Predicting pneumococcal community-acquired pneumonia in the emergency department
Evaluation of clinical parameters
Abstract
The aim of this study was to quantify the value of clinical predictors available in the emergency department (ED) in predicting Streptococcus pneumoniae as the cause of community-acquired pneumonia (CAP). A prospective, observational, cohort study of patients with CAP presenting in the ED was performed. Pneumococcal aetiology of CAP was based on either bacteraemia, or S. pneumoniae being cultured from sputum, or urinary immunochromatographic assay positivity, or positivity of a novel serotype-specific urinary antigen detection test. Multivariate logistic regression was used to identify independent predictors and various cut-off values of probability scores were used to evaluate the usefulness of the model. Three hundred and twenty-eight (31.0%) of 1057 patients with CAP had pneumococcal CAP. Nine independent predictors for pneumococcal pneumonia were identified, but the clinical utility of this prediction model was disappointing, because of low positive predictive values or a small yield. Clinical criteria have insufficient diagnostic capacity to predict pneumococcal CAP. Rapid antigen detection tests are needed to diagnose S. pneumoniae at the time of hospital admission.
Introduction
Streptococcus pneumoniae is the most common causative pathogen of community-acquired pneumonia (CAP), being responsible for 20–38% of CAP cases, depending on the population, use of microbiological tests, and definition of ‘causative pathogen’ 1-4. Antibiotic treatment recommendations for CAP have changed in recent years. The current treatment recommendations for CAP are based on the clinical severity of disease, rather than on the presumed aetiology, and recommend combinations of β-lactams and macrolides, or monotherapy with quinolones, for patients hospitalized with CAP 5-8. This implies that most patients with pneumococcal pneumonia now receive broad-spectrum antibiotics. Extensive use of antibiotics could lead to superinfections with antibiotic-resistant pathogens or selection of antibiotic resistance, and will increase healthcare costs 9, 10. Ideally, patients with pneumococcal pneumonia would be treated with narrow-spectrum antibiotics, which would necessitate accurate prediction of pneumococcal CAP at the time of antibiotic prescription. Prediction of CAP aetiology at the time of clinical presentation has been attempted before, with huge heterogeneity in approaches and results 11-16.
Recently, a new serotype-specific urinary antigen detection test (UAD) with high sensitivity and specificity has been clinically validated 17, 18. Using this test, in combination with the immunochromatographic assay (ICA) of BinaxNOW (Alere, Waltham, MA, USA), increased the proportion of patients diagnosed with pneumococcal CAP from 23.5% to 32.6%. With this optimized diagnostic approach for pneumococcal CAP, we aimed to develop a clinical prediction rule for diagnosing pneumococcal CAP in the emergency department (ED) in order to allow empirical treatment with narrow-spectrum antibiotics.
Materials and Methods
Patients
We conducted a prospective, observational, cohort study between January 2008 and April 2009. Adult patients (≥18 years) with a clinical suspicion of CAP presenting at the EDs of 23 Dutch hospitals were eligible. A clinical suspicion of CAP was defined as the presence of at least two of the following criteria: fever/hypothermia, cough/change in chronic coughing pattern, dyspnoea/tachypnoea/hypoxia, findings on percussion/auscultation consistent with pneumonia, leukocytosis/leukopenia, and/or shift to the left or an infiltrate(s) on the chest X-ray. Patients with recent hospitalization or residing in a nursing home, with known bronchial obstruction or a history of post-obstructive pneumonia, patients with primary lung cancer or another malignancy metastatic to the lungs, patients with AIDS, known or suspected Pneumocystis jerovicii pneumonia or known or suspected tuberculosis and unconscious patients were excluded. The study was approved by all local Research Ethics Committees, and written informed consent was obtained from all participants or family members.
A subset of the patients in this study with radiographically confirmed CAP and strict inclusion and exclusion criteria (n = 776) were used to validate the novel UAD 18.
Diagnostic approach
Standard diagnostic procedures included microbiological cultures of blood, sputum, and pleural fluid (if present), and, for study purposes, a urine sample was collected within 48 h after admission.
Urine samples were processed locally: samples were frozen at −70°C until being processed in the laboratory of Pfizer Vaccine Research (Pearl River, NY, USA). Here, both the UAD and the commercially available ICA (Alere) were performed batchwise by two technicians blinded to any clinical information. A third analyst interpreted the results when the first two analysts did not agree. The UAD is a Luminex technology-based multiplex urinary antigen detection assay that can simultaneously detect 13 different serotypes of S. pneumoniae by capturing serotype-specific polysaccharides secreted in human urine. In addition to an enhanced capacity to determine infections caused by one of those 13 pneumococcal serotypes (as compared with episodes yielding pneumococci from blood or other sterile body sites for serotyping), this test also has 41% higher sensitivity than the conventional ICA 17, 18. However, at the moment, this test is only available for research purposes.
Microbiological testing (blood, sputum cultures and BinaxNOW Legionella on urine, if clinically applicable) was performed in local laboratories according to local and manufacturers' protocols, with technicians unaware of the patient's clinical condition. When no urine sample was stored for central processing, the ICA results of the local laboratory were used to define aetiology.
Determinants
Data were collected from the medical chart, during admission or shortly afterwards, and documented in a standardized case record form by trained research nurses and/or physicians.The possible predictors were selected from the literature and because they were easily available in the ED (for a complete overview, see Table 2) 13, 14, 16. Information on previous pneumococcal vaccination was also collected, but this was not considered to be a potential predictor, as it is rarely used in The Netherlands.
Definition of CAP
CAP was defined as the presence of an infiltrate on the chest X-ray at the moment of presentation to the ED, according to the judgement of the local radiologist together with at least two of the following signs or symptoms: cough, sputum production, temperature of >38°C or <36.1°C, auscultatory findings consistent with pneumonia, leukocytosis (>10.0 × 109 white blood cells (WBCs)/L) or leukopenia (<4.5 × 109 WBCs/L), a C-reactive protein level more than three times the upper limit of normal, hypoxaemia with Po2 < 60 mmHg while the patient was breathing room air, or dyspnoea/tachypnoea.
The causative microorganism of CAP was considered to be ‘definite’ if it was cultured from blood or any other sterile fluid, or if the urinary antigen test was positive (either for Legionella or pneumococcal antigen with the ICA or with the UAD). A causative microorganism of CAP was considered to be ‘probable’ in the absence of a definite pathogen, and if it was present as the dominant flora in the sputum culture. Pneumococcal CAP was defined as CAP with S. pneumoniae as the ‘definite’ or ‘probable’ causative microorganism. If S. pneumoniae was cultured/diagnosed together with another pathogen, CAP was not considered to be pneumococcal.
Data analysis
The SPSS statistical package (version 20.0; SPSS, Chicago, IL, USA) was used for the analyses. Potential predictors were analysed in the groups with and without pneumococcal CAP by univariate analysis followed by multivariate analysis, to select the independent predictors. The group ‘without pneumococcal CAP’ consisted intentionally of a combination of patients with CAP caused by other pathogens and with unknown aetiology, because the prediction model was developed for use in the ED. At the moment of presentation, it is unknown whether the CAP patient will belong to the ‘known’ or ‘unknown’ aetiology group, and adjustment of the study domain on the basis of this knowledge is therefore not reasonable. For univariate analysis, Pearson's chi-square test, a two-sample t-test or the Mann–Whitney U-test were used, depending on the data and distribution. All continuous variables were checked for the linearity assumption, and used as such whenever possible. The variable ‘leukocyte count’ had to be converted to a categorical variable in three categories (<4.5 × 109 WBCs/L, 4.5–10.0 × 109 WBCs/L, and >10.0 × 109 WBCs/L).
The variable ‘respiratory rate’ was converted to a dichotomous variable, because of many missing values (approximately 57%). It was assumed that this value would have been documented if the respiratory rate had been ≥30 breaths/min. Therefore, all missing values were considered as respiratory rates of <30 breaths/min. For all other missing values for the determinants, single imputation by regression methods was used.
The potential predictor variables were entered into a multivariate model to determine the independent predictors of pneumococcal CAP by using backward logistic regression based on the likelihood ratio, with a p-value of 0.05 being considered significant. This resulted in a probability score that was checked with the probability of pneumococcal pneumonia in a calibration plot. Also, the Hosmer–Lemeshow test was used to check calibration. A receiver operating characteristic curve was used to investigate discrimination. The model was evaluated for different probability cut-off values in order to create a high chance of predicting pneumococcal pneumonia for a reasonable proportion of patients. The relevance of this model in daily practice was evaluated by presenting the sensitivity, specificity and positive and negative predictive values for the various cut-off values.
Results
Study population
A total of 1758 patients with a clinical suspicion of CAP were included, of whom 630 did not have an infiltrate on the chest X-ray at presentation, three did not fulfil the inclusion criteria, 65 had one or more exclusion criteria, and three did not have one of the reference tests performed. This resulted in a study population of 1057 patients with radiographically confirmed CAP.
Aetiology
Blood cultures were available for 894 patients (84.6%), sputum cultures for 545 patients (51.6%), Legionella urinary antigen test results for 703 patients (66.5%), ICA results for 1032 patients (97.6%) (978 from centrally processed samples and 54 from locally processed samples), and UAD results for 979 cases (92.6%). Urine samples from 78 subjects were not available for central processing.
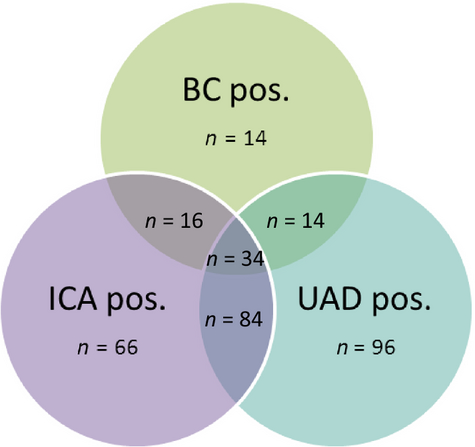
An aetiological cause of CAP could be determined in 461 patients (43.6%) (Table 1). The most common causative pathogen was S. pneumoniae (328 patients, 31.0%), followed by Haemophilus influenzae. In total, there were 228 UADs with a positive result and 200 ICAs with a positive result; the overlap of the various diagnostic modalities for S. pneumoniae is shown in Fig. 1. Three CAP episodes had a positive urine antigen test result for both S. pneumoniae and Legionella, and one episode had an Escherichia coli bacteraemia and a positive pneumococcal urinary antigen test result. In all four episodes, both the ICA and the UAD test gave positive results. These four cases were not considered to be true pneumococcal CAP (but were counted as ‘mixed aetiology’), as the ultimate goal of our prediction rule was to identify patients eligible for narrow-spectrum antibiotics.
| Pathogen | Definite pathogena | Probable pathogenb | Total (n = 1057), no. (%) |
|---|---|---|---|
| Streptocccus pneumoniae | 321 | 7 | 328 (31.0) |
| Haemophilus influenzae | 1 | 37 | 38 (3.6) |
| Legionella pneumophila | 27 | – | 27 (2.6) |
| Pseudomonas aeruginosa | 4 | 4 | 8 (0.8) |
| Moraxella catarrhalis | – | 6 | 6 (0.6) |
| Klebsiella species | 3 | 7 | 10 (0.9) |
| Escherichia coli | 3 | 6 | 9 (0.9) |
| Staphylococcus aureus | 4 | 6 | 10 (0.9) |
| Mixed aetiologyc | 6 | – | 6 (0.6) |
| Other | 6 | 13 | 19 (2.4) |
| Unknown | 596 (56.4) |
- a Positive blood culture or other sterile site or positive urinary antigen test result (either immunochromatographic assay (ICA) or urinary antigen detection test (UAD)).
- b In the absence of a definite pathogen and if the pathogen is the dominant flora in the sputum culture.
- c Consisting of: two blood cultures with two pathogens, one S. pneumoniae positive blood culture with a positive Legionella antigen test, one E. coli positive blood culture with a positive ICA and UAD, two positive ICA and UAD tests with a positive Legionella antigen test.
Predictors for pneumococcal CAP
In univariate analysis, 11 determinants were associated with pneumococcal CAP (Table 2). Pneumococcal immunization occurred in only three subjects, all in the ‘non-pneumococcal CAP’ group. In multivariate logistic regression analysis, nine predictors for pneumococcal CAP were identified (Table 3). The mean predicted probabilities correlated reasonably well with the observed probabilities, and the Hosmer–Lemeshow test had a p-value of 0.258, both indicating fair calibration of the model. The area under the receiver operating characteristic curve was 0.692 (95% CI 0.657–0.726). With a probability cut-off value of ≥0.50, the positive predictive value was 68%. Decreasing the cut-off value resulted in higher sensitivity and a higher negative predictive value, and increasing the cut-off value had the opposite effect (Table 4). However, no satisfying cut-off value could be determined for the use of this model in daily clinical practice.
| Determinants | Pneumococcal CAP (n = 328) | Other CAP (n = 729) | p-Value |
|---|---|---|---|
| Male gender, no. (%) | 182 (55.5) | 480 (65.8) | 0.002a |
| Present smoker, no. (%) | 102 (31.1) | 221 (30.3) | 0.829 |
| Comorbidities, no. (%) | |||
| COPD | 119 (36.3) | 263 (36.1) | 0.945 |
| Cardiovascular diseases | 188 (57.3) | 424 (58.2) | 0.840 |
| Diabetes mellitus | 48 (14.6) | 114 (15.6) | 0.713 |
| Renal failure | 9 (2.7) | 36 (4.9) | 0.137 |
| Malignancyb | 23 (7.0) | 60 (8.2) | 0.538 |
| Immunocompromised | 31 (9.5) | 69 (9.5) | 1.000 |
| Drug use, no. (%) | |||
| Proton-pump inhibitors | 82 (25.0) | 195 (26.7) | 0.597 |
| NSAIDs; not acetylsalicylic acid | 21 (6.4) | 68 (9.3) | 0.121 |
| Psychoactive drugs or benzodiazepines | 59 (18.0) | 145 (19.8) | 0.501 |
| Antibiotic pretreatmentc, no. (%) | 78 (23.8) | 258 (35.4) | 0.000a |
| Recurrent pneumoniasd, no. (%) | 55 (16.8) | 139 (19.1) | 0.392 |
| Pneumococcal immunization, no. (%) | 0 (0.0) | 3 (0.4) | 0.456 |
| Auscultation consistent with pneumonia, no. (%) | 258 (78.7) | 562 (77.1) | 0.633 |
| Symptoms at presentation, no. (%) | |||
| Altered mental status | 51 (15.5) | 102 (13.9) | 0.509 |
| Cough | 267 (81.4) | 555 (76.1) | 0.066 |
| Sputum production | 148 (45.1) | 319 (43.8) | 0.688 |
| Fever | 207 (63.1) | 427 (58.3) | 0.175 |
| Dyspnoea | 255 (77.7) | 575 (78.9 | 0.686 |
| Pleuritic pain | 82 (25.0) | 120 (16.5) | 0.001a |
| Chills | 50 (15.2) | 107 (14.6) | 0.852 |
| Respiratory rate of > 30 breaths/min | 42 (12.8) | 63 (8.6) | 0.045a |
| Duration of complaints before admission (days) median (IQR) | 3 (2–7) | 4 (2–8) | 0.002a |
| Age (years) median (IQR) | 67 (55–77) | 70 (57–79) | 0.047a |
| Systolic blood pressure (mmHg) mean (SD) | 127 ± 26.2 | 133 ± 23.6 | 0.001a |
| O2 saturation (%) median (IQR) | 94 (90–96) | 94 (91–96) | 0.286 |
| Pulse rate (beats/min) mean (SD) | 103 ± 20.8 | 99 ± 20.6 | 0.007a |
| White blood cells (×109/L) median (IQR) | 15.8 (11.7–21.4) | 12.8 (9.5–16.5) | 0.000a |
| CRP (mg/L) median (IQR) | 186 (80–323) | 128 (52–229) | 0.000a |
| Urea/BUN (mM) median (IQR) | 7.4 (5.2–11.2) | 6.6 (4.7–9.7) | 0.009a |
| Sodium (mM) median (IQR) | 136 (134–139) | 137 (134–140) | 0.967 |
| Glucose (mM) median (IQR) | 7.3 (6.2–8.8) | 7.1 (6.0–8.5) | 0.163 |
| Haematocrit median (IQR) | 0.39 (0.36–0.43) | 0.40 (0.36–0.43) | 0.788 |
- BUN, blood urea nitrogen; CAP, community-acquired pneumonia; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein IQR, interquartile range;; NSAID, non-steroidal anti-inflammatory drug; SD, standard deviation.
- a Significant difference between the group with ‘pneumococcal CAP’ and the group with ‘other CAP’.
- b Active or diagnosed in the year before admission.
- c In the 2 weeks before admission.
- d Two or more pneumonias requiring hospitalization during the last 5 years.
| B | p-Value | OR | 95% CI for OR | |
|---|---|---|---|---|
| Male gender | −0.470 | 0.001 | 0.625 | 0.471–0.830 |
| Antibiotic pretreatment | −0.516 | 0.001 | 0.597 | 0.435–0.818 |
| Cough | 0.435 | 0.014 | 1.544 | 1.091–2.186 |
| Pleuritic pain | 0.415 | 0.016 | 1.515 | 1.079–2.128 |
| Days complaints | −0.019 | 0.028 | 0.981 | 0.964–0.998 |
| Systolic BP (mmHg) | −0.008 | 0.005 | 0.992 | 0.986–0.998 |
| CRP (mg/L) | 0.003 | 0.000 | 1.003 | 1.002–1.004 |
| Sodium (mM) | 0.035 | 0.027 | 1.036 | 1.004–1.069 |
| WBC: 4.5–10 × 109/L (ref) | NA | NA | NA | NA |
| WBC: ≤4.5 × 109/L | 0.960 | 0.047 | 2.611 | 1.013–6.731 |
| WBC: ≥10 × 109/L | 0.791 | 0.000 | 2.206 | 1.516–3.212 |
| Constant | −5.582 | 0.014 | 0.004 |
- B, coefficient in prediction formula; BP, blood pressure; CRP, C-reactive protein; NA, not applicable; ref, reference; WBC, white blood cell.
| Cut-off value | N | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| ≥0.3 | 502 | 65.9 | 60.8 | 43.0 | 79.8 |
| ≥0.4 | 265 | 42.1 | 82.6 | 52.1 | 76.0 |
| ≥0.5 | 120 | 25.0 | 94.8 | 68.3 | 73.7 |
| ≥0.6 | 54 | 13.1 | 98.5 | 79.6 | 71.6 |
| ≥0.7 | 16 | 4.9 | 100.0 | 100.0 | 70.0 |
- N, number of patients with cut-off value above the set limit; NPV, negative predictive value; PPV, positive predictive value.
Discussion
This study of 1057 patients failed to demonstrate that clinical criteria can be used to predict a pneumococcal aetiology in patients with CAP presenting at the ED. The clinical value of predicting a pneumococcal aetiology of CAP lies in the possibility of minimizing empirical broad-spectrum antibiotic treatment before the microbiological test results are available. For this purpose, a prediction rule should have a high positive predictive value, in order to avoid inappropriate therapy in severely ill patients. However, in this study, the combination of predictors and the cut-off value needed to reach a 100% positive predictive value implied that only 1.5% of all CAP patients in the ED would be classified as having pneumococcal CAP. Using lower cut-off values would inevitably result in misclassification of patients and the potential prescription of incorrect antibiotic treatment.
So, despite the inclusion of 1057 patients with CAP, with 328 diagnosed as having pneumococcal CAP, we were unable to develop a useful clinical prediction rule. Previously, Bohte et al. 16 developed a scoring system to predict S. pneumoniae as the causative organism of CAP. However, that rule can only be applied if a sputum Gram stain result is available, which is not the case in c. 50% of all CAP patients 19. Furthermore, this rule was designed by comparing data from 79 patients with pneumococcal CAP with those from 83 patients with CAP caused by other pathogens, excluding the information from 106 patients in whom a causative pathogen could not be detected. From our point of view, this selection of patients does not reflect daily practice, because it is not known beforehand whether a pathogen can be detected.
Other investigators have also failed to accurately predict the aetiology of CAP on the basis of clinical patterns or radiographic features 14, 15, 20, 21. Ruiz-Gonzales et al. 11 developed a bedside rule to distinguish ‘virus-like’ pneumonia (including Mycoplasma pneumoniae and Legionella pneumophila) and bacterial pneumonia with three clinical predictors. They used different scores with sensitivities between 57% and 89% and specificities between 63% and 94%. However, this rule was developed with a study population of 103 patients only (48 with virus-like pneumonia and 37 with bacterial pneumonia), excluding patients with unknown aetiology (n = 18), which, as mentioned above, does not correspond to daily practice in the ED.
We had expected to be more successful in our efforts to predict pneumococcal CAP, because of our large sample size and high proportion of patients with pneumococcal CAP, owing to the addition of the UAD, but the nine independent predictors had no useful predictive value for applying this rule in daily practice. This leaves rapid pneumococcal antigen detection in urine as the only opportunity to start narrow-spectrum antibiotics directly at admission. In our cohort, the ICA could have allowed narrow-spectrum antibiotics to be started in 18.9% of the study population (n = 200) when used as a ‘point-of-care’ test in the ED.
In a study using the ICA as a treatment decision strategy, amoxycillin was prescribed to 48 of 219 patients with CAP on the basis of ICA results, and appeared to be as effective as clarithromycin in all other patients 22. In two other observational studies, only 31.5% and 35% of the patients with positive ICA results switched to (more) targeted antibiotic treatment 23, 24.
In another study, patients with CAP were—after reaching clinical stabilization—randomized to empirical treatment (broad-spectrum β-lactam + macrolide/levofloxacin) or targeted treatment (amoxycillin) 25. Of the 88 patients in the targeted treatment arm, 22 (25%) were switched to amoxycillin after an average of 5 days, because of a positive pneumococcal ICA result at admission. Three of those patients experienced a clinical relapse after antibiotic switch, and the authors concluded that narrowing treatment on the basis of ICA results carries a higher risk of clinical relapse. However, this conclusion was based on relatively few patients, and amoxycillin was started after the patient was considered to be clinically stable. Therefore, we feel that these conclusions should be interpreted with caution.
The strengths of our study include the size of our study population, the uniform methods for data collection, and the high proportion of episodes categorized as pneumococcal CAP owing to the addition of a new, highly sensitive, diagnostic test. Our study also has some limitations. First, we were not able to perform the UAD in all cases, which might have created a partial verification bias. However, this accounts for only 78 cases (7.4%), and we used the combination of results of the other diagnostic tools (ICA, blood culture, and sputum culture) from the local laboratory. Therefore, we believe that the risk of bias is very limited. Furthermore, there were some missing data among the determinants, which were imputed with regression methods.
In conclusion, clinical criteria cannot be used to diagnose pneumococcal CAP, but urinary antigen testing could offer the possibility of safely avoiding broad-spectrum antibiotic therapy in c. 20% of episodes of CAP.
Acknowledgements
The authors would like to acknowledge the substantial contribution to this study made by M. Peeters (St Elisabeth Hospital, Tilburg, The Netherlands), who sadly passed away in June 2011, and of R. Veenhoven (Spaarne Hospital, Hoofddorp, The Netherlands), who sadly passed away in October 2013. Furthermore, we would like to thank all CAP diagnostic investigators for their time and effort. The preliminary results of this study without the UAD data were presented at ISPPD 2010, Tel Aviv, Israel.
Authorship
S. M. Huijts and M. J. M. Bonten were responsible for study design, data collection, and writing the manuscript. D. E. Grobbee reviewed the manuscript. W. C. Gruber, K. U. Jansen, M. W. Pride and C. Webber reviewed the study protocol and the manuscript. W. G. Boersma, J. A. J. W. Kluytmans, A. F. Kuipers and F. Palmen were involved in data collection and reviewed the manuscript.
Transparency Declaration
This study was sponsored by Wyeth Pharmaceuticals Inc., which was acquired by Pfizer in October 2009. Role of funder: financial support and review of the protocol and article. M. Pride, K. U. Jansen, C. Webber and W. C. Gruber report being employees of Pfizer Inc., and hold stock in Pfizer Inc.