altering the cellular morphology.
Our study findings revealed that eight α subunits and four β subunits of integrin were encoded in the genome of M. separata. Our results demonstrated that MysIntα2 is essential for the cellular immune response of M. separata, particularly during encapsulation. This function is likely achieved through the regulation of hemocyte spreading activity, facilitating the formation of multilayered capsules around large invaders. Although some integrin genes were upregulated in response to the presence of invaders in the larval hemocoel, the number of components used in the experiments, both biological and nonbiological, was limited. Therefore, the mechanisms by which this expression is regulated should be carefully discussed. Also, more precise experiments are required to determine the causal relationship between changes in hemocyte shape and the function of MysIntα2. There may be some possible limitations in this study, however, it is true that integrins have an important role in M. separata, and our findings elucidate the function of the integrin family in insects and enhance our comprehension of the role of integrins in insect cellular immunity. Several crucial aspects such as the identification of the partner β-integrin of MysIntα2, the ligands recognized, and the intracellular signaling pathways activated by this heterodimer remain unexplored. Further examination of the function of MysIntα2, and other integrins, will aid in comprehending the significance of integrins in insects.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science KAKENHI [Grant Number JP17K08157].
Disclosure
The authors declare they have no conflict of interest in this work.




