Effects of host plants on aphid feeding behavior, fitness, and Buchnera aphidicola titer
Abstract
Aphids are sap-feeding plant pests that depend on their symbiotic relationships with the primary endosymbiont Buchnera aphidicola to adapt to impoverished diets. However, how the host plant affects the aphid primary symbiont and aphid adaptation to host plant transfer are poorly known. In this study, aphid symbiont screening and genotype identification were used to establish 2 aphid strains (Rhopalosiphum maidis [Rm] and Rhopalosiphum padi [Rp] strains) containing only Buchnera without any secondary symbionts for both wheat aphid species (R. maidis and R. padi). Aphid fitness and Buchnera titers were unstable on some of these host plants after transferring to novel host plants (G1–G5), which were influenced by host plant species and generations; however, they stabilized after prolonged feeding on the same plants for 10 generations. The electropenetrography (EPG) records showed that the allocation of aphid feeding time was significantly distinct in the 6 host plants; aphids had more intracellular punctures and spent more nonprobing time on green bristlegrass which was not conducive to its growth compared with other plants. The content of soluble sugar, soluble protein, and amino acid in the leaves of the 6 host plants were also clearly separated. The correlation coefficient analysis showed that the nutrient contents of host plants had significant correlations with aphid feeding behaviors, fitness, and Buchnera titers. In the meantime, aphid fitness, and Buchnera titers were also affected by aphid feeding behaviors. Also, Buchnera titers of aphid natural populations on 6 host plants showed a visible difference. Our study deepened our understanding of the interaction among aphids, endosymbionts, and host plants, indicating that the host plant nutrient content is a predominant factor affecting aphid adaptation to their diet, initially affecting aphid feeding behaviors, and further affecting aphid fitness and Buchnera titers, which would further contribute to exploiting new available strategies for aphid control.
Introduction
Buchnera aphidicola (Gammaproteobacteria, referred to here as Buchnera) was found in almost all aphids as the primary symbiont, which could provide aphids with essential amino acids (EAAs) that are rare in the plant phloem sap (Hansen & Moran, 2011). In addition to providing essential nutrients, recent studies discovered that Buchnera heat resistance contributes to aphid thermal tolerance (Zhang et al., 2019) and found an association between Buchnera density and the susceptibility of Aphis gossypii Glover to a pesticide (Guo et al., 2020). It is obvious that the versatility of Buchnera was closely linked to the survival of aphids, while Buchnera abundance was affected by several factors.
Host plants affect aphid Buchnera titers and feeding behaviors. Buchnera titers of A. gossypii varied significantly among host plants (Ma et al., 2011), and the Buchnera titer decreased as Aphis glycines Matsumura fed on virus-infected soybeans (Cassone et al., 2015). Further study showed that when Buchnera was being disrupted, aphids performed delayed acceptance of host plants and encountered a problem with stylet penetration into plants (Machado-Assefh et al., 2015), and barley varieties also led to an alteration in aphid feeding behaviors (Leybourne et al., 2019). Further, a follow-up study confirmed the participation of Buchnera in aphid–host plant interactions (Machado-Assefh & Alvarez, 2018).
Pertinent studies have discovered a close relationship between host plant nutrient concentration and aphid ecology, and aphids prefer plants with high nitrogen quality (Alkhedir et al., 2013; Liu et al., 2016). The concentrations of soluble sugars and proteins were reduced in Acyrthosiphon pisum under the disruption of Buchnera (Lv et al., 2018). Buchnera was more active in EAAs metabolizing during the aphid nymphal stage (Russell et al., 2013; Pers & Hansen, 2021), and a higher proportion of EAAs appeared to be conducive to both aphid reproduction on host plants and Buchnera growth (Liu et al., 2016). Additionally, EAAs proportion in host plants influenced the obligate endosymbiont titers in Bemisia tabaci (Gennadius) as well (Liu et al., 2020).
Variation in Buchnera titers will also affect aphid fitness. Reduced Buchnera titers resulted in a negative effect on cowpea aphid reproduction (Chen et al., 2009). Infection with secondary symbionts (S-symbionts) may cause the variation of Buchnera titers and eventually lead to a different performance of aphid fitness (Liu et al., 2023). However, previous studies showed that the Buchnera titers could not influence the host specificity of A. gossypii (Guo et al., 2022) and intermediate Buchnera titers were fit for the natural population of A. pisum (Hansen & Moran, 2011).
Both Rhopalosiphum maidis (Fitch) and Rhopalosiphum padi (L.) are capable of transmitting plant viruses, ultimately leading to crop yield losses due to impaired photosynthesis (Parry et al., 2012; Stewart et al., 2020; Guo et al., 2023), while Buchnera is a key factor which contributes to aphid adaptation to changing surroundings (Douglas, 2015). However, it is unclear which aspects of the aphids are influenced by host plants, and the interaction between host plant species, aphid ecology, and Buchnera titers remains unexplored in R. maidis and R. padi. Therefore, our study recorded the aphid fitness, measured Buchnera titers, monitored aphid feeding behaviors, determined the host plant nutrient content, and ultimately analyzed their correlations. Overall, our study aimed to have a better understanding of the interaction among aphids, endosymbionts, and host plants.
Materials and methods
Rearing and characterization of aphid strains
Aphid samples were collected from a maize field in Langfang City, Hebei Province, China. At first, to identify the species of all the aphid samples, aphid total DNA was extracted with a mixed solution of 0.1 mol/L Tris–hydrochloride buffer (1 mL) and proteinase K (8 μL), according to the previous method described by Myint et al. (2021) with minor modifications. Aphids were separately crushed in a mixed solution (30 μL) and centrifuged at 8 000×g for 1 min at room temperature. The homogenate was subsequently incubated at 65 °C for 30 min, 25 °C for 2 min, 96 °C for 10 min, and a final hold at 4 °C. The species of aphid samples were identified based on the mitochondrial cytochrome oxidase I (COI) gene sequences. Polymerase chain reaction (PCR) cycling conditions were 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min, 72 °C for 10 min for final extension, and holding at 10 °C.
Then, all the aphid samples were screened for the primary symbiont and all the known S-symbionts with PCR, using universal primers and specific primers based on the symbionts' 16S ribosomal RNA (rRNA) gene sequences. Only aphids containing the primary symbiont were left for further aphid genotype identification. Lastly, aphids were genotyped using 4 microsatellite loci: R3.171, R5.10, R5.50, and S17b (Wilson et al., 2004; Leybourne et al., 2020a). The PCR cycling conditions for endosymbiont detection and genotype identification were the same as those for aphid species identification, with modifications in the annealing temperatures. All the primers are shown in Table S1, and all the required gene sequences have been deposited in GenBank under accession numbers listed in Table S2.
R. maidis (Rm) and R. padi (Rp) strains were established initially from a different single aphid, only containing Buchnera without any S-symbionts. Both strains were reared indoors on barley seedlings (Hordeum vulgare L.) at a constant 25 ± 1 °C with 75% relative humidity and a 16-h daily light cycle.
Plant materials
Six host plants, namely, barley, wheat (Triticum aestivum L.), maize (Zea mays L.), sorghum (Sorghum bicolor L. Moench), rice (Oryza sativa L.), and green bristlegrass (Setaria viridis L. Beauv), were used in this study. Plant seeds were sown in soil (peat : vermiculite ratio = 2 : 1) in a pot. After germination, maize seedlings were allowed to grow into a 4-leaf stage (35 cm in height) in the pot (10 cm in diameter), then surrounded by transparent plastic buckets with their tops covered by gauze. Other plant seedlings were placed in Petri dishes with roots and a dash of soil wrapped in wet cotton. All the plants were kept in a growth chamber under the same conditions as aphid rearing.
Aphid sample collection
To understand the variation of Buchnera titers in aphid natural populations, aphid samples were collected from 6 host plants in the field for 2 consecutive years in the same location where both aphid strains were collected. At least 15 3rd instar aphids were collected for each species on each host plant. All samples were preserved in absolute ethanol.
Aphid strain fitness measurement
To minimize any confounding effects from host plant alteration, aphid strains were initially reared on barley for at least 5 generations before being subjected to further experiments.
In the beginning, adult aphids were transferred onto new barley seedlings, producing offspring termed G0, which were allowed to become adults. Then, the G0 adult aphids were transferred onto new barley or 1 of the different novel host plants to produce offspring termed G1. Adult aphids were removed after being transferred for 12 h. Newborn aphid survival was recorded daily for G1 until the 7th d, and on the 5th d for G2 to G5, G10, and G20. Aphid mature duration (days from birth to first reproduction), fecundity, and weight (20 newly matured adults) of each generation were recorded, and fresh plant seedlings were replaced every 5 d. Three biological replicates were performed with at least 20 aphids per replicate for every host plant in each aphid generation.
Determination of aphid Buchnera titers
To understand the effect of host plants on aphid Buchnera titers, DNA was extracted from the 3rd instar aphids of natural, G0 to G5, G10, and G20 aphid population samples. The Buchnera titers were calculated by the ratio of the copy number of the Buchnera 16S rRNA gene to that of the ef1α gene. Primers are shown in Table S1, and quantitative PCR was performed according to 1 previous method (Liu et al., 2023). For each treatment, at least 5 aphids were pooled as a biological replicate, and 3 of them were performed, with 4 technical replicates for each.
Aphid feeding behaviors
The Giga-8 DC electropenetrography (EPG) amplifier (EPG-Systems, Wageningen, Netherlands) was used to monitor aphid feeding behaviors after prolonged feeding on the same host plant. The EPG system was connected to a Faraday cage. In the beginning, newly matured aphids were starved for 2 h in Petri dishes. Then, the aphid dorsum was pasted to a piece of gold wire (18 μm diameter × 4–5 cm length) with conductive water-soluble silver glue (weight of glue : silver : water = 3 : 3 : 2), the free end of the wire was adhered to a copper nail which was inserted into the EPG amplifier. After that, 8 channels of aphids were placed onto plant seedlings separately, and the plant electrode was inserted into the soil. The EPG signals of 12 aphids for each treatment were recorded with Stylet+d software for 8 h under light conditions at a constant 25 ± 1 °C. EPG waveforms were sorted by Stylet+a software according to the method described by Tjallingii (1994). Waveforms were assigned to np (nonprobing), C (intercellular stylet pathway), pd (intracellular punctures), E1 (saliva secretion into phloem), E2 (saliva secretion and passive phloem ingestion), and G (xylem ingestion) parameters. EPG waveforms were transformed into time-series data and calculated by “Workbook”, which can be found in Sarria et al. (2009).
Nutrient contents of host plants
Plant seedlings used for nutrient content analysis were at the same stage as those used for aphid fitness measurement. Fresh leaves of 6 host plants were measured for soluble sugars, soluble proteins, and 20 free amino acids with 3 replicates. The soluble sugars and proteins were determined according to the methods of DuBois et al. (1956) and Bradford (1976), respectively.
For the determination of amino acid concentration, plant leaves (0.4 g) were put in the centrifuge tube and 0.1 mol/L HCl (2 mL) was added. After shaking the mixture, the plant seedlings were extracted with ultrasound for 40 min and shaken 3 times during the extraction. Then, the mixture was centrifuged for 10 min at a high speed of 9600 xg at 4 °C. After the centrifugation, the supernatant of the mixture was taken to another centrifuge tube, 0.1 mol/L HCl (1 mL) was added, and reserved for later use. Following the instructions above, the supernatant was extracted twice for amino acid analysis. To get ready for the amino acid analysis, 2 volumes of the supernatant were combined and filtered. Amino acids were analyzed by high performance liquid chromatography – mass spectrometry (HPLC-MS) with the chromatographic column, ACQUITY UPLC BEH Amide (1.7 μm, 2.1 mm × 100 mm; Waters, Milford, USA). Mobile phase A was an aqueous solution containing 20 mmol/L formic acid, and mobile phase B was an acetonitrile solution containing 20 mmol/L formic acid, with a flow rate of 0.25 mL/min at 30 °C. Positive electron spray ionization was at 450 °C and 5.5 kV, with medium collision gas, 30 psi curtain gas, and 50 psi for GS1 and GS2. All the acquired data were quantified based on the standard curves and standard solution concentrations.
Statistical analysis
The survival rates of aphid strain G1 were visualized as Kaplan–Meier survival curves and assessed with Cox's model by Chi-squared test (P < 0.05) with GraphPad Prism 9.0.0.121. Two-way analysis of variance (ANOVA) was applied to explore the effects of host plant and generation on aphid fitness and Buchnera titers. One-way ANOVA, with Tukey's test at a 0.05 level with post facto multiple comparisons of means, was employed to explore the differences in aphid fitness among generations, aphid Buchnera titers among generations and natural populations, Buchnera titers of aphid natural populations among host plants, EPG parameters, and host plant nutrient contents. Both ANOVAs were conducted with IBM SPSS Statistics 26.0.
Principal component analysis (PCA) of amino acid concentrations was performed and plotted by Metware Cloud online tools, which can be accessed online at https://cloud.metware.cn/#/tools/tool-form?toolId = 230. The data were square root transformed and standardized before analysis. Pearson's correlation coefficient was used to measure the relationship between aphid EPG parameters, aphid fitness, Buchnera titers, and host plant nutrient content in Origin 2022 software.
Results
Genotypes of aphid strains
Based on the pattern of microsatellite loci sizes, R. maidis was grouped into 4 genotypes (labeled A–D) (Liu et al., 2023), and R. padi was grouped into 3 genotypes (labeled E–G) (Table S3). To maintain the same aphid genotypes in our further research, aphids with genotypes D or E were selected to build Rm and Rp, respectively, for the following experiments.
Effects of host plant transfer on aphid fitness
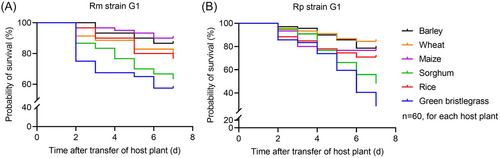
Rm and Rp generation 1 (G1) survival in 7 d was significantly influenced by host plants (Rm: χ2 = 21.09, df = 5; Rp: χ2 = 45.71, df = 5; both P < 0.001) (Fig. 1). Rm had the highest survival rate at 90.0% on the 7th d on maize, and had a survival rate of over 70% on barley, wheat, and rice, while having a survival rate of less than 65% on sorghum and green bristlegrass. A similar situation has arisen in the Rp survival rate. The result showed that Rp survived better on wheat, barley, maize, and rice, while only having a survival rate of less than 50% on sorghum on the 7th d, and even less than 30% on green bristlegrass. Rm and Rp fitness (G1–G5) was significantly affected by the host plant, generation, and their interaction. Rm and Rp fitness (G10–G20) was only strongly influenced by host plants; also, their weight was also observably affected by their interaction (Tables 1–2).
| G1 to G5 | G10, G20 | ||||||
|---|---|---|---|---|---|---|---|
| Fitness indices | Source | df | F | P | df | F | P |
| Survival rate* | H | 5 | 99.02 | <0.001 | 5 | 118.95 | <0.001 |
| G | 4 | 26.15 | <0.001 | 1 | 0.19 | 0.666 | |
| H × G | 20 | 5.08 | <0.001 | 5 | 0.26 | 0.929 | |
| Mature duration | H | 5 | 189.99 | <0.001 | 5 | 33.87 | <0.001 |
| G | 4 | 132.10 | <0.001 | 1 | 0.22 | 0.640 | |
| H × G | 20 | 31.52 | <0.001 | 5 | 1.17 | 0.354 | |
| Fecundity | H | 5 | 2.42 × 103 | <0.001 | 5 | 1.02 × 103 | <0.001 |
| G | 4 | 50.43 | <0.001 | 1 | 0.11 | 0.744 | |
| H × G | 20 | 6.84 | <0.001 | 5 | 0.51 | 0.767 | |
| Weight† | H | 5 | 1.71 × 104 | <0.001 | 5 | 8.10 × 103 | <0.001 |
| G | 4 | 350.96 | <0.001 | 1 | 20.60 | <0.001 | |
| H × G | 20 | 87.56 | <0.001 | 5 | 6.11 | <0.01 | |
- * Aphid survival rate on the 5th d.
- † Weight of 20 newly matured adults.
| Fitness indices | Source | G1 to G5 | G10, G20 | ||||
|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | ||
| Survival rate* | H | 5 | 107.82 | <0.001 | 5 | 38.13 | <0.001 |
| G | 4 | 2.46 | <0.001 | 1 | <0.01 | 0.961 | |
| H × G | 20 | 3.63 | <0.001 | 5 | 0.07 | 0.996 | |
| Mature duration | H | 5 | 150.58 | <0.001 | 5 | 75.23 | <0.001 |
| G | 4 | 23.93 | <0.001 | 1 | 0.46 | 0.503 | |
| H × G | 20 | 5.87 | <0.001 | 5 | 1.02 | 0.430 | |
| Fecundity | H | 5 | 1.68 × 103 | <0.001 | 5 | 787.48 | <0.001 |
| G | 4 | 24.95 | <0.001 | 1 | 1.41 | 0.248 | |
| H × G | 20 | 22.96 | <0.001 | 5 | 2.08 | 0.103 | |
| Weight† | H | 5 | 8.52 × 103 | <0.001 | 5 | 2.90 × 103 | <0.001 |
| G | 4 | 36.18 | <0.001 | 1 | 0.27 | 0.607 | |
| H × G | 20 | 60.89 | <0.001 | 5 | 5.90 | <0.01 | |
- * Aphid survival rate on the 5th d.
- † Weight of 20 newly matured adults.
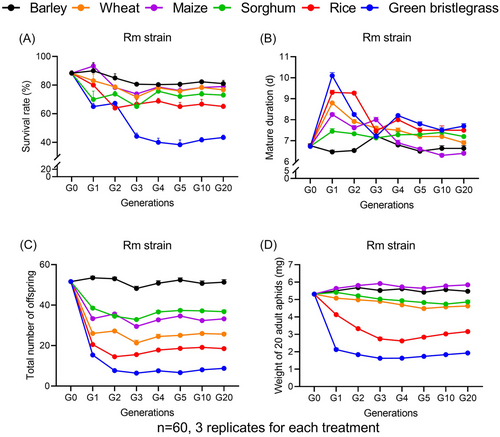
Rm survival showed significant fluctuation at G1–G3 on wheat (F6, 14 = 2.50, P = 0.074), maize (F6, 14 = 12.17, P < 0.001), rice (F6, 14 = 5.60, P < 0.01) and green bristlegrass (F6, 14 = 27.92, P < 0.001), while remaining stable on barley (F6, 14 = 1.80, P = 0.170) and sorghum (F6, 14 = 1.31, P = 0.316) (Fig. 2A). Interestingly, Rm mature duration remained similar among generations on sorghum (F6, 14 = 1.13, P = 0.396), but displayed significant fluctuation on barley (F6, 14 = 5.27, P < 0.01), wheat (F6, 14 = 51.34, P < 0.001), maize (F6, 14 = 67.06, P < 0.001), rice (F6, 14 = 38.82, P < 0.001) and green bristlegrass (F6, 14 = 85.76, P < 0.001) (Fig. 2B). Rm fecundity had no significant difference among generations on barley (F6, 14 = 2.52, P = 0.07), but diminished dramatically at G1 and showed significant fluctuation on wheat (F6, 14 = 6.29, P < 0.01), maize (F6, 14 = 12.23, P < 0.001), sorghum (F6, 14 = 6.78, P < 0.01), rice (F6, 14 = 48.95, P < 0.001) and green bristlegrass (F6, 14 = 545.67, P < 0.001) (Fig. 2C). Rm weight varied greatly throughout generations on all the host plant (barley, F6, 14 = 15.95, P < 0.001; wheat, F6, 14 = 202.26, P < 0.001; maize, F6, 14 = 13.23, P < 0.001; sorghum, F6, 14 = 47.89, P < 0.001; rice, F6, 14 = 295.03, P < 0.001; green bristlegrass, F6, 14 = 71.62, P < 0.001) (Fig. 2D). Overall, Rm survived better on barley and maize, with higher survival rates, greater numbers of offspring, heavier weights and shorter mature durations; however, they performed poorly on rice and green bristlegrass.
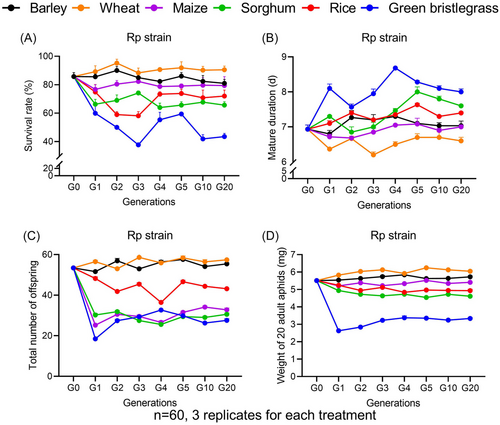
Rp survival showed no significant difference between generations on barley (F6, 14 = 1.48, P = 0.255), wheat (F6, 14 = 0.51, P = 0.794), maize (F6, 14 = 0.23, P = 0.962), and sorghum (F6, 14 = 1.55, P = 0.233); however, it varied greatly on rice (F6, 14 = 3.62, P < 0.05) and green bristlegrass (F6, 14 = 5.60, P < 0.01) (Fig. 3A). The Rp mature duration was stable on barley (F6, 14 = 1.58, P = 0.23), but showed fluctuation across generations on wheat (F6, 14 = 3.88, P < 0.05), maize (F6, 14 = 3.42, P < 0.05), sorghum (F6, 14 = 16.71, P < 0.001), rice (F6, 14 = 14.76, P < 0.001), and green bristlegrass (F6, 14 = 14.02, P < 0.001) (Fig. 3B). Rp fecundity and weight exerted fluctuation among generations (fecundity: barley, F6, 14 = 8.67, P < 0.001; wheat, F6, 14 = 4.01, P < 0.05; maize, F6, 14 = 19.63, P < 0.001; sorghum, F6, 14 = 10.42, P < 0.001; rice, F6, 14 = 35.31, P < 0.001; green bristlegrass, F6, 14 = 73.69, P < 0.001; Fig. 3C) (weight : barley, F6, 14 = 11.78, P < 0.001; wheat, F6, 14 = 53.23, P < 0.001; maize, F6, 14 = 42.11, P < 0.001; sorghum, F6, 14 = 26.58, P < 0.001; rice, F6, 14 = 29.89, P < 0.001; green bristlegrass, F6, 14 = 73.43, P < 0.001; Fig. 3D). In general, Rp survived better on wheat and barley, with higher survival rates, more offspring, heavier weights and shorter mature durations; however, they performed poorly on sorghum and green bristlegrass.
Host plant transfer influence on aphid Buchnera titers
Aphid Buchnera titer was the ratio of the copy number of the Buchnera 16S rRNA gene to that of the ef1α gene. Rm and Rp Buchnera titers (G1–G5) were significantly affected by the host plant, generation and their interaction, while their Buchnera titers (G10–G20) were only significantly influenced by the host plant (Table 3).
| Aphid strain | Source | G1 to G5 | G10, G20 | ||||
|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | ||
| Rm strain | H | 5 | 135.14 | <0.001 | 5 | 142.34 | <0.001 |
| G | 4 | 4.34 | <0.01 | 1 | 2.02 | 0.168 | |
| H × G | 20 | 6.01 | <0.001 | 5 | 0.51 | 0.764 | |
| Rp strain | H | 5 | 90.11 | <0.001 | 5 | 26.15 | <0.001 |
| G | 4 | 21.80 | <0.001 | 1 | 0.37 | 0.550 | |
| H × G | 20 | 6.06 | <0.001 | 5 | 1. 75 | 0.205 | |
Rm Buchnera titers only remained stable on barley (F6, 14 = 0.33, P = 0.91) but not on the other host plants (wheat, F6, 14 = 7.20, P < 0.01; maize, F6, 14 = 4.30, P < 0.05; sorghum, F6, 14 = 30.51, P < 0.001; rice, F6, 14 = 6.34, P < 0.01; green bristlegrass, F6, 14 = 5.51, P < 0.01; Fig. 4A). Rp Buchnera titers showed significant variation across generations on all the host plants (barley, F6, 14 = 6.38, P < 0.01; wheat, F6, 14 = 8.95, P < 0.001; maize, F6, 14 = 22.61, P < 0.001; sorghum, F6, 14 = 3.16, P < 0.05; rice, F6, 14 = 6.41, P < 0.01; green bristlegrass, F6, 14 = 6.56, P < 0.01; Fig. 4B). Both Rm and Rp Buchnera titers remained stable at G10–G20.
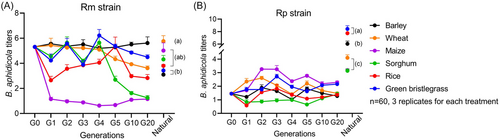
The host plants exerted a notable influence on aphid natural population Buchnera titers (R. maidis, F5, 12 = 5.43, P < 0.01; R. padi, F5, 12 = 59.44, P < 0.001). When compared to long-term indoor aphid strains (G10–G20), all the R. padi natural population Buchnera titers were strongly higher (both P < 0.001), while R. maidis natural population Buchnera titers were observably higher on wheat (F2, 6 = 19.55, P < 0.01), maize (F2, 6 = 38.66, P < 0.001), sorghum (F2, 6 = 60.57, P < 0.001), and rice (F2, 6 = 9.83, P < 0.05), but lower on barley (F2, 6 = 8.00, P < 0.05) and green bristlegrass (F2, 6 = 25.48, P < 0.01) (Fig. 4).
Host plants affect aphid feeding behaviors
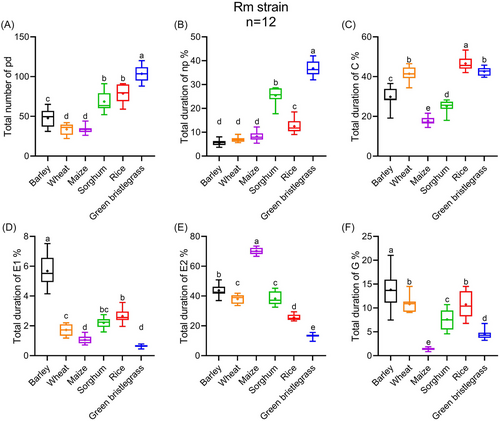
During the 8 h EPG recording, the allocation of Rm and Rp feeding time was totally different among the 6 host plants. Rm had the highest total number of intracellular punctures (pd) (F5, 66 = 96.59, P < 0.001) and nonprobing duration (np) (F5, 66 = 322.02, P < 0.001) on green bristlegrass, spent nearly half of its time in intercellular stylet pathway (C) on rice, markedly longer than the others (F5, 66 = 151.89, P < 0.001) (Fig. 5A–C). Rm had the longest saliva secretion duration (E1) (F5, 66 = 148.10, P < 0.001) and xylem ingestion duration (G) (F5, 66 = 53.77, P < 0.001) on barley, and took the longest passive phloem ingestion duration (E2) (F5, 66 = 480.34, P < 0.001) on maize when compared with other plants (Fig. 5D–F).
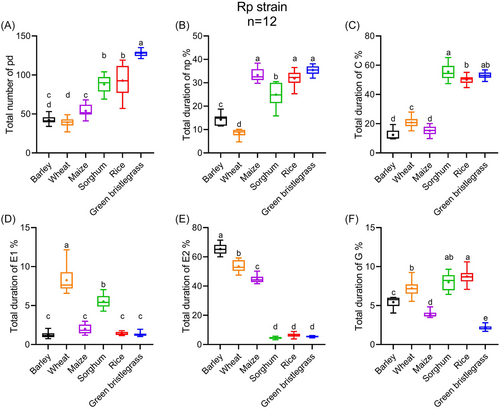
Like Rm, Rp also had the highest total number of intracellular punctures (pd) (F5, 66 = 137.59, P < 0.001) and nonprobing duration (np) (F5, 66 = 157.27, P < 0.001) on green bristlegrass (Fig. 6A–B). Rp required only 12.4% of its time in intercellular stylet pathway (C) on barley which did not differ from maize but was lower than the others (F5, 66 = 406.04, P < 0.001) (Fig. 6C). Rp took the longest saliva secretion duration (E1) on wheat (F5, 66 = 160.70, P < 0.001) and passive phloem ingestion (E2) on barley (F5, 66 = 1.50 × 103, P < 0.001) (Fig. 6D–E). Rp took 8.7% of its feeding time in xylem ingestion, slightly longer than sorghum, but notably longer than the others (F5, 66 = 122.55, P < 0.001) (Fig. 6F).
Nutrient contents of 6 host plants
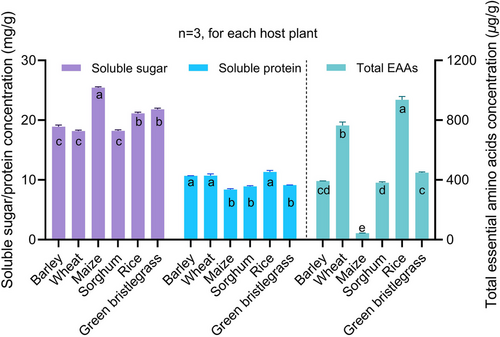
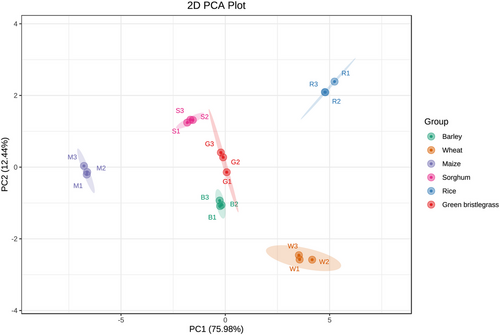
The nutrient contents of the leaves of the 6 host plants were determined. Maize had the highest soluble sugar at 25.43 mg/g (F5, 12 = 195.58, P < 0.001). No significant difference was found in soluble protein among barley, wheat, and green bristlegrass, which were markedly higher than the others (F5, 12 = 42.82, P < 0.001). The total EAA concentrations varied greatly among host plants, with maize having only 43.52 C, extremely lower than the others (F5, 12 = 546.40, P < 0.001) (Fig. 7). PCA indicated lower variations in amino acid composition in the samples of aphids from the same host plant and revealed a clear separation among host plants. The first and second principal components explained 75.9% and 12.4% of the total variation, respectively (Fig. 8).
Correlation coefficient analysis
The correlation matrixes were constructed to understand the relationship between host plant nutrient contents, aphid feeding behaviors and fitness, and Buchnera titers of 3rd instar aphids (Fig. 9).
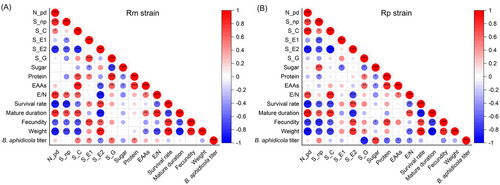
For Rm, soluble sugar had significant negative correlations with xylem ingestion (S_G) (r = −0.76, P < 0.001), while soluble protein was positively correlated with it (r = 0.84, P < 0.001) and intercellular stylet pathway duration (S_C) (r = 0.68, P < 0.01). EAAs were positively correlated with intercellular stylet pathway duration (S_C) and xylem ingestion (S_G) (r = 0.89, P < 0.001; r = 0.62, P < 0.01); however, they were negatively correlated with phloem ingestion (S_E2) (r = −0.64, P < 0.05). The ratio of total EAAs concentration to total nonessential amino acids concentration (E/N) had significant positive correlations with the number of intracellular punctures (N_pd) (r = 0.84, P < 0.001); however, they negatively correlated with phloem ingestion (S_E2) (r = −0.87, P < 0.001). For Rm fitness, both EAAs and E/N were positively correlated with mature duration (r = 0.62, P < 0.01; r = 0.85, P < 0.001); meanwhile, they were negatively correlated with weight (r = 0.50, P < 0.05; r = 0.72, P < 0.001). Rm mature duration was positively correlated with intracellular punctures (N_pd) (r = 0.82, P < 0.001), nonprobing duration (S_np) (r = 0.68, P < 0.01) and intercellular stylet pathway duration (S_C) (r = 0.73, P < 0.001), but were negatively correlated with phloem ingestion (S_E2) (r = −0.89, P < 0.001). The correlations between survival rate, fecundity or weight and these 4 EPG parameters were inversely different from mature duration (|r| ≥ 0.54, P < 0.05). Rm Buchnera titer was positively correlated with intercellular stylet pathway duration (S_C) (r = 0.52, P < 0.05), saliva secretion (S_E1) (r = 0.47, P < 0.05), and xylem ingestion (S_G) (r = 0.58, P < 0.05), and also the soluble protein (r = 0.51, P < 0.05), but negatively correlated with phloem ingestion (S_E2) (r = −0.51, P < 0.05) (Fig. 9A).
For Rp, soluble protein was negatively correlated with nonprobing duration (S_np) (r = −0.47, P < 0.05) and positively correlated with xylem ingestion (S_G) (r = 0.54, P < 0.05), while the correlations between soluble sugar and these 2 EPG parameters were conversely different from soluble protein (r = 0.72, P < 0.001; r = −0.56, P < 0.05). EAAs were positively correlated with aphid xylem ingestion (S_G) (r = 0.61, P < 0.01). Similar to Rm, E/N was also positively correlated with the number of aphid intracellular punctures (N_pd) and intercellular stylet pathway duration (S_C) (r = 0.73, P < 0.001; r = 0.84, P < 0.001), while being negatively correlated with aphid phloem ingestion (S_E2) (r = −0.73, P < 0.001). For Rp fitness, fecundity was positively correlated with soluble protein (r = 0.76, P < 0.001). E/N was found negatively correlated to the survival rate and weight (r = −0.59, P < 0.01; r = −0.61, P < 0.01), while being positively correlated to mature duration (r = 0.71, P < 0.001). Rp intracellular punctures (N_pd), nonprobing duration (S_np), intercellular stylet pathway duration (S_C), and xylem ingestion (S_G) were largely correlated with Rp fitness (|r| ≥ 0.65, P < 0.01). Rp Buchnera titer was positively correlated with soluble sugar (r = 0.76, P < 0.001) and negatively correlated with xylem ingestion (S_G) (r = −0.84, P < 0.001) (Fig. 9B).
Discussion
Buchnera provides aphids with nutritional supplementation that is absent in their diet (Baumann, 2005). Host plants had a substantial effect on aphid Buchnera titers (Ma et al., 2011), and Buchnera was involved in aphid–host plant interaction by participating in aphid feeding behaviors (Machado-Assefh & Alvarez, 2018). So far, the connection between host plants, feeding behaviors, and Buchnera titers in R. maidis and R. padi is still not evident at this time. Our study investigated the effect of host plant alternation on aphid fitness and Buchnera titers, monitored aphid feeding behaviors, and determined host plant nutrient contents. Host plants had a profound influence on aphid feeding behaviors, fitness, and Buchnera titers, which in all probability may be on account of host plant nutrient contents.
Aphid growth was influenced by host plant species and appeared to have observable changes once they were transferred onto novel plants (Maremela et al., 2013; Ali et al., 2021). Our result illustrated that wheat aphid fitness can be affected due to host plant alternation and will become stable after prolonged feeding on the same host plant. Buchnera was closely related to aphid adaptation, and evidence about A. fabae has shown that Buchnera titers were always variable with plant species (Wilkinson et al., 2001).
Host plants have a large influence on endosymbiont abundance (Liu et al., 2020). In our study, observable differences were found among aphid natural population Buchnera titers from different host plants. Further, both aphid Buchnera titers became erratic after host plant transfer, with pronounced fluctuations in the following generations, and remained stable at G10–G20. Likewise, previous studies reported that the Buchnera abundance in Aphis citricidus (Kirkaldy) was associated with host plants (Guidolin & Consoli, 2017) and aphid survival became less variable with Buchnera titer stabilization after aphids fed on novel plants for 2 to 3 generations (Zhang et al., 2016). A similar phenomenon has been found in our study, where aphid fitness and Buchnera titers were steady, which were accompanied by prolonged feeding on the same host plant after 10 generations. It is reported that Buchnera titers were linked to aphid reproduction (Chen et al., 2009), while our result showed that there was no significant connection between aphid fitness and Buchnera titers, which may be due to the different host plants, and aphids need several generations to adapt to novel host plants. In natural populations, aphids favored the intermediate Buchnera titers (Vogel & Moran, 2011), while our study found that the Buchnera titers of some aphid natural populations were markedly higher than long-term indoor aphid strains (G10–G20) and some were not, which may be due to the challenging environment (Vogel & Moran, 2011). However, flexible Buchnera abundance was a regulatory modality that helped aphids adapt to their surroundings (Neiers et al., 2021).
In our study, EPG parameters revealed that host plant species had a great influence on aphid feeding behaviors. The reduction in ingesting phloem time possibly contributed to decreasing aphid fitness on partially resistant barley (Leybourne et al., 2020b). Buchnera was also known to influence aphid stylet penetration on plants, probably through the specific protein GroEL (Machado-Assefh & Alvarez, 2018). We speculated that plant defenses altered aphid feeding behaviors, along with the variation of Buchnera titers, which may together affect aphid fitness. The plant water-soluble carbohydrate concentration affected aphid reproduction and mortality (Alkhedir et al., 2013); higher nitrogen quality and EAAs favored aphid adaptation to plants (Liu et al., 2016), while the Buchnera abundance was correlated with plant nutrients (Wilkinson et al., 2007; Liu et al., 2016). Our results agree with the previous study that phloem amino acid composition varies among different plant species (Wilkinson & Douglas, 2003), and demonstrated that aphid feeding behaviors, fitness, and Buchnera titers were discrepant among host plants, inferring that they were all influenced by plant nutrient contents.
Correlative studies verified that reduced aphid nymph growth was caused by the substantial decline in aphid phloem ingestion (S_E2) (Leybourne et al., 2020b). We assumed that aphid feeding behaviors could be a key factor that contributes to aphid fitness. A previous study documented the positive correlation between aphid performance and sucrose (Jensen et al., 2013). However, aphid adaptation to sucrose was species-specific, which may be related to its host range (Puterka et al., 2017). An inferior E/N may lead to extra growth costs for aphids (Simpson et al., 1995), as we discovered that E/N was positively correlated with aphid mature duration and negatively correlated with aphid weight. In addition, the soluble sugar and protein concentrations were correlated with Rp fecundity, indicating that the plant nutrient contents could be another pivotal factor affecting aphid fitness.
Aphids can benefit from plant phloem containing a higher proportion of E/N (Liu et al., 2016); meanwhile, a reduction in phloem ingestion (S_E2) may likely be adverse to aphid growth (Leybourne et al., 2020b), implying that aphids may take longer in phloem ingestion (S_E2) involving better nitrogen quality. Interestingly, our result revealed that E/N was negatively correlated to phloem ingestion (S_E2) and positively correlated to the number of intracellular punctures (N_pd) or intercellular stylet pathway duration (S_C). Additionally, both soluble protein and EAA total concentrations were positively correlated to aphid xylem ingestion (S_G). Occasionally, the latter was recognized as a mechanism by which aphids deal with the osmotic effects of large amounts of phloem ingestion (Escudero-Martinez et al., 2021). Our results suggested that various host plant nutrient contents account for the differences in aphid feeding time allocation.
Aphid Buchnera abundance was positively correlated with dietary nitrogen levels (Wilkinson et al., 2007) and Buchnera participated in aphid colonization on host plants (Machado-Assefh & Alvarez, 2018). The relationships of Buchnera titers with aphid feeding behaviors and host plant nutrient contents were divergent in these 2 aphid strains, and converse correlations between Buchnera titers and aphid xylem ingestion (S_G) or soluble protein concentration were found in both aphid strains. Further, numerous studies revealed that Buchnera titers were correlated with aphid fitness (Chen et al., 2009; Zhang et al., 2019), and aphid survival relied heavily on EAAs supplied by Buchnera (Shigenobu et al., 2000). Nonetheless, we did not find any correlations between them. Indeed, Buchnera titers were also influenced by host plant secondary metabolites. For example, gossypol in cotton suppressed the Buchnera density in aphids while cucurbitacin from cucurbit increased Buchnera density (Zhang et al., 2016).
Our study characterized the interaction between host plants and aphids, elucidated that host plants had a large influence on aphid ecology due to its diverse nutrient contents, as it affected aphid feeding behaviors, fitness and Buchnera titers. Meanwhile, aphid fitness and Buchnera titers were also affected by aphid feeding behaviors. Overall, we could conclude that host plant nutrient contents play a critical role in the process of aphid adaptation to plants, affecting aphid feeding behaviors, and further affecting aphid fitness and Buchnera titers.
Acknowledgments
This study was funded by the programs of the China Agriculture Research System of MOF and MARA (CARS–02) and the Agricultural Science and Technology Innovation Program (ASTIP) of CAAS.
Disclosure
All authors have seen and agree with the contents of the manuscript and there is no conflict of interest, including specific financial interest and relationships and affiliations relevant to the subject of the manuscript.




