Larval diet impacts black soldier fly (Diptera: Stratiomyidae) thermal tolerance and preference
Abstract
Thermal tolerance and preference are key parameters impacting agricultural production systems. In this study, the impact of larval diet on black soldier fly thermal tolerance and preference across life-stages and sexes was examined. Larvae were fed either a low-protein high-carbohydrate synthetic diet (i.e., P7C35), a high-protein low-carbohydrate synthetic diet (i.e., P35C7), or the Gainesville diet (i.e., C) as a control and reference. Our results demonstrate that the impacts of larval diet on black soldier fly thermal tolerance and preference could be stage and sex specific. The mean heat knockdown temperatures (HKT) ranged between 46.6 and 47.9 °C. Synthetic diets resulted in greater HKT and the difference decreased form larvae (e.g., ∼1 °C) to adults (e.g., ∼0.2 °C). The mean chill-coma recovery time (CCRT) ranged between 8.3 and 21.6 min. Not much differences were detected between diets, but CCRT became longer from larvae to adults. The mean thermal preference ranged between 13.6 and 29.5 °C. Larvae fed synthetic diets preferred much lower temperatures than the control diet. A bimodal distribution was observed for adults regardless of sex. Differences on body mass, lipid, and protein contents were detected among diets; however, more research should be done before any conclusions can be linked to their thermal traits. These findings highlight the importance of considering the ingredients and nutritional makeup of larval diets when optimizing temperature management protocols for mass production of black soldier flies. Conversely, specific diets can be developed to promote survival under extreme rearing temperatures.
Introduction
Sustainable means of feed production for livestock are needed due in part to environmental concerns (Viana et al., 2022) as well as increased costs of feed ingredients (Jannathulla et al., 2019). Because of such pressures on livestock production, identifying alternative protein sources for use in feed formulations is crucial. In fact, production of insects as such feed ingredients has gained tremendous interest (Jensen et al., 2021) due to its numerous benefits addressing these issues.
The black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae), appears to be the leading choice as it has been designated the “crown jewel” of the insects as feed sector due to several key traits unique to this system (Tomberlin & Van Huis, 2020). The larvae are nutrient packed (e.g., containing 38%–62% protein and 7%–39% lipid; Barragan-Fonseca et al., 2017; Surendra et al., 2020), which can be a substitute of the protein (which is typically recommended as 5% of the diet) or fat (which can usually be 100% replacement without negative effects) sources used for poultry (Abd El-Hack et al., 2020) and aquaculture production (Rana et al., 2015). Beyond macro nutrients, the relatively high amounts of calcium (e.g., ∼5%, used as calcium supplements; Barragan-Fonseca et al., 2017) and lauric acid (e.g., 30%–60% of lipid (Surendra et al., 2020) with noted antiviral properties (Harlystiarini et al., 2020)) positions the larvae for their development as functional additives in feeds for mass-produced animals.
There are numerous variables (e.g., temperature, nutrient, water, radiation, etc.) that producers must regulate to optimize black soldier fly production (Whitman & Agrawal, 2009). Temperature is one of the most important and dynamic variables that black soldier fly producers need to regulate (Tomberlin et al., 2009; Harnden & Tomberlin, 2016; Shumo et al., 2019a; Hosseini & Arast, 2021). Questions commonly being addressed with each new facility developed for optimal production of this species are, “what is the best room temperature” and “how frequently and accuracy should one adjust the room temperature?”
Tolerance and preference are two important traits for measuring how organisms react to temperature. Thermal tolerances, usually refer to the upper and lower thermal limits (Addo-Bediako et al., 2000), can be measured in many ways (e.g., heat/cold knockdown temperature, median lethal temperature, chill-coma recovery times, and super cooling points, etc.) (Addo-Bediako et al., 2000; Jørgensen et al., 2021). However, each trait mentioned provides different insights as to the ecological and physiological drivers for the targeted organism (Andersen et al., 2015). In this study heat knockdown temperature and chill-coma recovery time were measured for heat and cold tolerance, respectively.
Thermal preference is the temperature organisms select when given choices (Dillon et al., 2009). Though it is a bit difficult to determine why an organism prefers specific temperatures, thermal preferences are usually temperatures that overlap with various thermal optima (e.g., flight speed, development rates, or energy assimilation) that contribute to an increased fitness (Martin & Huey, 2008). As with other farmed animals (Pankhurst & King, 2010; Adamo & Lovett, 2011), such information is critical for maximizing production of the black soldier fly used industrially (Chia et al., 2018).
Previous studies have determined that stage, age, size, and sex impact black soldier fly thermal tolerance, however, with a single diet (i.e., the Gainesville diet) (Addeo et al., 2022; Li et al., 2022). From a sustainability perspective, black soldier fly larvae have a broad dietary range including but not limited to manure (Hao et al., 2023), food waste (Kim et al., 2021), solid fraction of digestate (after anaerobic digestion of chicken manure mixed with rapeseed straw) (Elsayed et al., 2020), and contaminated food (Bosch et al., 2017) that cannot be consumed by humans or livestock depending on regulations in place. In fact, the larvae in many instances can substantially convert wastes to biomass (e.g., 10%–70%) (Liu et al., 2019; Surendra et al., 2020) for use as feed. In addition, the larvae can be used to bioremediate contaminants such as mycotoxins (e.g., aflatoxin, deoxynivalenol, and fumonisin) and antibiotics (e.g., tylosin and enrofloxacin) (Mei et al., 2022), while concurrently producing the previously discussed products of value (e.g., insect biomass and frass for use as fertilizer).
Shifts in types of substrates fed to larvae impact development traits of the black soldier fly. For example, larvae fed poultry feed, manure, or digested sludge resulted in prepupal weights of ∼251, ∼164, and ∼70 mg, respectively (Lalander et al., 2019). Shifting protein and carbohydrate ratios impact larval duration. Development of larvae fed a diet either comprised of low protein in combination with high carbohydrates (i.e., 7 : 35) or the opposite (i.e., 35 : 7) increased development by ∼10 d versus the control diet (i.e., Gainesville diet) (Cammack & Tomberlin, 2017), which could due to the nutrient deficient as the Gainesville diet meet the larval macronutrient demand better.
Diets have prolonged effects, which not only impact larval traits but also adult reproductive traits (e.g., the number of egg mass and total egg yield) (Barragan-Fonseca et al., 2021) and antimicrobial peptides related genes (Vogel et al., 2018; Candian et al., 2023). More recent studies determined not only the ratios of protein and carbohydrate, but also the concentration impacts the life-history traits tested (i.e., growth rate, body weight, and body composition throughout the lifecycle) (Cheon et al., 2022).
Considering the great diversity of larval diets use for production as previously discussed, the universal optimum rearing temperature suggested (e.g., 27–30 °C) (Chia et al., 2018; Shumo et al., 2019) may not always be the best choice as what is appropriate for the black soldier fly can vary depending on diets. Thus, the objective was to test how diets could impact black soldier fly thermal tolerance and preference. In addition, factors such as sex and stage (Addeo et al., 2022; Li et al., 2022), which impact black soldier fly thermal traits, were included for better biological understanding. Data provided in the current study potentially can help the industry notice shifting diets could potentially impact survival during extreme temperatures.
Materials and methods
Colony maintenance
The black soldier fly strain used in this study was established from eggs purchased from Phoenix Worm, Inc. (Tifton, GA, USA) since January 2014, which originated from a laboratory colony maintained at the Coastal Plains Experiment Station (University of Georgia, Tifton, GA, USA since 1998). This strain was then maintained at the Forensic Laboratory for Investigative Entomological Sciences (F.L.I.E.S. Facility) at Texas A&M University (College Station, TX, USA) as well as at EVO Conversion System LLC (College Station, TX, USA).
For this study, a BulletTM (Tomberlin et al., 2021) consisting of ∼15 000 seven-day-old black soldier fly larvae was received from EVO Conversion Systems, LLC and raised to adults according to a procedures outlined by Li et al. (2022). Resulting adults were released in a cage maintained in a greenhouse at the F.L.I.E.S Facility. Resulting eggs were placed in a Kerr® wide-mouth 946 mL Mason Jar (Hearthmark LLC, Daleville, IN, USA) in a laboratory walk-in Rheem® environmental chamber (Asheville, NC, USA, at ∼27.0 ± 0.5 °C, ∼60.0% ± 5.1% relative humidity (RH), 14 h light/10 h dark) for hatching. Newly hatched larvae (i.e., < 24 h) were collected and provided with the Gainesville diet (containing 50% wheat bran, 30% alfalfa meal, 20% corn meal, and moisturized to 70% water content) (Hogsette, 1992) as a starter feed. Seven-day old larvae were sieved (i.e., to separate frass), weighed, and counted to obtain an average larval weight. Five hundred 7-d-old larvae were weighed accordingly and transferred to larger (∼1 893 mL) cylinder plastic containers (Airlite® 6451, Omaha, NE, USA) with different diets provided (i.e., 3 replicates within a trial × 2 trials with eggs from different generations, in total 6 containers for each diet).
The three diets used were: two synthetic diets with nutrient shifts specific to protein and carbohydrates, which were (1) a low-protein (7% ww) high-soluble-carbohydrate (35% ww) diet (i.e., P7C35) and (2) a high-protein (35% ww) low-soluble-carbohydrate (7% ww) diet (i.e., P35C7), and (3) the plant-based Gainesville diet (∼15% protein and ∼ 36% soluble carbohydrate on dry matter basis; Bellezza Oddon et al., 2022) as a control diet according to the black soldier fly nutritional requirement determined (Barragan-Fonseca et al., 2021; Cheon et al., 2022). Synthetic diets were prepared according to the method published previously (Cammack & Tomberlin, 2017). Diets (i.e., P7C35 and P35C7) chosen were those determined to be able to support black soldier flies grow from larvae to adults while having significant impacts on flies life-history traits (Cammack & Tomberlin, 2017). The feeding procedures were the same across tested diets as described by Cammack & Tomberlin (2017). For each, 16 g of the dry diet with 70% moisture were provided daily until reaching 40% prepupae and no more feed were provided afterward. On the seventh day after the first prepupa was observed, a net was placed over the container and fastened with a rubber band to prevent adult escape. In each stage (e.g., 14-d-old larvae, 1-d-old prepupae, 1-d-old females, 1-d-old males), for each test (i.e., heat tolerance, cold tolerance, and preference, see below), 10 individuals were collected, weighed, and thermal tolerance and preference were determined. Another 30 individuals (i.e., larvae, prepupae, and adults) were collected, oven dried (∼60 °C, Thelco laboratory 6540, Thermo Fisher Scientific Inc., Marietta, OH, USA) and stored in freezer (Frigidaire FFFC25M4TW, Mississauga, Ontario, Canada) for later lipid and protein analysis (see below). The first ∼10 prepupae and adults were discarded during prepupal and adults’ collection in order to eliminate the extremes in each cohort.
Heat tolerance
Heat knockdown temperatures were measured according to methods modified from Li et al. (2022). A heating water bath (PolyScience® WB10, Niles, IL, USA) was used to regulate the water temperature. Glass tubes (Corning® 99447–20 PYREX® 20 × 125mL, Durham, NC, USA) with wooden lids were put into water bath to ensure homogenous temperature inside the tubes. Tubes submerged under water were placed horizontally during larval and prepupal tests, while placed vertically for adults to better determine their behavior during knockdown as described. Black soldier flies were individually placed into a tube where they were then subjected to heating at constant rates of 1 °C/min. The water inside the bath was periodically stirred to ensure thermal homogeneity. The starting temperature was 27 °C and ramped up to 53 °C. In each round of testing (i.e., heating up the water), individuals from three diets were randomized in 12 tubes, four digital k-type thermocouples (Model A0188598; Gain Express Inc., Kowloon, Hongkong, China) were inserted in tubes (the 1st, 4th, 7th, 10th tube count from left to right) right above the tested individuals to ensure accurate measurement, and videos (iPhone® 8, 12-megapixel wide-angle f/1.8 camera, Cupertino, CA, USA) were taken until all the flies were knocked down. Individual heat knockdown temperatures were recorded. A larva or prepupa was considered knocked down when it failed to locomote, while an adult was considered knocked down when it failed to cling to the side of the tube or hold itself upright (i.e., lost ability to effectively locomote) (Huey et al., 1992). In total 720 individuals were tested (i.e., 3 for diets × 4 for stages and sexes × 2 for trials with eggs from different generations × 3 for replicates within a trial × 10 for replicates within containers = 720 total individuals)
Cold tolerance
Chill-coma recovery times were measured as indices for cold tolerances. Black soldier flies were individually placed into a tube (i.e., same as those used in the heat tolerance test). Tubes were placed on a metal plate and then the plate was put in a –20 °C freezer (mentioned above). The plate was removed 10 min later and videos (methods described above) were taken for 40 min in the lab around 25 °C. For tubes with adults, each tube was carefully rolled to a position that the adult was upside down. Individual chill-coma recovery time was then recorded. A larva or prepupa was considered recovered when half of the body obviously moved, while an adult was considered recovered when it oriented its body right-side up. In total 720 individuals were tested (i.e., 3 for diets × 4 for stages and × 2 for trials with eggs from different generations × 3 for replicates within a trial × 10 for replicates within containers = 720 total individuals).
Thermal preference
Thermal preferences were measured following similar methods from Malawey et al. (2021) and Addeo et al. (2022). The thermal gradient was made with an aluminum plate (85 × 20 cm) with both ends bent at 90° to create a “U” shape with 10 cm arms. With one arm inserted in a water bath (i.e., same as the one used for heat tolerance test) heated to 60 °C and the other arm inserted in a polystyrene box (∼27 × 22 × 22 cm) cooled to ∼0 °C with ice water, a thermal gradient with temperature ranged from 45 °C to 10 °C was maintained. By spacing four digital k-type thermocouples (i.e., same as those mentioned before) at 2, 18, 34, and 52 cm along the surface of the aluminum gradient, the thermal gradient surface temperature was measured. Ten individuals (i.e., larvae, prepupae, female adults, or male adults) were released at the middle of the thermal gradient and allowed to explore and adjust to the condition for 5 min. Cameras (SPORTS HD DV nimi camera SQ11, Shenzhen, China) were set right above the plate and videos were taken for 1 h following the exploration time. A virtual ruler was set up on the computer desktop and the spatial position of each individual was recorded from the video that paused at 5 min intervals by reading the virtual ruler. Temperature where the flies touched were calculated individually based on their location and the temperature recorded along the plate simultaneously during the experiment. In total 720 individuals were tested (i.e., 3 for diets × 4 for stages and sexes × 2 for trials × 3 for replicates within trials × 10 for replicates within containers = 720 total individuals).
Lipid content
The method used for lipid content determination was based on a published paper (Loveridge, 1973). For each replicate, six 60 °C-oven-dried larvae, prepupae, adult females, or adult males were weighed and washed with chloroform (i.e., submerged under chloroform for 24 h) at least three times until no more changes occurred in the color of chloroform (i.e., from yellowish to clear liquid that similar to the unused chloroform). Washed samples were dried again at 60 °C until weights no more decreased. Lipid contents were then calculated using the following formula:
Lipid content (%) = (dry weight of larvae pre-chloroform – dry weight of larvae postchloroform)/dry weight of larvae pre-chloroform × 100.
Protein content
To determine the dried black soldier fly protein content, samples were sent to the Soil, Water and Forage Testing Laboratory in Texas A & M University, College Station, TX, USA. As required, 0.5 g minimum of sample per replicate were ground (JueyingbailiTM portable spice grinder, 5 cm diameter, Shenzhen, China) and stored in sealed sample bags before delivery. Total nitrogen is determined by high-temperature combustion process (Sweeney, 1989).
Statistical analysis
All the data were analyzed in R version 4.0.5 (R Core Team, 2021) with the significance level set at P < 0.05. For heat tolerance, generalized linear models (GLM, with family set as Gaussian) were applied to test how stage (or adult sex) and diet impacted black soldier fly heat knockdown temperatures. For significant predictors in the best model, pairwise comparisons among different levels were done by function emmeans in the R package “emmeans” (Lenth, 2023). Means and standard error of means (SEM) were provided in results.
For cold tolerance, the chill-coma recovery time is a censored dataset since some individuals did not recover within 40 min, therefore Cox regressions were applied to test how stage (or adult sex) and diets impacted black soldier fly chill-coma recovery times with the function coxph in the package “survival” (Therneau & Grambsch, 2000; Therneau, 2022). Pairwise comparisons for significant predictors among different levels were done by function emmeans in the package “emmeans” (Lenth, 2023). Means and standard error of means (SEM) were provided in results.
For thermal preference, linear mixed effects model, the lme function from the nlme package (Pinheiro & Bates, 2023), was applied to test how diet and stage (i.e., larvae, prepupae, and adults) or diet and sex (for adult only) impacted black soldier fly thermal preferences. Group (i.e., those ten individuals measured together were treated as a group) was included as a random effect to account for repeat measurement across time. The model used is as follow: lme (thermal preference ∼ diet × stage + trial, random = ∼1|group), with restricted maximum likelihood (REML) as the parameter estimation methods. Pairwise comparisons for significant predictors among different levels were done by function emmeans in the package “emmeans” (Lenth, 2023). Medians and standard error of medians were provided in the result. The 25% quartile, median, and 75% quartile were reported for the thermal preference of each group to better describe the data distribution.
For body nutrients, GLMs (with family set as Gamma and link function set as identity) were applied to test how stage (i.e., larvae, prepupae, adult males, and adult females) and diets impacted black soldier fly lipid content and protein content. Model assumptions were checked by diagnostic plots. Pairwise comparisons among different levels were done by function emmeans in the package “emmeans” (Lenth, 2023).
Body weights were recorded individually during tolerance tests and included in the GLM model (i.e., heat knockdown temperature ∼ diet × weight; cold coma recovery time ∼ diet × weight) for thermal tolerance test within each stage during the first try. Since body weight neither interacted with diet nor impacted thermal tolerance significantly in any of the model tested, body weight was removed in the final model used for thermal tolerance test to avoid weakening the power of the model. The test statistic and P-value for body weight in the GLM model were provided as Supplementary Materials (Table S1). Though body weight was not the aim of this study, to provide more information, GLM was used to analyze the impacts of diets and stage on body weight and the correlations between body weights and knockdown temperatures were analyzed with Spearman's correlation. Means and standard error of means (SEM) were provided as Supplementary Materials.
Results
Heat tolerance
No difference (F = 0.6958, df = 1, P = 0.405) between trials was determined. The impact of diets on black soldier fly heat knockdown temperatures was stage dependent (F = 10.157, df = 4, P < 0.001 for the diet × stage interaction predictor). In general, synthetic diets improved larval heat tolerance and the effect of high-protein synthetic diet lasted longer to adult stage. Within-stage pairwise comparisons showed that larvae developed on the control diet had lower heat knockdown temperatures (46.9 ± 0.1 °C) than larvae developed on P35C7 (47.6 ± 0.1 °C, t. ratio = –6.902, df = 699, P < 0.001) and P7C35 (47.6 ± 0.1 °C, t. ratio = –7.332, df = 699, P < 0.001), resulting in ∼40% deference in knockdown resistance at ∼47 °C. Similarly, prepupae developed on the control diet had lower heat knockdown temperatures (47.2 ± 0.1 °C) than prepupae developed on P35C7 (47.9 ± 0.1 °C, t. ratio = –6.869, df = 699, P < 0.001) and P7C35 (47.6 ± 0.1 °C, t. ratio = –4.237, df = 699, P < 0.001). Heat knockdown temperatures of adults developed on the control diet were slightly lower (46.7 ± 0.1 °C) than adults developed on P35C7 (46.9 ± 0.1 °C, t. ratio = –2.971, df = 699, P = 0.009) but similar to adults developed on P7C35 (46.7 ± 0.1 °C, t. ratio = –0.359, df = 699, P = 0.932). Therefore, differences between P35C7 and P7C35 on black soldier fly heat knockdown temperatures were negligible for larvae (t. ratio = –0.430, df = 699, P = 0.903), but slightly greater for prepupae (t. ratio = 2.632, df = 688, P = 0.024) and adults (t. ratio = 2.639, df = 688, P = 0.023) (Figs. 1 and S5, and Table S2).
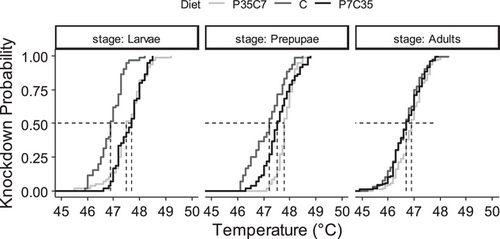
The impact of diets on black soldier fly heat knockdown temperatures was also sex dependent (F = 5.806, df = 2, P = 0.003 for the diet × sex interaction predictor) and no difference (F = 2.604, df = 1, P = 0.107) between trials was determined. Within-sex pairwise comparisons showed that females developed on P35C7 had slightly greater heat knockdown temperatures (47.1 ± 0.1 °C) than those developed on control diet (46.6 ± 0.1 °C, t. ratio = 4.499, df = 342, P < 0.001) and P7C35 (46.8 ± 0.1 °C, t. ratio = 3.055, df = 342, P = 0.007), which resulting in ∼30% increment in heat knockdown resistance at ∼46.5 °C. But no differences (t. ratio ≤ 1.284, df = 342, P ≥ 0.405) were found in males between P35C7 (46.7 ± 0.1 °C), P7C35 (46.7 ± 0.1 °C), and control (46.8 ± 0.1 °C). (Fig. S5 and Table S2)
Greater body weights did not always result in greater heat knockdown temperatures. Only larvae developed on the control diet (r = 0.29, P = 0.026), prepupae developed on P7C35 (r = 0.40, P = 0.001), and adults developed on P35C7 (r = 0.35, P < 0.001) showed significant but weak correlation (Fig. S1).
Cold tolerance
In total 8% of the samples were censored, within which 7%, 12%, and 6% of the samples were censored for diet C, P35C7, and P7C35 respectively. No significant impacts from trials (χ2 = 3.161, df = 1, P = 0.075) and the interaction between stage and diet (χ2 = 4.273, df = 4, p = 0.370) were detected. Black soldier fly chill-coma recovery times increased ontogenically (χ2 = 59.810, df = 2, P < 0.001). Larvae recovered faster (8.7 ± 1.7 min) than prepupae (14.6 ± 1.6 min, z. ratio = 6.080, P < 0.001) and adults (17.4 ± 0.6 min, z. ratio = –8.625, P < 0.001), regardless of diet. Though an overall diet effect on the black soldier fly chill-coma recovery time was detected (χ2 = 6.845, P = 0.033), no pairwise differences were determined between P35C7 (16.7 ± 10.8 min), P7C35 (14.3 ± 0.6 min), and the control (15.0 ± 0.6 min) (Figs. 2 and S6, and Table S2).
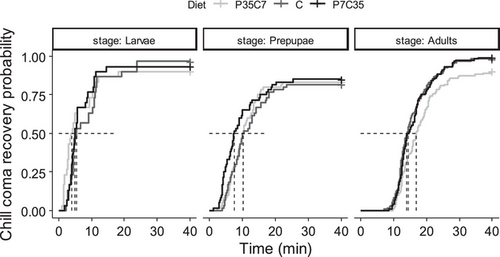
For adult data pooled by sex, no significant impacts from trials (χ2 = 1.335, df = 1, P = 0.248) and the interaction between sex and diets (χ2 = 0.862, df = 2, P = 0.650) were detected. A diet effect (χ2 = 16.466, df = 2, P < 0.001) was determined, which adults developed on P35C7 (19.6 ± 1.1 min) recovered slightly slower than those developed on control (16.1 ± 0.8 min, z. ratio = –3.588, P = 0.001) and P7C35 (16.4 ± 0.7 min, z. ratio = –3.397, P = 0.002). For sex effect, male (18.7 ± 0.6 min) black soldier flies took longer (χ2 = 13.519, df = 1, P < 0.001) to recover than females (16.0 ± 0.5 min) regardless of diets (Fig. S6 and Table S2).
Greater body weights did not always result in shorter chill-coma recovery times. Only adults developed on the control diet (r = –0.20, P = 0.031) and P35C7 (r = –0.23, P = 0.001) showed significant but weak correlation (Fig. S2).
Thermal preference
According to the lme model output, no significant differences (F = 0.0057, df = 1, P = 0.940) between trials were detected. Stage interacted with the diet in determining black soldier fly thermal preferences (F = 45.251, df = 4, P < 0.001). The data distributions were much similar in prepupal and adult stage than in larval stage. Larvae developed on the control diet preferred greater temperatures (median = 29.4 °C, Q1–Q3 range: 21.4–38.7 °C) than those developed on P7C35 (median = 20.1 °C, Q1–Q3 range: 12.8–29.4 °C, t. ratio = 6.958, df = 8049, P < 0.001) and P35C7 (median = 15.9 °C, Q1–Q3 range: 11.2–30.7 °C, t. ratio = 7.962, df = 8049, P < 0.001). There was no difference between P7C35 and P35C7 on larval thermal preferences (t. ratio = 0.803, df = 8049, P = 0.7012). Similar to larvae but with smaller differences, prepupae developed on the control diet preferred greater temperatures (median = 18.1 °C, Q1–Q3 range: 11.8–30.7 °C) than those developed on P7C35 (median = 13.8 °C, Q1–Q3 range: 11.5–26.1 °C, t. ratio = 3.978, df = 8 049, P = 0.0002) and P35C7 (median = 13.5 °C, Q1–Q3 range: 11.1–24.4 °C, t. ratio = 5.614, df = 8 049, P < 0.001). There was no difference between P7C35 and P35C7 on prepupal thermal preferences (t. ratio = 1.704, df = 8049, P = 0.2037). On the contrary, adults developed on the control diet preferred lower temperatures (median = 20.4 °C, Q1–Q3 range: 12.2–33.4 °C) than those developed on P7C35 (median = 24.8 °C, Q1–Q3 range: 13.5–34.7 °C, t. ratio = –4.409, df = 8049, P < 0.001) and P35C7 (median = 28.1 °C, Q1–Q3 range: 13.8–36.7 °C, t. ratio = –8.873, df = 8049, P < 0.001). Adults developed on P35C7 preferred greater temperature than those on P7C35 (t. ratio = 4.570, df = 8049, P < 0.001). Therefore, effects of synthetic diets flipped from larvae to adults and P35C7 seemed to have stronger effects for adults compared to P7C35 (Fig. 3 and Table S2).
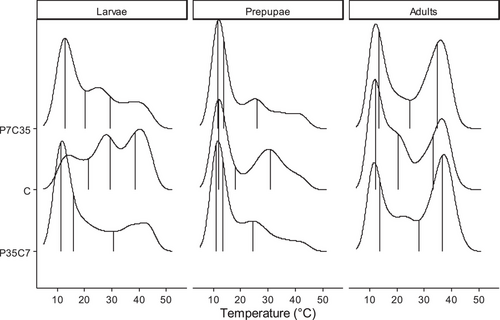
For adult data pooled by sex, trials (F = 0.087, df = 1, P = 0.783) were not found to be significant, while the interaction between sexes and diets tend to be significant (F = 2.599, df = 2, P = 0.075). Males developed on P35C7 (median = 26.8 °C, Q1–Q3 range: 12.4–36.3) preferred greater temperature than those on control (median = 21.3 °C, Q1–Q3 range: 12.2–33.4 °C, t. ratio = 4.760, df = 4218, P < 0.001) and P7C35 (median = 24.1 °C, Q1–Q3 range: 12.8–34.7 °C, t. ratio = 2.900, df = 4218, P = 0.011). Similarly, females developed on P35C7 (median = 26.8 °C, Q1–Q3 range: 12.4–36.3) preferred greater temperature than those on control (median = 20.1 °C, Q1–Q3 range: 12.8–34.3 °C, t. ratio = 4.760, df = 4218, P < 0.001) and P7C35 (median = 25.4 °C, Q1–Q3 range: 14.6–34.7 °C, t. ratio = 2.900, df = 4218, P = 0.011). The only difference between male and female is female developed on P7C35 preferred greater temperature than control (t. ratio = 4.334, df = 4218, P < 0.001) while no differences were found for males between these two diets (t. ratio = 1.888, df = 4218, P = 0.1422) (Table S2).
Lipid content
The impact of diets on black soldier fly lipid content was stage dependent (F = 2.212, df = 60, P < 0.001 for the interaction predictor). Within-stage pairwise comparisons showed that larvae developed on P7C35 had greater lipid content (17.4% ± 1.3%) than those reared on control (9.7 ± 0.7%, t. ratio = –5.048, df = 60, P < 0.001) and P35C7 (5.7% ± 0.4%, t. ratio = –8.390, df = 60, P < 0.001) diets. Prepupae developed on P7C35 had similar lipid content (16.2% ± 1.2%) to those reared on control (14.5% ± 1.1%, t. ratio = –1.047, df = 60, P = 0.551), but greater than those reared on P35C7 (12.1% ± 0.9%, t. ratio = –2.689, df = 60, P = 0.025) diets. For adults, males had similar lipid contents (|t. ratio| ≤ 2.277, df = 60, P ≥ 0.067) when reared on P7C35 (27.7% ± 2.1%), control (29.0% ± 2.1%), and P35C7 (22.9% ± 1.7%). Females developed on P7C35 had similar lipid content (25.3% ± 1.9%) to females developed on control diets (28.3% ± 2.4%, t. ratio = 0.976, df = 60, P = 0.595), but greater than those developed on P35C7 diets (17.2% ± 1.3%, t. ratio = –3.474, df = 60, P = 0.003). Therefore, black soldier flies developed on the diet P35C7 had the lowest (in number) lipid contents across stage and sex though not always significantly lower than the other two diets due to smaller in differences between diets for prepupae and adult males. Diet P7C35 only resulted in significantly greater lipid contents during larval stage but became similar to that of the control afterward (Fig. S4A).
Protein content
The impact of diets on black soldier fly protein content was stage dependent (F = 4.154, df = 60, P = 0.005 for the interaction predictor). Within-stage pairwise comparisons showed that when developed on P35C7, black soldier flies had the greatest (t. ratio ≥ 2.930, df = 60, P ≤ 0.019) protein contents (57.4% ± 1.2% for larvae, 56.3% ± 1.1% for prepupae, 66.0% ± 1.3% for adult males, 71.6% ± 1.4% for adult females) regardless of stage and sex. While for the other two diets, diet P7C35 only resulted in significantly lower protein contents during larval stage (42.6% ± 0.9% for P7C35 and 48.8% ± 1.0% for control, t. ratio = 4.712, df = 60, P < 0.001) but were similar (|t. ratio| ≤ 1.067, df = 60, P ≥ 0.543) to control diets afterward (49.4% ± 1.0% vs. 50.2% ± 1.0% for prepupae, 60.7% ± 1.2% vs. 60.1% ± 1.2% for adult males, and 62.1% ± 1.2% vs. 64.0% ± 1.3% for adult females, for P7C35 and control respectively) (Fig. S4B).
Body weight
Body weights were between 0.02 and 0.14 g (Figs. S1 and S2). The interaction between diet and stage on black soldier fly body weights were significant (F = 26.345, df = 4, P < 0.001). Stage impacts (F = 3669.337, df = 2, P < 0.001) black soldier fly body weights much stronger than diet (F = 70.409, df = 2, P < 0.001). In general, individuals used for test were the lightest in their adult stage (e.g., ∼0.05 g), followed by the larval stage (e.g., ∼0.09 g) and prepupal stage (e.g., ∼0.10 g), and females (e.g., ∼0.049 g) were heavier (F = 105.675, df = 1, P < 0.001) than males (e.g., ∼0.041 g) regardless of diets. In larval stage, diet P35C7 resulted in the heaviest weight (t. ratio ≥ 4.689, df = 1310, P < 0.001), followed by control and P7C35. While in prepupal stage, the control diet resulted in the heaviest weight (t. ratio ≥ 6.055, df = 1310, P < 0.001) followed by P35C7 and P7C35. In adult stage, the control diet continued resulted in heavier weight (t. ratio ≥ 4.780, df = 1310, P < 0.001) compared to the other two diets, while no differences were detected between the two synthetic diets (t. ratio = 1.065, df = 1310, P = 0.536).
Discussion
This study determined the impact of larval diets on black soldier fly thermal tolerance and preference was mostly stage and sex dependent. Differences were observed not only between the nutrition-balanced control diet and nutrition-imbalanced synthetic diets, but also between the two synthetic diets with different protein to carbohydrate ratio. Shifting from the control diet to synthetic diets increased the maximum critical knockdown temperatures of the black soldier fly (e.g., maximally ∼1 °C greater for those developed on P35C7 and P7C35); though such responses decreased as the black soldier fly progressed through its life cycle (Fig. 1), the effect of P35C7 lasted longer. Diet did not impact chill-coma recovery time (e.g., differed less than 5 min) within stage but stage did, with adults (e.g., 17.4 min) taking longer to recovery than prepupae (e.g., 14.6 min) and larvae (e.g., 8.7 min) (Fig. 2). While body weight (e.g., ranged from 0.02 to 0.14 g) of the individual could play a role (Figs. S1 and S2), the amount of lipid (e.g., ranged from 4.1% to 33.8%, Fig. S4A) or protein (e.g., ranged from 40.7% to 73.9%, Fig. S4B) acquired through growth can also have downstream effects on thermal responses, which would be studied in detail in the future. For thermal preference, shifting from the control diet to either synthetic diet lowered the larval thermal preference (e.g., at least 9 °C) but increased adult thermal preference (e.g., at least 4 °C), within which the diet P35C7 had stronger effects (Fig. 3). This study provides data related to the impact of larval diet on thermal responses of the black soldier fly across stage and sex. Unfortunately, analysis on body weights and body nutrients were limited in terms of inferences made; however, it is evident such traits reveal a high degree of physiological complexity with regards to phenotypic variation as related to the thermal traits measured.
Heat tolerance
Diet type or nutrition balance may impact insect heat tolerance. Compared to the control diets, both synthetic diets were nutritional imbalanced for black soldier fly larvae. Nutrient restriction was determined to be able to increase tolerance to stressors such as heat stress (Wenzel, 2006). Ingredient also leads to differences in stress tolerance (Colinet & Renault, 2014). The different ingredients used in the control diet may also count for the different thermal response in this study, which need further experiment in the future to clarify.
Comparing the two synthetic diets, the effect of heat tolerance improvement lasted longer to the adult stage when feeding larvae with P35C7. Being fed a high-protein diet could be responsible for instances of greater thermal tolerance. For example, Drosophila melanogaster Meigen (Diptera: Drosophilidae) adults fed a protein-enriched diet as larvae were determined to be more heat tolerance (e.g., knocked down at least 15% slower) than those developed on a carbohydrate-enriched diet (Andersen et al., 2010). They hypothesized heat shock proteins (Hsp70) were upregulated for D. melanogaster larvae fed a protein-enriched diet but not for those on protein-deficient diet (Andersen et al., 2010). In the case of the current study, adult females resulting from larvae fed the P35C7 diet had the greatest protein content (Fig. S4B), which was correlated with the greatest heat tolerance among all the adult treatments (Table S2). In this case, if diet had an impact on adult body nutrients, and subsequently on their heat tolerances, the high protein content seemed to be more important in heat tolerance than the high lipid content. As the high-protein diet enhanced heat tolerance by adult females, the expression of heat shock proteins across diets in black soldier flies may be of interest for further exploration.
Size of individuals also impacted thermal tolerance. This result has been documented frequently in nature and is not a surprise considering that larger adults have a greater volume to surface area ratio resulting in a slower heating rate (Angilletta, 2009). For example, for various army ant (Hymenoptera: Formicidae) species, heat tolerance increased with increasing body size (Baudier et al., 2015). It is important to point out that when both diets and stages were controlled, body weights had negligible effects on black soldier fly heat tolerance (Fig. S1). The body weight (which is highly correlated to the body size) determined in the current study could be a good indicator for differences in heat tolerance responses between stages due to the significant differences of body weight across stage. The adult body weight, which was significantly lower than prepupae and larvae, was correlated with the lowest heat tolerance determined by stage (Fig. S1). Interestingly, such correlations were stronger (i.e., moderate with Spearman's r between 0.3 and 0.5) for P35C7 and P7C35 than the control (i.e., negligible with Spearman's r below 0.3). Therefore, body weight seems to be more important when rearing with the nutrition-imbalanced synthetic diets used in this study.
Energy allocation tradeoffs may explain in part the differences in heat tolerance throughout the life-history of the black soldier fly. The priority of energy utilization (i.e., lipid, protein, and carbohydrate) and allocation (i.e., growth vs. reproduction) should vary by stage and sex, which was supported by the decreasing differentiation in lipid and protein contents in the prepupal stage compared to the larval stages as well as in males compare to females (Fig. S4A). Similar to the findings presented from this study, female D. melanogaster developed on a protein-enriched media had greater heat tolerances (i.e., persisted ∼26% longer in time before entering heat knockdown coma) and had greater egg production (i.e., ∼16%) than those developed on a sucrose (i.e., carbohydrate)-enriched media (Andersen et al., 2010). Both the protein source and energy source (i.e., lipid and carbohydrate) are known to be essential for the egg formation (Mirth et al., 2019). In addition, the limitation of protein synthesis at high temperatures usually results in protein deposition deficient (Renaudeau et al., 2012). Therefore, the protein provided by control diet (i.e., ∼15%) and P7C35 (i.e., ∼7%) may not be enough particular for the adult black soldier flies in balancing heat stress resistance and reproduction simultaneously, which need to be determined with further experiment. These data demonstrating the complexity of nutrient acquisition and utilization in flies require further detailed analysis on the body composition to better understand the physiological mechanisms.
Cold tolerance
Few differences were found in chill-coma recovery time between diets within stage for the black soldier fly, meanwhile stage instead of diet significantly altered chill-coma recovery time. In this study, the chill-coma recovery time determined increased from larvae (8.7 min), prepupae (14.6 min), to adults (17.4 min). For sex differences, male black soldier flies were determined to recover 2.7 min slower than females regardless of diets. The tiny differences determined by diet and sex may not have biological impacts (Fig. 2 and Table S2). It is worthwhile to note that the temperature (e.g., –20 °C) used for cold shock was much lower than the temperature black soldier flies would experience in nature. But considering the expansion of black soldier fly mass rearing globally, such a low temperature could provide information for those in high latitudes or those who need a short transportation with cold storage.
High-carbohydrate diets are well known to increase body lipid contents (Mayntz et al., 2005; Barragan-Fonseca et al., 2021), which are correlated with better cold tolerance by insects. Shifting lipid content in Drosophila spp. from ∼10% to ∼20% increased cold tolerance survival from ∼0% up to ∼100%, respectively (Hoffmann et al., 2001). In the current study, the high-carbohydrate diet P7C35 did result in greater body lipid content within stage (Fig. S4A) but did not correlate with their cold tolerance (i.e., no differences in cold tolerance between diets, Fig. 2). Though the flies’ lipid profile was not analyzed in detail, body lipid profile has determined to be heavily impacted by diets (Hadj Saadoun et al., 2020). In this study, neither lipid content nor lipid profile in general altered flies cold tolerance. More detail, such as metabolome (Colinet et al., 2016) or lipidome (Trenti et al., 2022), would be required in the future for physiological explanation.
As with thermal tolerance, body size of black soldier flies also impacted chill-coma recovery responses. Larger body sizes are usually linked to greater thermal inertia, which takes longer to warm up. However, opposite trends were recorded here. Some significant but small differences (e.g., ∼14% of body weight) in body weights (Fig. S2) were determined within stage, which were much smaller compared to the effect of stage on body weight within diet (e.g., ∼50% of body weight) (Fig. 2). The body weight was significantly lower for adults regardless of diets, which was moderately negative correlated with the chill-coma recovery time determined (Fig. S2). In addition, the greater body weights determined for females than males (Fig. S2) were also correlated with faster (though only 0.9 min in difference) recovery from chill coma in females (Table S2).
When considering what is known with regards to thermal biology, three schools of thought explain how organisms cope with extreme temperatures: (1) avoidance through behavioral regulation (e.g., crawl/walk/fly away), (2) adaptation through evolution (e.g., thick fur), and (3) acclimation through temporary physiological modification (e.g., upregulation of heat-shock proteins) (Hardison et al., 2021). Therefore, a less mobile stage, for example, larvae and prepupae in the case of the current study, may have physiological adaptations to allow greater survival when experiencing extreme temperature than adults, leading to slightly greater heat knockdown temperatures (Fig. 1) and shorter recovery time (Fig. 2) than adults. Such responses have been documented for other dipteran. The blow fly, Calliphora vicina Robineau-Desvoidy (Diptera: Calliphoridae) (Coleman et al., 2015), kelp fly, Paractora dreuxi Séguy (Diptera: Helcomyzidae) (Terblanche et al., 2007), and the Antarctic midge, Belgica antarctica Jacobs (Diptera: Chironomidae) (Lee Jr et al., 2006) all have larvae that are capable of surviving more extreme temperatures than the adult.
Thermal preference
Temperature and diet interactions impact insect growth (Clissold et al., 2013; Gligorescu et al., 2018). Diet composition can alter animal thermal preferences; for example, locusts, Locusta migratoria (L.) (Orthoptera: Acrididae), that developed on a carbohydrate-deficient diet (e.g., 32 °C) preferred 6 °C lower than those developed on a protein-deficient diet (Clissold et al., 2013), which is similar to our finding that larvae developed on P35C7 preferred lower temperature than larvae fed on P7C35 (Fig. 3). Clissold et al. (2013) further determined L. migratoria nymphs had greater carbohydrate conversion efficiency at 32 °C, while greater protein conversion efficiency at 38 °C. Of course, this study was conducted with nonhost kangaroo grass Themeda triandra Forssk (Poales: Poaceae) as the food substrate. Interestingly, when developed on wheat Triticum aestivum L. (Poales: Poaceae), which is its host plant, L. migratoria ingested more material at a higher temperature (i.e., 38 °C) than a lower temperature (i.e., 32 °C), but with equal conversion efficiencies for both protein and carbohydrate regardless of temperatures. Therefore, the digestibility of different nutrients by an insect such as the black soldier fly or L. migratoria may have different thermal optima depends on the diet as related to its natural history (Kutz et al., 2019).
Both the metabolism and growth rate can increase with increasing temperatures, which have been determined for the locust L. migratoria (Clissold et al., 2013) and black soldier fly (Gligorescu et al., 2018). So, there is obviously a tradeoff between optimum metabolic efficiency and optimum growth rate. Coggan et al. (2011) determined L. migratoria tented to maximize metabolic efficiency when diet was limited but to maximize growth rate when diet was in excess. Given greater metabolic costs (i.e., at least 1 µW/mg) were determined in black soldier fly larvae when shifting from the control diet to two synthetic diets (i.e., 86.1% protein + 12.3% carbohydrate and 8.7% protein + 85.6% carbohydrate) (Gligorescu et al., 2018). It is possible that black soldier fly larvae developed on synthetic diets, as in this study, preferred a lower temperature to maximize metabolic efficiency, while larvae fed the control diet favored higher temperatures to maximize growth.
Comparisons to previous studies
Stage, age, size, and sex impact black soldier fly thermal tolerance and preference were determined previously; however, a single diet (i.e., control diet) was used (Addeo et al., 2022; Li et al., 2022). In general, the results of the control diet from the current study were cross validated with these other studies. Fortunately, results were consistent across studies with any minor differences determined being explained by method modifications. For example, the observation of heat knockdown temperatures in the current study was done under water instead of removing the tubes from the water as in the previous studies in order to increase accuracy (i.e., avoid cooling due to removal); the chill-coma recovery time was used instead of cold knockdown temperatures to avoid a short observation window; the temperature range on the thermal gradient was adjusted to 10–45 °C instead of 15–60 °C to avoid heat killed individuals.
Limitations of current study
The synthetic diets were formulated with chemically defined ingredients, which differs from the control diet (i.e., Gainesville is comprised of plant materials). Diet ingredients (e.g., fatty acids, Holmbeck & Rand, 2015), which have been determined to impact insect thermal traits, may also play a role in our results. Future studies should consider using a formulated diet with a similar macro nutrients composition to Gainesville. By doing so, one could separate the impact of type of ingredients from the nutrient effects.
Ramping rate is one of the well-known factors causing variations in thermal tolerance experiments, which fast ramping rates may result in an overestimate of the upper critical thermal tolerance (Overgaard et al., 2012; Peralta-Maraver & Rezende, 2021). The ramping rate (i.e., 1 °C/min) used in the heat tolerance experiment was relatively fast within published range of ramping rate, which may overestimate black soldier fly heat tolerance in an industrialized setting where the ramping rate is potentially slower.
Last but not least, only one genotype of black soldier fly was tested in the current study. This genotype has a long adaptive history in College Station, TX, USA, which may not represent other genotypes as well as colonies adapted to other climates.
Applications and recommendations
This study provides the first records of diet-dependent stage or sex impacts on black soldier fly thermal tolerance (in terms of heat knockdown temperature and chill-coma recovery time) and preference. The inclusion of body weights and body composition measurements indicate physiological complexities as related to phenotypic variation of the black soldier fly and its thermal traits. Note that extreme temperatures frequently happen in black soldier fly mass rearing facilities, for example, larval rearing substrate temperature can easily go up above 45 °C (Li et al., 2023; Fig. S7). The adult body temperature can also increase rapidly under sunshine (e.g., Fig. S8 showed how the adult body temperature went above 45 °C within 2 min under direct sunshine). Larval diet in an industrial facility producing black soldier fly larvae can be highly variable and impact biomass production and colony success if temperature responses as those examined in the current study are not taken into account. The impacts of diets should be carefully considered when setting the optimal rearing temperatures and threshold temperatures as a means to avoid such pitfalls and limitations.
Acknowledgments
This is part of the author's PhD project done in Texas A&M University. The authors would like to thank Thomas M. Chappell and Ada Szczepaniec for suggested edits during the initial development of this manuscript. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The conclusions in this report are those of the authors and do not necessarily represent the views of the USDA. USDA is an equal opportunity provider and employer.
Disclosure
Material for use in this project was purchased from EVO Conversion Systems LLC, a company with which Dr. Tomberlin has a significant financial interest. This conflict of interest is managed by a plan submitted to and approved by Texas A&M University and Texas A&M AgriLife.




